Alterations of the Adipo–Myokine Irisin in Sepsis and Septic Shock: Diagnostic and Prognostic Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Evaluation
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients and Controls
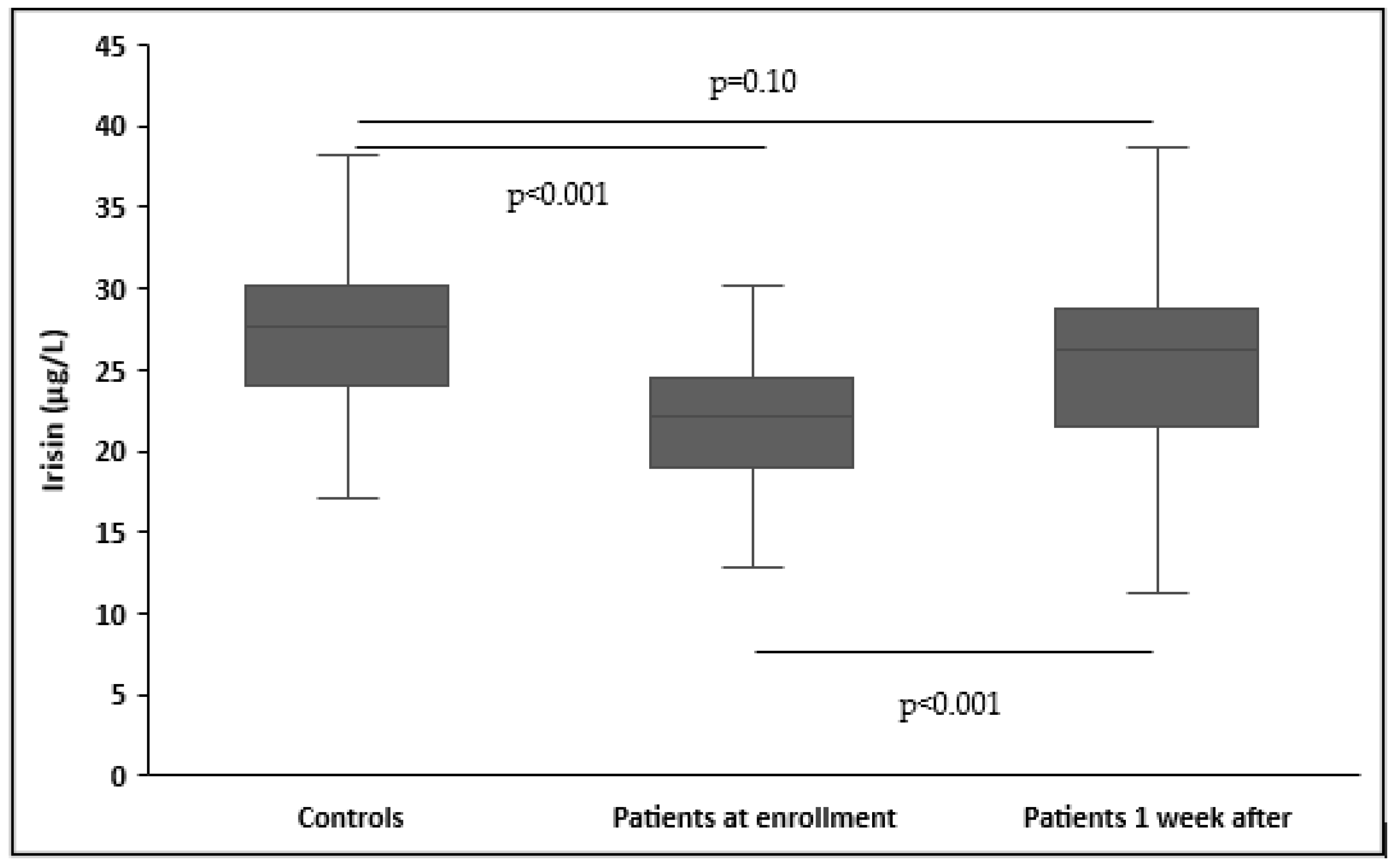
3.2. Circulating Irisin in Patients and Controls
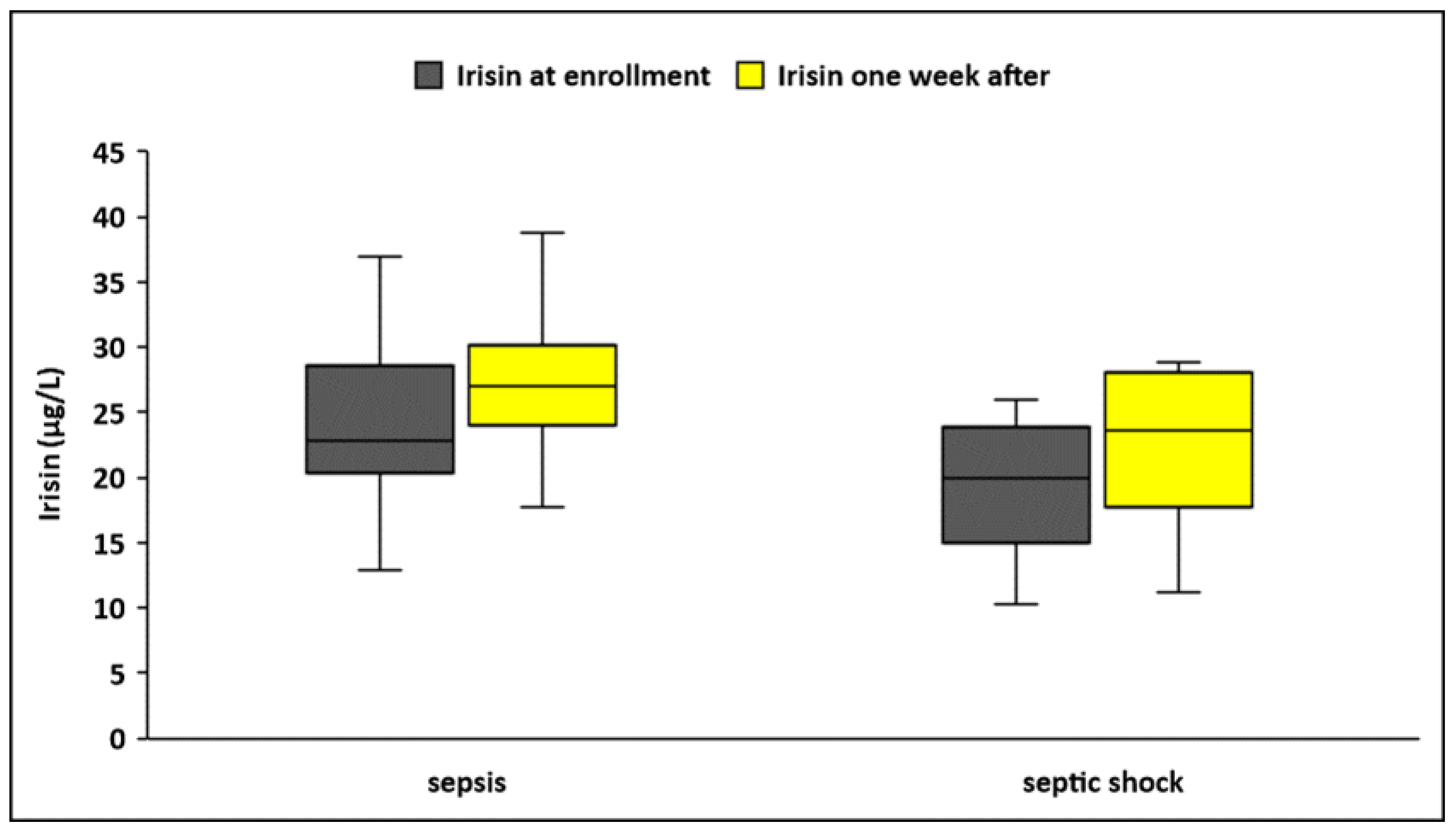
3.3. Circulating Irisin in Sepsis and Septic Shock
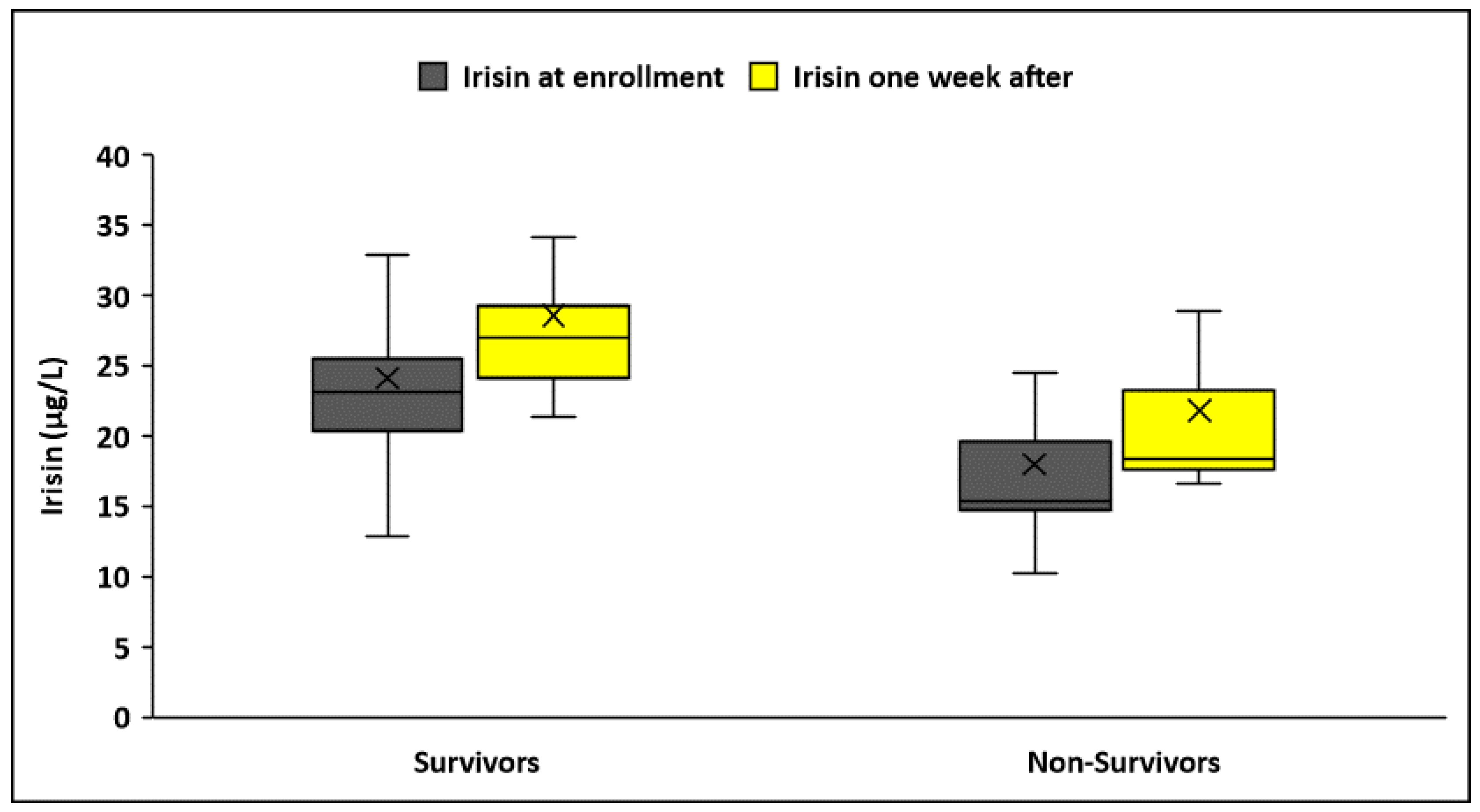
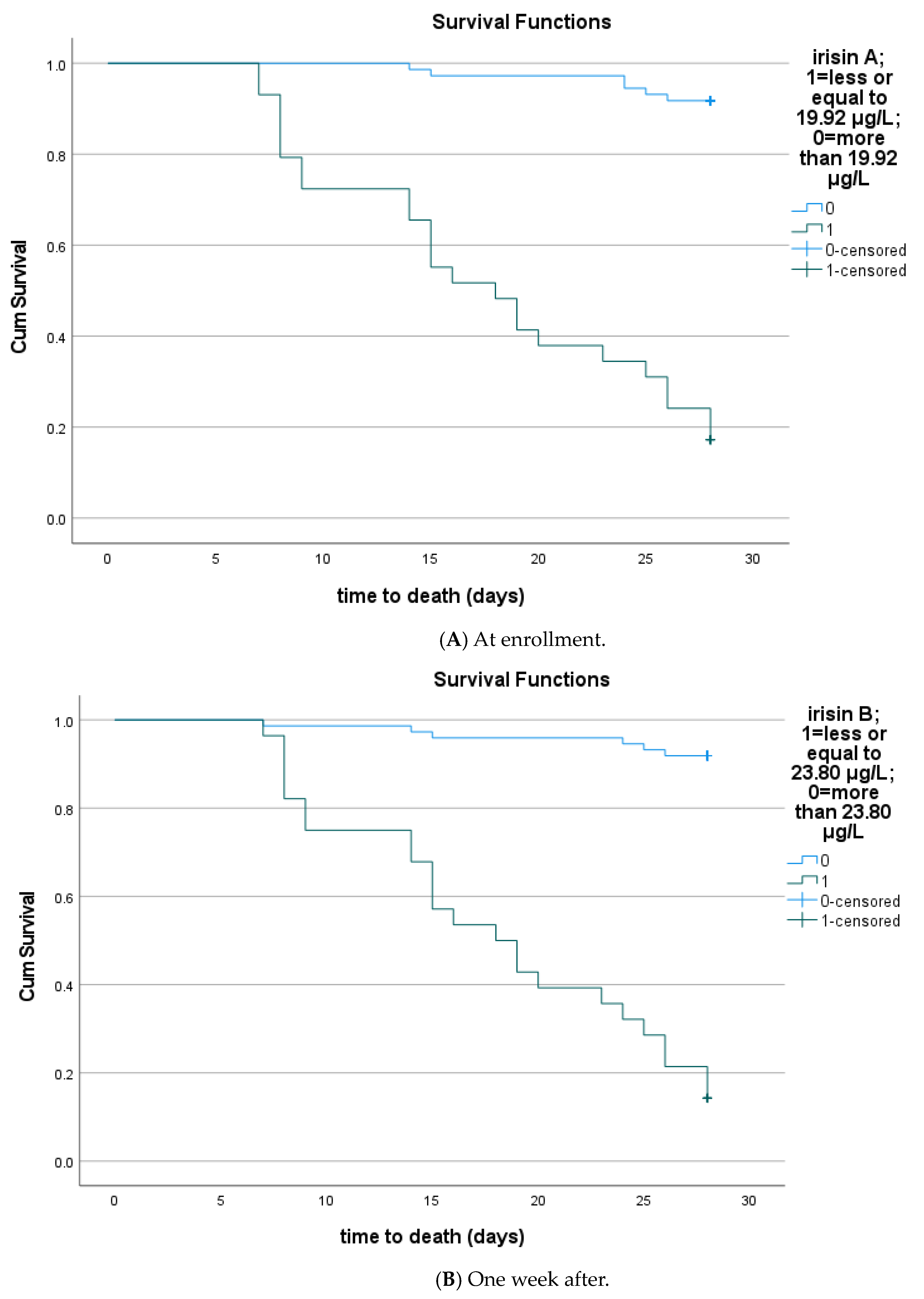
3.4. Circulating Irisin According to Sepsis Outcomes
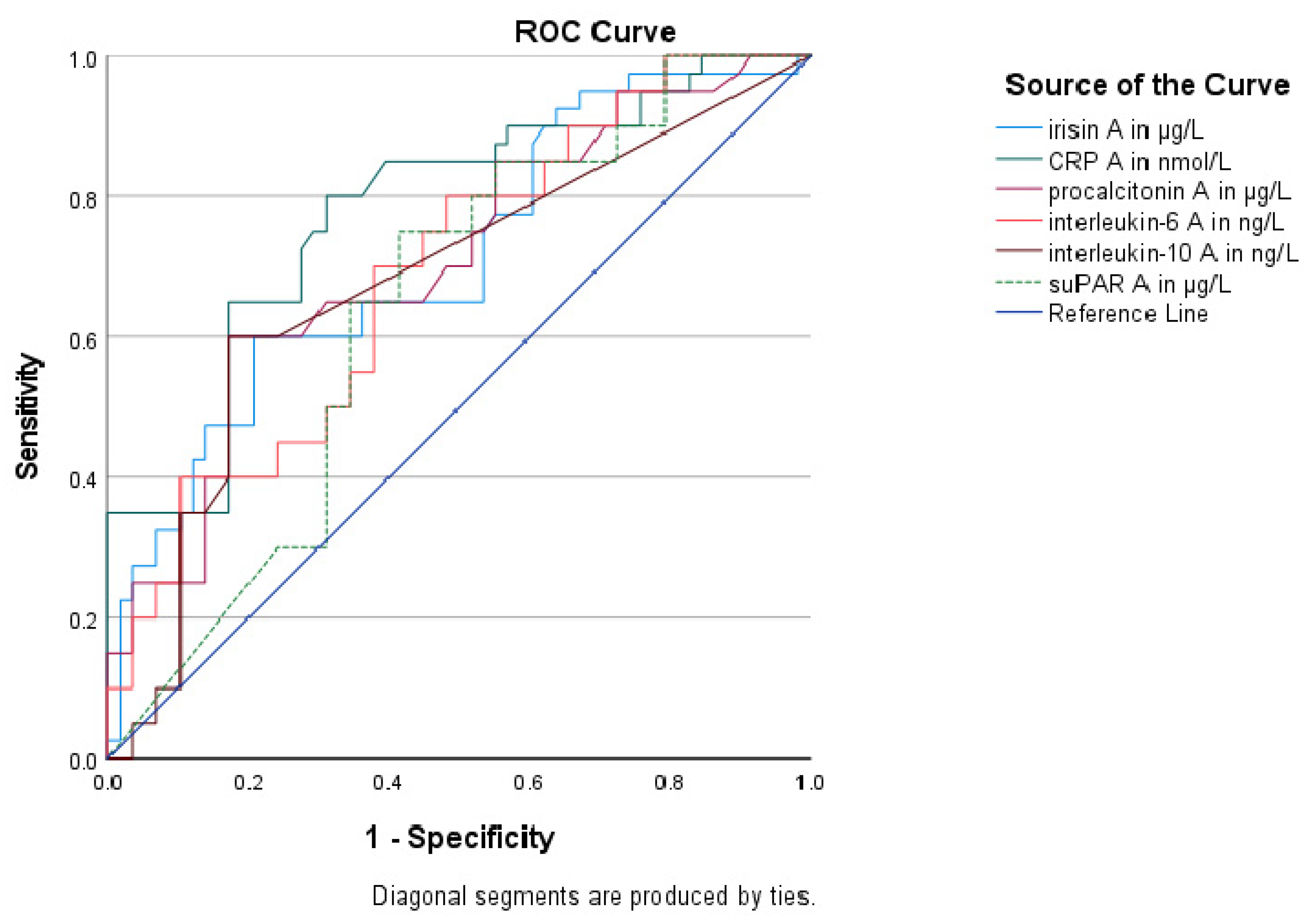
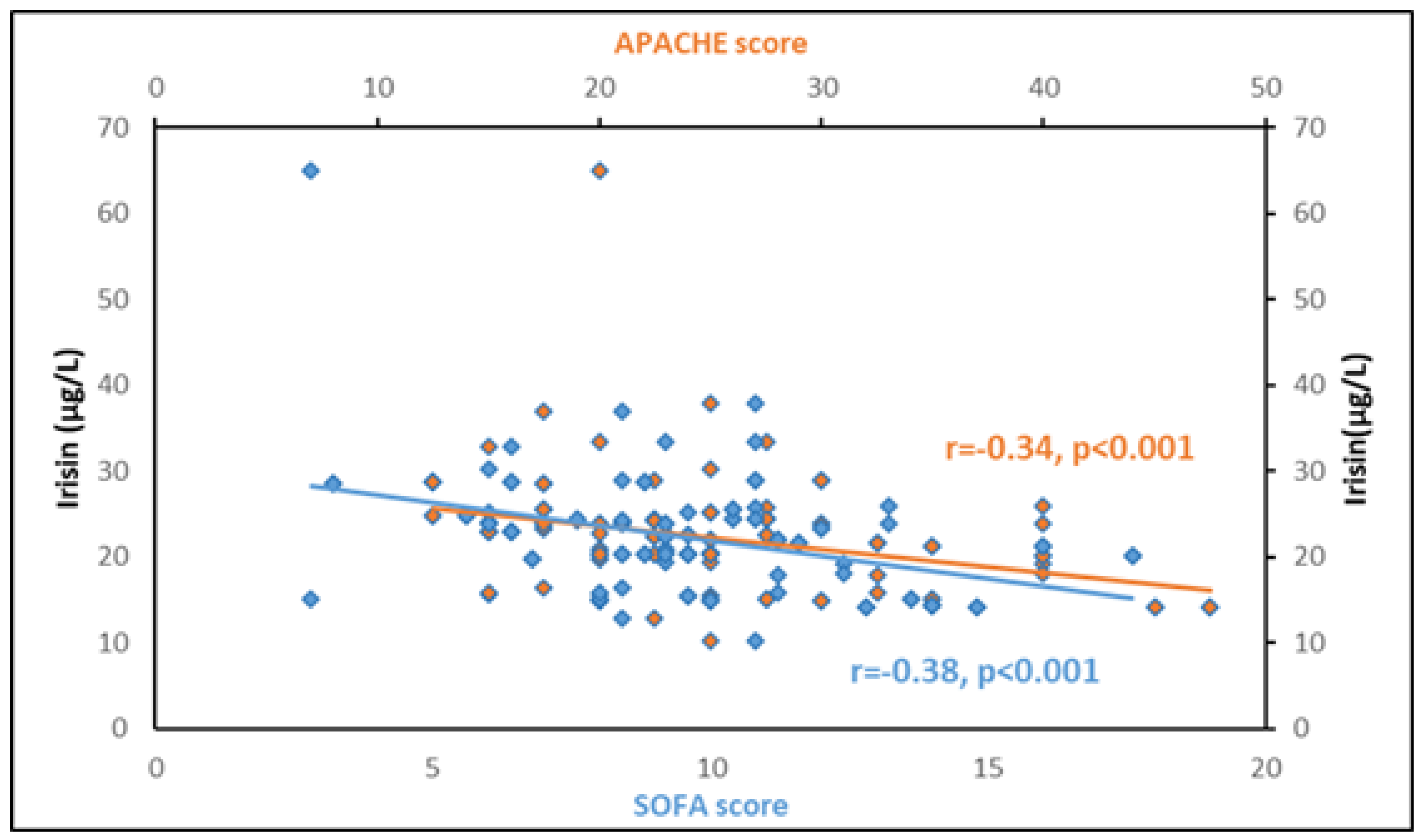
3.5. Association of Circulating Irisin with Other Biomarkers
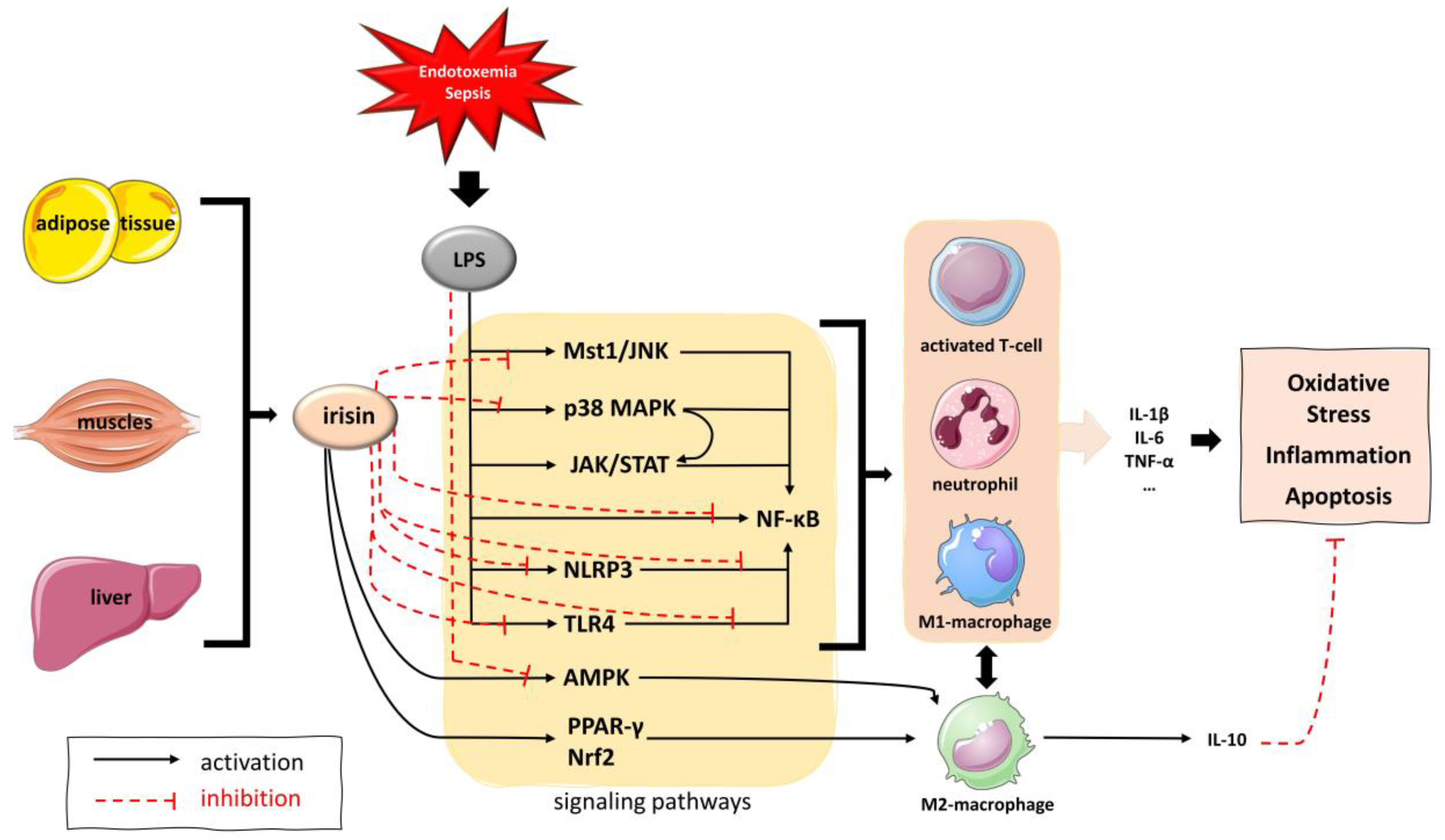
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/Irisin: Physiology and Pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef]
- Hofmann, T.; Elbelt, U.; Stengel, A. Irisin as a muscle-derived hormone stimulating thermogenesis—A critical update. Peptides 2014, 54, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.N.; Moreno, M.; Polyzos, S.A.; Moreno-Navarrete, J.M.; Ricart, W.; Delgado, E.; de la Hera, J.; Sahin-Efe, A.; Chamberland, J.P.; Berman, R.; et al. Circulating irisin levels and coronary heart disease: Association with future acute coronary syndrome and major adverse cardiovascular events. Int. J. Obes. 2015, 39, 156–161. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Liu, J.; Dalamaga, M. Could exercise hormone irisin be a therapeutic agent against Parkinson’s and other neurodegenerative diseases? Metabol. Open 2023, 17, 100233. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.C.; Vella, J.P.; Cafeo, F.R.; Affonso Fonseca, F.L.; Bacci, M.R. Association between irisin and major chronic diseases: A review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4072–4077. [Google Scholar] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Yu, F.; Wei, W.P.; Yang, P.; Zhang, R.; Sheng, Y.; Shi, Y.Q. Relationship between circulating irisin levels and overweight/obesity: A meta-analysis. World J. Clin. Cases 2019, 7, 1444–1455. [Google Scholar] [CrossRef]
- Elizondo-Montemayor, L.; Gonzalez-Gil, A.M.; Tamez-Rivera, O.; Toledo-Salinas, C.; Peschard-Franco, M.; Rodríguez-Gutiérrez, N.A.; Silva-Platas, C.; Garcia-Rivas, G. Association between Irisin, hs-CRP, and Metabolic Status in Children and Adolescents with Type 2 Diabetes Mellitus. Mediat. Inflamm. 2019, 2019, 6737318. [Google Scholar] [CrossRef] [PubMed]
- Stratigou, T.; Dalamaga, M.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Christodoulatos, G.S.; Karampela, I.; Papavassiliou, A.G. Hyperirisinemia is independently associated with subclinical hypothyroidism: Correlations with cardiometabolic biomarkers and risk factors. Endocrine 2018, 61, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Vliora, M.; Nintou, E.; Karligiotou, E.; Ioannou, L.G.; Grillo, E.; Mitola, S.; Flouris, A.D. Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 9971. [Google Scholar] [CrossRef]
- Christodoulatos, G.S.; Spyrou, N.; Kadillari, J.; Psallida, S.; Dalamaga, M. The Role of Adipokines in Breast Cancer: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 413–433. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Geng, Z.; Zhou, B.; Zhang, F.; Han, Y.; Zhou, Y.B.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism 2018, 83, 31–41. [Google Scholar] [CrossRef]
- Ouyang, H.; Li, Q.; Zhong, J.; Xia, F.; Zheng, S.; Lu, J.; Deng, Y.; Hu, Y. Combination of melatonin and irisin ameliorates lipopolysaccharide-induced cardiac dysfunction through suppressing the Mst1-JNK pathways. J. Cell. Physiol. 2020, 235, 6647–6659. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, J.; Kong, D.; Guo, X.; Li, J.; Long, Z.; Peng, J.; Wang, Z.; Wu, H.; Liu, P.; et al. Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARγ and Nrf2 signaling. Free Radic. Biol. Med. 2023, 201, 98–110. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, Y.; Zhou, Y.; Chen, J.; Sun, C.; Chen, Z.; Jing, C.; Xu, L.; Liu, F.; Ni, W.; et al. Irisin protects female mice with LPS-induced endometritis through the AMPK/NF-κB pathway. Iran. J. Basic Med. Sci. 2021, 24, 1247–1253. [Google Scholar] [CrossRef]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin reduces inflammatory signaling pathways in inflammation-mediated metabolic syndrome. Mol. Cell. Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Zhang, Y.; Liu, C.; Wang, Y.; Zhang, L.; Shi, Z.; Wu, Z.; et al. Exercise hormone irisin mitigates endothelial barrier dysfunction and microvascular leakage-related diseases. JCI Insight 2020, 5, e136277. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Tan, Y.; Ouyang, H.; Xiao, X.; Zhong, J.; Dong, M. Irisin ameliorates septic cardiomyopathy via inhibiting DRP1-related mitochondrial fission and normalizing the JNK-LATS2 signaling pathway. Cell Stress Chaperones 2019, 24, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, M.; Zhao, Y.; Dong, M. Irisin Protects Against LPS-Stressed Cardiac Damage Through Inhibiting Inflammation, Apoptosis, and Pyroptosis. Shock 2021, 56, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Q.; Wang, Y.; Peng, J.; Shao, L.; Li, X. Irisin protects against sepsis-associated encephalopathy by suppressing ferroptosis via activation of the Nrf2/GPX4 signal axis. Free Radic. Biol. Med. 2022, 187, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Qiongyue, Z.; Xin, Y.; Meng, P.; Sulin, M.; Yanlin, W.; Xinyi, L.; Xuemin, S. Post-treatment with Irisin Attenuates Acute Kidney Injury in Sepsis Mice Through Anti-Ferroptosis via the SIRT1/Nrf2 Pathway. Front. Pharmacol. 2022, 13, 857067. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Li, Z.Y.; Jiang, X.Q.; Wu, F.; Li, Z.T.; Chen, H.; Xi, D.; Zhang, Y.Y.; Chen, Z.Q. Irisin alleviates renal injury caused by sepsis via the NF-κB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6470–6476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liao, J.; Jin, L.; Lin, Y. NLRP3 inflammasome involves in the pathophysiology of sepsis-induced myocardial dysfunction by multiple mechanisms. Biomed. Pharmacother. 2023, 167, 115497. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Bi, J.; Yang, L.; Zhang, J.; Wan, Y.; Chen, X.; Wang, Y.; Wu, Z.; Lv, Y.; Wu, R. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin. Transl. Med. 2020, 10, e173. [Google Scholar] [CrossRef] [PubMed]
- Vanhorebeek, I.; Gunst, J.; Casaer, M.P.; Derese, I.; Derde, S.; Pauwels, L.; Segers, J.; Hermans, G.; Gosselink, R.; Van den Berghe, G. Skeletal Muscle Myokine Expression in Critical Illness, Association with Outcome and Impact of Therapeutic Interventions. J. Endocr. Soc. 2023, 7, bvad001. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Christodoulatos, G.S.; Kandri, E.; Antonakos, G.; Vogiatzakis, E.; Dimopoulos, G.; Armaganidis, A.; Dalamaga, M. Circulating eNampt and resistin as a proinflammatory duet predicting independently mortality in critically ill patients with sepsis: A prospective observational study. Cytokine 2019, 119, 62–70. [Google Scholar] [CrossRef]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef]
- Karampela, I.; Kandri, E.; Antonakos, G.; Vogiatzakis, E.; Christodoulatos, G.S.; Nikolaidou, A.; Dimopoulos, G.; Armaganidis, A.; Dalamaga, M. Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: A prospective study. J. Crit. Care 2017, 41, 78–85. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Vallianou, N.; Tsilingiris, D.; Chrysanthopoulou, E.; Skyllas, G.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; et al. Circulating Chemerin and Its Kinetics May Be a Useful Diagnostic and Prognostic Biomarker in Critically Ill Patients with Sepsis: A Prospective Study. Biomolecules 2022, 12, 301. [Google Scholar] [CrossRef]
- Sotiropoulos, G.P.; Kotopouli, M.; Karampela, I.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Lekka, A.; Papavassiliou, A.G.; Dalamaga, M. Circulating plasminogen activator inhibitor-1 activity: A biomarker for resectable non-small cell lung cancer? J. BUON 2019, 24, 943–954. [Google Scholar]
- Dalamaga, M.; Nikolaidou, A.; Karmaniolas, K.; Hsi, A.; Chamberland, J.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: A case-control study. Oncology 2007, 73, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hroussalas, G.; Kassi, E.; Dalamaga, M.; Delimaris, I.; Zachari, A.; Dionyssiou-Asteriou, A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas 2008, 59, 339–349. [Google Scholar] [CrossRef]
- Karampela, I.; Chrysanthopoulou, E.; Skyllas, G.; Christodoulatos, G.S.; Kandri, E.; Antonakos, G.; Stratigou, T.; Armaganidis, A.; Dalamaga, M. Circulating leptin, soluble leptin receptor and free leptin index in critically ill patients with sepsis: A prospective observational study. Minerva Anestesiol. 2021, 87, 880–890. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karmaniolas, K.; Papadavid, E.; Pelekanos, N.; Sotiropoulos, G.; Lekka, A. Hyperresistinemia is associated with postmenopausal breast cancer. Menopause 2013, 20, 845–851. [Google Scholar] [CrossRef]
- Pavlidou, A.; Dalamaga, M.; Kroupis, C.; Konstantoudakis, G.; Belimezi, M.; Athanasas, G.; Dimas, K. Survivin isoforms and clinicopathological characteristics in colorectal adenocarcinomas using real-time qPCR. World J. Gastroenterol. 2011, 17, 1614–1621. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. Res. 2021, 41, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Lu, L.; Wang, Z.; Ma, J.; Shao, Y.; Liu, Y.; Zhai, M.; Jin, P.; Yang, J.; Zheng, Q.; et al. Irisin attenuates sepsis-induced cardiac dysfunction by attenuating inflammation-induced pyroptosis through a mitochondrial ubiquitin ligase-dependent mechanism. Biomed. Pharmacother. 2022, 152, 113199. [Google Scholar] [CrossRef]
- Jiang, X.; Cai, S.; Jin, Y.; Wu, F.; He, J.; Wu, X.; Tan, Y.; Wang, Y. Irisin Attenuates Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in the H9C2 Cellular Model of Septic Cardiomyopathy through Augmenting Fundc1-Dependent Mitophagy. Oxid. Med. Cell Longev. 2021, 2021, 2989974. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X.; Yuan, C.; Li, R.; Ma, Y.; Tang, X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: A cross-sectional study. Sci. Rep. 2020, 10, 16093. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Dalamaga, M. The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox. Curr. Obes. Rep. 2019, 8, 434–457. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Chrysanthopoulou, E.; Christodoulatos, G.S.; Dalamaga, M. Is There an Obesity Paradox in Critical Illness? Epidemiologic and Metabolic Considerations. Curr. Obes. Rep. 2020, 9, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Matsumoto, H.; Matsubara, T.; Matsuura, H.; Hirose, T.; Shimizu, K.; Ogura, H.; Kang, S.; Tanaka, T.; Shimazu, T. Adipocytokine Profile Reveals Resistin Forming a Prognostic-Related Cytokine Network in the Acute Phase of Sepsis. Shock 2021, 56, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Luedde, M.; Benz, F.; Niedeggen, J.; Vucur, M.; Hippe, H.J.; Spehlmann, M.E.; Schueller, F.; Loosen, S.; Frey, N.; Trautwein, C.; et al. Elevated Omentin Serum Levels Predict Long-Term Survival in Critically Ill Patients. Dis. Markers 2016, 2016, 3149243. [Google Scholar] [CrossRef]
- Hillenbrand, A.; Weiss, M.; Knippschild, U.; Wolf, A.M.; Huber-Lang, M. Sepsis-Induced Adipokine Change with regard to Insulin Resistance. Int. J. Inflamm. 2012, 2012, 972368. [Google Scholar] [CrossRef]
- Horn, P.; Metzing, U.B.; Steidl, R.; Romeike, B.; Rauchfuß, F.; Sponholz, C.; Thomas-Rüddel, D.; Ludewig, K.; Birkenfeld, A.L.; Settmacher, U.; et al. Chemerin in peritoneal sepsis and its associations with glucose metabolism and prognosis: A translational cross-sectional study. Crit. Care 2016, 20, 39. [Google Scholar] [CrossRef]
- Karampela, I.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; Dalamaga, M. Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients. Medicina 2023, 59, 833. [Google Scholar] [CrossRef]
| Parameters | Patients (N = 102) | Controls (N = 102) | p-Value |
|---|---|---|---|
| Demographic parameters | |||
| Age a, years | 64.7 ± 15.6 | 66.4 ± 10.3 | 0.35 |
| Gender, male, n (%) | 57 (55.9) | 57 (55.9) | 0.56 |
| BMI a, kg/m2 | 29.9 ± 8.5 | 28.1 ± 5.01 | 0.06 |
| Clinical parameters | |||
| APACHE II a | 23 ± 7.2 | - | - |
| SOFA a | 10 ± 3.3 | - | - |
| Septic shock, n (%) | 42 (41.2) | - | - |
| Death at 28 days, n (%) | 30 (29.4) | - | - |
| Hematologic and coagulation parameters | |||
| Hemoglobin a, g/L | 93 ± 20 | 147.9 ± 16.3 | <0.001 |
| White Blood Cells a ×109/L | 14.1 ± 8.4 | 6.97 ± 1.8 | <0.001 |
| Platelets a ×109/L | 216.2 ± 118.8 | 243.8 ± 46.9 | 0.03 |
| Prothrombin time a, s | 14.3 ± 4.7 | 11.9 ± 0.8 | <0.001 |
| aPTT a, s | 38.9 ± 9.4 | 34.4 ± 7.3 | <0.001 |
| Fibrinogen a, μmol/L | 14.49 ± 5.26 | 9.06 ± 1.3 | <0.001 |
| D-dimer a, μg/L | 7278 ± 8158 | - | - |
| Organ dysfunction biomarkers | |||
| Lactate b, mmol/L | 2.1 (1–9) | - | |
| Total Protein a, g/L | 50 ± 9 | 71 ± 4.2 | <0.001 |
| Albumin a, g/L | 24.6 ± 5.9 | 46.7 ± 5.6 | <0.001 |
| Creatinine a, μmol/L | 124 ± 71 | 74 ± 12 | 0.08 |
| AST b, U/L | 33 (8–896) | 17.5 (7–34) | <0.001 |
| ALT b, U/L | 31 (9–965) | 22.5 (9–42) | 0.002 |
| Metabolic parameters | |||
| Glucose a, mmol/L | 7.97 ± 2.9 | 5.32 ± 1.16 | <0.001 |
| HOMA-IR b | 8.9 (3.24–34.5) | 2.3 (0.65–23.5) | <0.001 |
| Inflammatory biomarkers | |||
| CRP b, nmol/L | 1257 (67–4104) | 32 (1–104) | <0.001 |
| Procalcitonin b, μg/L | 0.9 (0.1–100) | - | - |
| IL-1β b, ng/L | 5.9 (5.9–206) | - | - |
| IL-6 b, ng/L | 27.4 (6–444) | - | - |
| IL-10 b, ng/L | 5 (5–300) | - | - |
| suPAR b, μg/L | 13 (2.1–16.8) | - | - |
| Irisin a, μg/L | 22.3 ± 6.8 | 28.1 ± 6.7 | <0.001 |
| Baseline | One Week After | |||||
| Sepsis (n = 60) | Septic Shock (n = 42) | p-Value | Sepsis (n = 60) | Septic Shock (n = 42) | p-Value | |
| White Blood Cells a ×109/L | 12.5 ± 5.9 | 16.3 ± 10.7 | 0.02 | 8.5 ± 3.2 | 16.2 ± 11.1 | <0.001 |
| Platelets a ×109/L | 230.4 ± 117.6 | 195.8 ± 118.8 | 0.15 | 252.7 ± 120.3 | 174.6 ± 97.9 | 0.001 |
| Albumin a, g/L | 26 ± 5.6 | 22.6 ± 5.7 | 0.004 | 25.1 ± 4.8 | 22.5 ± 4.2 | 0.005 |
| Lactate b, mmol/L | 1.2 (1–5) | 2.4 (2.1–9) | <0.001 | 1 (1–2.7) | 1.9 (0.7–19) | <0.001 |
| CRP b, nmol/L | 848 (67–2076) | 1667 (344–4105) | <0.001 | 524 (76–2686) | 962 (124–2410) | 0.01 |
| Procalcitonin b, μg/L | 0.7 (0.09–47.7) | 4.8 (0.14–100) | 0.002 | 0.5 (0.06–15) | 1.4 (0.14–83) | 0.001 |
| IL-1β b, ng/L | 5.9 (5.9–207) | 8.8 (5.9–44.8) | 0.18 | 17 (5.9–499) | 8.8 (5.9–45) | 0.13 |
| IL-6 b, ng/L | 16.5 (6–385) | 74.4 (10–444) | 0.001 | 25 (4.6–419) | 20.5 (6–487) | 0.34 |
| IL-10 b, ng/L | 5 (5–300) | 6.9 (5–87) | 0.001 | 5 (5–300) | 5 (5–66) | 0.02 |
| suPAR b, μg/L | 10.5 (2.2–16.8) | 14.1 (4.4–16.8) | 0.04 | 11.3 (2.6–16.8) | 12.9 (5.2–16.8) | 0.68 |
| Irisin a, μg/L | 24.2 ± 7.3 | 19.6 ± 5.1 | <0.001 | 28.7 ± 10.5 | 23.6 ± 7 | 0.004 |
| Biomarkers | AUC (95% CI) | p Value | Sensitivity | Specificity | Youden Index | Cutoff Value | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|---|---|---|
| Irisin | 0.72 (0.62–0.82) | <0.001 | 62% | 80% | 0.42 | 20.32 μg/L | 68.4% | 75% |
| CRP | 0.78 (0.68–0.87) | <0.001 | 80% | 69% | 0.49 | 1257 nmol/L | 64.4% | 83.1% |
| PCT | 0.71 (0.60–0.81) | 0.001 | 60% | 83% | 0.43 | 4.30 μg/L | 70.9% | 74.7% |
| IL-6 | 0.69 (0.58–0.79) | 0.001 | 70% | 62% | 0.32 | 24.50 ng/L | 56.4% | 74.7% |
| IL-10 | 0.68 (0.57–0.79) | 0.003 | 60% | 83% | 0.43 | 5.88 ng/L | 70.9% | 74.7% |
| suPAR | 0.64 (0.53–0.75) | 0.02 | 75% | 59% | 0.34 | 11.79 μg/L | 55.9% | 77% |
| Biomarkers | AUC (95% C.I.) | p Value | Sensitivity | Specificity | Youden’s Index | Cutoff Value | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|---|---|---|
| Irisin | 0.81 (0.69–0.93) | <0.001 | 77% | 93% | 0.63 | 19.92 μg/L | 88.5% | 86.1% |
| CRP | 0.72 (0.60–0.83) | 0.001 | 67% | 74% | 0.4 | 1462 nmol/L | 63.9% | 75.9% |
| PCT | 0.65 (0.54–0.78) | 0.02 | 53% | 76% | 0.29 | 4.60 μg/L | 61.3% | 70% |
| IL-6 | 0.65 (0.53–0.78) | 0.02 | 53% | 83% | 0.36 | 110.3 ng/L | 68.6% | 71.7% |
| IL-10 | 0.65 (0.53–0.77) | 0.02 | 64% | 84% | 0.39 | 5.68 ng/L | 63.7% | 74.8% |
| suPAR | 0.55 (0.44–0.67) | 0.39 ‡ | - | - | - | - | - | - |
| b | SEb | Wald | df | p-Value | HR | 95% for C.I. | |
|---|---|---|---|---|---|---|---|
| Independent predictors at enrollment | |||||||
| Irisin | −0.81 | 0.28 | 8.41 | 1 | 0.004 | 0.44 | 0.26–0.77 |
| CRP | −0.008 | 0.17 | 0.002 | 1 | 0.96 | 0.99 | 0.70–1.39 |
| IL-6 | 0.29 | 0.18 | 2.66 | 1 | 0.10 | 1.34 | 0.94–1.90 |
| APACHE II | 0.37 | 0.19 | 3.94 | 1 | 0.04 | 1.44 | 1.01–2.08 |
| Independent predictors one week after enrollment | |||||||
| Irisin | −1.0 | 0.23 | 18.24 | 1 | <0.001 | 0.37 | 0.23–0.58 |
| CRP | −0.19 | 0.19 | 1.11 | 1 | 0.29 | 0.82 | 0.57–1.19 |
| IL-6 | 0.52 | 0.20 | 6.70 | 1 | 0.01 | 1.68 | 1.13–2.49 |
| APACHE II | 0.77 | 0.24 | 9.94 | 1 | 0.002 | 2.16 | 1.34–3.47 |
| Enrollment | One Week after | |||
|---|---|---|---|---|
| r | p | r | p | |
| Hematologic biomarkers | ||||
| White Blood Cells | −0.21 | 0.03 | −0.15 | 0.11 |
| Platelets | 0.06 | 0.53 | 0.14 | 0.15 |
| Coagulation biomarkers | ||||
| Prothrombin time | −0.14 | 0.14 | −0.17 | 0.09 |
| aPTT | −0.26 | 0.008 | −0.15 | 0.12 |
| Fibrinogen | −0.13 | 0.19 | 0.000 | 0.99 |
| D-dimer | 0.12 | 0.4 | 0.53 | 0.006 |
| Metabolic biomarkers | ||||
| Lactate | −0.48 | <0.001 | −0.29 | 0.003 |
| AST | −0.25 | 0.01 | −0.04 | 0.67 |
| ALT | −0.23 | 0.01 | 0.06 | 0.49 |
| Albumin | 0.11 | 0.24 | 0.14 | 0.15 |
| Creatinine | −0.07 | 0.48 | 0.03 | 0.72 |
| HOMA-IR | −0.27 | 0.006 | - | - |
| BMI | −0.07 | 0.46 | - | - |
| Inflammatory biomarkers | ||||
| CRP | −0.21 | 0.03 | 0.06 | 0.54 |
| Procalcitonin | −0.26 | 0.008 | −0.21 | 0.03 |
| IL-1β | −0.09 | 0.37 | 0.18 | 0.06 |
| IL-6 | −0.21 | 0.03 | −0.001 | 0.99 |
| IL-10 | −0.29 | 0.003 | 0.07 | 0.48 |
| suPAR | −0.005 | 0.96 | 0.13 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampela, I.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Psallida, S.; Kounatidis, D.; Stratigou, T.; Marinou, I.; Vogiatzakis, E.; Dalamaga, M. Alterations of the Adipo–Myokine Irisin in Sepsis and Septic Shock: Diagnostic and Prognostic Implications. Biomolecules 2024, 14, 291. https://doi.org/10.3390/biom14030291
Karampela I, Vallianou NG, Tsilingiris D, Christodoulatos GS, Psallida S, Kounatidis D, Stratigou T, Marinou I, Vogiatzakis E, Dalamaga M. Alterations of the Adipo–Myokine Irisin in Sepsis and Septic Shock: Diagnostic and Prognostic Implications. Biomolecules. 2024; 14(3):291. https://doi.org/10.3390/biom14030291
Chicago/Turabian StyleKarampela, Irene, Natalia G. Vallianou, Dimitrios Tsilingiris, Gerasimos Socrates Christodoulatos, Sotiria Psallida, Dimitris Kounatidis, Theodora Stratigou, Ioanna Marinou, Evaggelos Vogiatzakis, and Maria Dalamaga. 2024. "Alterations of the Adipo–Myokine Irisin in Sepsis and Septic Shock: Diagnostic and Prognostic Implications" Biomolecules 14, no. 3: 291. https://doi.org/10.3390/biom14030291





