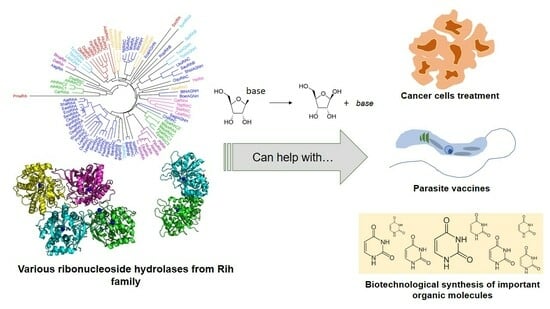
Ribonucleoside Hydrolases–Structure, Functions, Physiological Role and Practical Uses
Abstract
:1. Introduction
2. Physiological Role of Rih Family Proteins
3. Ribonucleoside Hydrolase Structure
3.1. Rih Amino Acid Sequences
3.2. Rih Crystal Structure
4. Catalytic Properties of Rih Hydrolases
5. Stability of Rih Hydrolases
6. Practical Importance of Rih Hydrolases
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ChemDraw PerkinElmer JS. Available online: https://chemdrawdirect.perkinelmer.cloud/js/sample/index.html# (accessed on 28 August 2023).
- Miller, R.L.; Sabourin, C.L.K.; Krenitsky, T.A. Nucleoside Hydrolases from Trypanosoma Cruzi. J. Biol. Chem. 1984, 259, 5073–5077. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.L.; Kidder, G.W. Inhibition of Growth and Purine-Metabolizing Enzymes of Trypanosomid Flagellates by N6-Methyladenine. Antimicrob. Agents Chemother. 1980, 17, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Vandemeulebroucke, A.; Minici, C.; Bruno, I.; Muzzolini, L.; Tornaghi, P.; Parkin, D.W.; Versées, W.; Steyaert, J.; Degano, M. Structure and Mechanism of the 6-Oxopurine Nucleosidase from Trypanosoma brucei brucei. Biochemistry 2010, 49, 8999–9010. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.W.; Horenstein, B.A.; Abdulah, D.R.; Estupinan, B.; Schramm, V.L. Nucleoside Hydrolase from Crithidia Fasciculuta. Metabolic Role, Purification, Specificity, and Kinetic Mechanism. J. Biol. Chem. 1991, 266, 20658–20665. [Google Scholar] [CrossRef]
- Suthar, N.; Goyal, A.; Dubey, V.K. Identification of Potential Drug Targets of Leishmania Infantum by In-Silico Genome Analysis. Lett. Drug Des. Discov. 2009, 6, 620–622. [Google Scholar] [CrossRef]
- Sales, E.M.; Sousa, G.S.; Belouezzane, C.; Almeida, F.C.L.; Figueroa-Villar, J.D. Expression, Purification and Spectrophotometric Analysis of Nucleoside Hydrolase from Leishmania chagasi (LcNH). Protein Expr. Purif. 2019, 161, 40–48. [Google Scholar] [CrossRef]
- Shi, W.; Schramm, V.L.; Almo, S.C. Nucleoside Hydrolase from Leishmania Major. Cloning, Expression, Catalytic Properties, Transition State Inhibitors, and the 2.5-Å Crystal Structure. J. Biol. Chem. 1999, 274, 21114–21120. [Google Scholar] [CrossRef]
- Degano, M.; Almo, S.C.; Sacchettini, J.C.; Schramm, V.L. Trypanosomal Nucleoside Hydrolase. A Novel Mechanism from the Structure with a Transition-State Inhibitor. Biochemistry 1998, 37, 6277–6285. [Google Scholar] [CrossRef]
- Fish, W.R.; Looker, D.L.; Marr, J.J.; Berens, R.L. Purine Metabolism in the Bloodstream Forms of Trypanosoma gambiense and Trypanosoma rhodesiense. Biochim. Biophys. Acta 1982, 719, 223–231. [Google Scholar] [CrossRef]
- Horenstein, B.A.; Parkin, D.W.; Estupifiiin, B.; Schramm, V.L. Transition-State Analysis of Nucleoside Hydrolase from Crithidia fasciculata. Biochemistry 1991, 30, 10788–10795. [Google Scholar] [CrossRef]
- Versées, W.; Barlow, J.; Steyaert, J. Transition-State Complex of the Purine-Specific Nucleoside Hydrolase of T. vivax: Enzyme Conformational Changes and Implications for Catalysis. J. Mol. Biol. 2006, 359, 331–346. [Google Scholar] [CrossRef]
- Versées, W.; Decanniere, K.; Pellé, R.; Depoorter, J.; Brosens, E.; Parkin, D.W.; Steyaert, J. Structure and Function of a Novel Purine Specific Nucleoside Hydrolase from Trypanosoma vivax. J. Mol. Biol. 2001, 307, 1363–1379. [Google Scholar] [CrossRef]
- Versées, W.; Decanniere, K.; Van Holsbeke, E.; Devroede, N.; Steyaert, J. Enzyme-Substrate Interactions in the Purine-Specific Nucleoside Hydrolase from Trypanosoma vivax. J. Biol. Chem. 2002, 277, 15938–15946. [Google Scholar] [CrossRef]
- Gopaul, D.N.; Meyer, S.L.; Degano, M.; Sacchettini, J.C.; Schramm, V.L. Inosine−Uridine Nucleoside Hydrolase from Crithidia fasciculata. Genetic Characterization, Crystallization, and Identification of Histidine 241 as a Catalytic Site Residue. Biochemistry 1996, 35, 5963–5970. [Google Scholar] [CrossRef]
- Degano, M.; Gopaul, D.N.; Scapin, G.; Schramm, V.L.; Sacchettini, J.C. Three-Dimensional Structure of the Inosine−Uridine Nucleoside N-Ribohydrolase from Crithidia fasciculata. Biochemistry 1996, 35, 5971–5981. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry: International Edition, 8th ed.; W. H. Freeman & Co., Ltd.: New York, NY, USA, 2021; ISBN 1319381499. [Google Scholar]
- Bzowska, A.; Kulikowska, E.; Shugar, D. Purine Nucleoside Phosphorylases: Properties, Functions, and Clinical Aspects. Pharmacol. Ther. 2000, 88, 349–425. [Google Scholar] [CrossRef]
- Bzowska, A. Purine and Pyrimidine Nucleoside Phosphorylases—Remarkable Enzymes Still Not Fully Understood. Postep. Biochem. 2015, 61, 260–273. [Google Scholar]
- Liang, L.; He, X.; Liu, G.; Tan, H. The Role of a Purine-Specific Nucleoside Hydrolase in Spore Germination of Bacillus thuringiensis. Microbiology 2008, 154, 1333–1340. [Google Scholar] [CrossRef]
- Todd, S.J.; Moir, A.J.G.; Johnson, M.J.; Moir, A. Genes of Bacillus cereus and Bacillus anthracis Encoding Proteins of the Exosporium. J. Bacteriol. 2003, 185, 3373–3378. [Google Scholar] [CrossRef]
- Guimarães, A.P.; Oliveira, A.A.; Da Cunha, E.F.F.; Ramalho, T.C.; França, T.C.C. Analysis of Bacillus anthracis Nucleoside Hydrolase via in Silico Docking with Inhibitors and Molecular Dynamics Simulation. J. Mol. Model. 2011, 17, 2939–2951. [Google Scholar] [CrossRef]
- Kopečná, M.; Blaschke, H.; Kopečný, D.; Vigouroux, A.; Končitíková, R.; Novák, O.; Kotland, O.; Strnad, M.; Moréra, S.; von Schwartzenberg, K. Structure and Function of Nucleoside Hydrolases from Physcomitrella patens and Maize Catalyzing the Hydrolysis of Purine, Pyrimidine, and Cytokinin Ribosides. Plant Physiol. 2013, 163, 1568–1583. [Google Scholar] [CrossRef]
- Stasolla, C.; Katahira, R.; Thorpe, T.A.; Ashihara, H. Purine and Pyrimidine Nucleotide Metabolism in Higher Plants. J. Plant Physiol. 2003, 160, 1271–1295. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and Purine Biosynthesis and Degradation in Plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef] [PubMed]
- Thicklin, L.; Shamsuddin, A.; Alahmry, F.; Gezley, C.; Brown, E.; Stone, J.; Burns-Carver, E.; Kline, P.C. Purification of a Non-Specific Nucleoside Hydrolase from Alaska Pea Seeds. Protein Expr. Purif. 2019, 154, 140–146. [Google Scholar] [CrossRef]
- Riegler, H.; Geserick, C.; Zrenner, R. Arabidopsis thaliana Nucleosidase Mutants Provide New Insights into Nucleoside Degradation. New Phytol. 2011, 191, 349–359. [Google Scholar] [CrossRef]
- Kurtz, J.E.; Exinger, F.; Erbs, P.; Jund, R. The URH1 Uridine Ribohydrolase of Saccharomyces cerevisiae. Curr. Genet. 2002, 41, 132–141. [Google Scholar] [CrossRef]
- Rizzatti, A.C.S.; Sandrim, V.C.; Jorge, J.A.; Terenzi, H.F.; Polizeli, M.D.L.T.M. Influence of Temperature on the Properties of the Xylanolytic Enzymes of the Thermotolerant Fungus Aspergillus phoenicis. J. Ind. Microbiol. Biotechnol. 2004, 31, 88–93. [Google Scholar] [CrossRef]
- Elshafei, A.M.; Mohamed, L.A.; Hassan, M.M. Degradation of Purine Ribonucleosides by Extracts of Penicillium viridicatum. Afr. J. Microbiol. Res. 2011, 5, 316–323. [Google Scholar]
- Elshafei, A.M.; Abu-Shady, M.R.; El-Beih, F.M.; Mohamed, L.A. Mode and Extent of Degradation of Adenosine and Guanosine by Extracts of Aspergillus terricola. Microbiol. Res. 1995, 150, 291–295. [Google Scholar] [CrossRef]
- Allam, A.M.; Hassan, M.M.; Ghanem, B.S.; Elzainy, T.A. Nature of the Enzymes That Catalyse the Cleavage of the N-Glycosidic Bond of Pyrimidine Ribonucleosides in Some Filamentous Fungi. Biochem. Syst. Ecol. 1987, 15, 515–517. [Google Scholar] [CrossRef]
- Abdel-Fatah, O.M.; Elsayed, M.A.; Elshafei, A.M. Hydrolytic Cleavage of Purine Ribonucleosides in Aspergillus phoenicis. J. Basic. Microbiol. 2003, 43, 439–448. [Google Scholar] [CrossRef]
- Versées, W.V.; Van Holsbeke, E.; De Vos, S.; Decanniere, K.; Zegers, I.; Steyaert, J. Cloning, Preliminary Characterization and Crystallization of Nucleoside Hydrolases from Caenorhabditis elegans and Campylobacter jejuni. Acta Cryst. 2003, 59, 1087–1089. [Google Scholar] [CrossRef]
- Singh, R.K.; Steyaert, J.; Versées, W. Structural and Biochemical Characterization of the Nucleoside Hydrolase from C. elegans Reveals the Role of Two Active Site Cysteine Residues in Catalysis. Protein Sci. 2017, 26, 985–996. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Valenzuela, J.G. The Salivary Purine Nucleosidase of the Mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2003, 33, 13–22. [Google Scholar] [CrossRef]
- Tarr, H.L.A. Fish Muscle Riboside Hydrolases. J. Biol. Chem. 1955, 127, 386–391. [Google Scholar] [CrossRef]
- Belenky, P.; Racette, F.G.; Bogan, K.L.; McClure, J.M.; Smith, J.S.; Brenner, C. Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD+. Cell 2007, 129, 473–484. [Google Scholar] [CrossRef]
- Shirai, H.; Kanatani, H. 1-Methyladenosine Ribohydrolase in the Starfish Ovary and Its Relation to Oocyte Maturation. Exp. Cell Res. 1972, 75, 79–88. [Google Scholar] [CrossRef]
- Tarr, H.L.A. 1-Methyladenosine Hydrolase of Starfish (Pisaster ochraceous). J. Fish. Res. Board. Can. 1973, 30, 1861–1866. [Google Scholar] [CrossRef]
- Roberts, M.F.; Wallert, G.R. N-Methyltransferases and 7-Methyl-N9-Nucleoside Hydrolase Activity in Coffea arabica and the Biosynthesis of Caffeine. Phytochemistry 1979, 18, 451–455. [Google Scholar] [CrossRef]
- Waller, G.R.; MacVean, C.D.; Suzuki, T. High Production of Caffeine and Related Enzyme Activities in Callus Cultures of Coffea arabica L. Plant Cell Rep. 1983, 2, 109–112. [Google Scholar] [CrossRef]
- Negishi, O.; Ozawa, T.; Imagawa, H. N-Methyl Nucleosidase from Tea Leaves. Agric. Biol. Chem. 1988, 52, 169–175. [Google Scholar] [CrossRef]
- Kanatani, H.; Shirai, H. Chemical Structural Requirements for Induction of Oocyte Maturation and Spawning in Stareishes. Dev. Growth Differ. 1971, 13, 53–64. [Google Scholar] [CrossRef]
- Petersen, C.; Møller, L.B. The RihA, RihB, and RihC Ribonucleoside Hydrolases of Escherichia coli. Substrate Specificity, Gene Expression, and Regulation. J. Biol. Chem. 2001, 276, 884–894. [Google Scholar] [CrossRef]
- Garau, G.; Muzzolini, L.; Tornaghi, P.; Degano, M. Active Site Plasticity Revealed from the Structure of the Enterobacterial N-Ribohydrolase RihA Bound to a Competitive Inhibitor. BMC Struct. Biol. 2010, 10, 14. [Google Scholar] [CrossRef]
- West, T.P. Degradation of Pyrimidine Ribonucleosides by Pseudomonas aeruginosa. Antonie van Leeuwenhoek 1996, 69, 331–335. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine Ribonucleoside Catabolic Enzyme Activities of Pseudomonas pickettii. Antonie van Leeuwenhoek 1994, 66, 307. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine Base and Ribonucleoside Catabolic Enzyme Activities of the Pseudomonas diminuta Group. FEMS Microbiol. Lett. 1992, 99, 305–310. [Google Scholar] [CrossRef]
- Porcelli, M.; Concilio, L.; Peluso, I.; Marabotti, A.; Facchiano, A.; Cacciapuoti, G. Pyrimidine-Specific Ribonucleoside Hydrolase from the Archaeon Sulfolobus solfataricus—Biochemical Characterization and Homology Modeling. FEBS J. 2008, 275, 1900–1914. [Google Scholar] [CrossRef]
- Porcelli, M.; De Leo, E.; Del Vecchio, P.; Fuccio, F.; Cacciapuoti, G. Thermal Unfolding of Nucleoside Hydrolases from the Hyperthermophilic Archaeon Sulfolobus solfataricus: Role of Disulfide Bonds. Protein Pept. Lett. 2012, 19, 369–374. [Google Scholar] [CrossRef]
- Porcelli, M.; De Leo, E.; Marabotti, A.; Cacciapuoti, G. Site-Directed Mutagenesis Gives Insights into Substrate Specificity of Sulfolobus solfataricus Purine-Specific Nucleoside Hydrolase. Ann. Microbiol. 2012, 62, 881–887. [Google Scholar] [CrossRef]
- Minici, C.; Cacciapuoti, G.; De Leo, E.; Porcelli, M.; Degano, M. New Determinants in the Catalytic Mechanism of Nucleoside Hydrolases from the Structures of Two Isozymes from Sulfolobus solfataricus. Biochemistry 2012, 51, 4590–4599. [Google Scholar] [CrossRef]
- Terada, M.; Tatibana, M.; Hayaishi, O. Purification and Properties of Nucleoside Hydrolase from Pseudomonas fluorescens. J. Biol. Chem. 1967, 242, 5578–5585. [Google Scholar] [CrossRef]
- Ueda, A.; Attila, C.; Whiteley, M.; Wood, T.K. Uracil Influences Quorum Sensing and Biofilm Formation in Pseudomonas aeruginosa and Fluorouracil Is an Antagonist. Microb. Biotechnol. 2009, 2, 62–74. [Google Scholar] [CrossRef]
- Savinova, O.S.; Glazunova, O.A.; Moiseenko, K.V.; Begunova, A.V.; Rozhkova, I.V.; Fedorova, T.V. Exoproteome Analysis of Antagonistic Interactions between the Probiotic Bacteria Limosilactobacillus reuteri Lr1 and Lacticaseibacillus rhamnosus f and Multidrug Resistant Strain of Klebsiella pneumonia. Int. J. Mol. Sci. 2021, 22, 999. [Google Scholar] [CrossRef]
- Khoroshkin, M.S.; Leyn, S.A.; Van Sinderen, D.; Rodionov, D.A. Transcriptional Regulation of Carbohydrate Utilization Pathways in the Bifidobacterium Genus. Front. Microbiol. 2016, 7, 120. [Google Scholar] [CrossRef]
- Mitsukawa, Y.; Hibi, M.; Matsutani, N.; Horinouchi, N.; Takahashi, S.; Ogawa, J. New Nucleoside Hydrolase with Transribosylation Activity from Agromyces sp. MM-1 and Its Application for Enzymatic Synthesis of 2′-O-Methylribonucleosides. J. Biosci. Bioeng. 2018, 125, 38–45. [Google Scholar] [CrossRef]
- Mitsukawa, Y.; Hibi, M.; Matsutani, N.; Horinouchi, N.; Takahashi, S.; Ogawa, J. A Novel Nucleoside Hydrolase from Lactobacillus buchneri LBK78 Catalyzing Hydrolysis of 2′-O-Methylribonucleosides. Biosci. Biotechnol. Biochem. 2016, 80, 1568–1576. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.H.; Lee, W.S.; Bang, W.G. Genes Encoding Ribonucleoside Hydrolase 1 and 2 from Corynebacterium ammoniagenes. Microbiology 2006, 152, 1169–1177. [Google Scholar] [CrossRef]
- Johansson, P.; Paulin, L.; Säde, E.; Salovuori, N.; Alatalo, E.R.; Björkroth, K.J.; Auvinen, P. Genome Sequence of a Food Spoilage Lactic Acid Bacterium, Leuconostoc gasicomitatum LMG 18811T, in Association with Specific Spoilage Reactions. Appl. Environ. Microbiol. 2011, 77, 4344–4351. [Google Scholar] [CrossRef]
- Hunt, C.; Gillani, N.; Farone, A.; Rezaei, M.; Kline, P.C. Kinetic Isotope Effects of Nucleoside Hydrolase from Escherichia coli. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1751, 140–149. [Google Scholar] [CrossRef]
- Hansen, M.R.; Dandanell, G. Purification and Characterization of RihC, a Xanthosine-Inosine-Uridine- Adenosine-Preferring Hydrolase from Salmonella enterica Serovar Typhimurium. Biochim. Biophys. Acta-Gen. Subj. 2005, 1723, 55–62. [Google Scholar] [CrossRef]
- Aučynaite, A.; Rutkiene, R.; Tauraite, D.; Meškys, R.; Urbonavičius, J. Identification of a 2′-O-Methyluridine Nucleoside Hydrolase Using the Metagenomic Libraries. Molecules 2018, 23, 2904. [Google Scholar] [CrossRef]
- Arivett, B.; Farone, M.; Masiragani, R.; Burden, A.; Judge, S.; Osinloye, A.; Minici, C.; Degano, M.; Robinson, M.; Kline, P. Characterization of Inosine-Uridine Nucleoside Hydrolase (RihC) from Escherichia coli. Biochim. Biophys. Acta-Proteins Proteom. 2014, 1844, 656–662. [Google Scholar] [CrossRef]
- Arevalo-Ferro, C.; Hentzer, M.; Reil, G.; Görg, A.; Kjelleberg, S.; Givskov, M.; Riedel, K.; Eberl, L. Identification of Quorum-Sensing Regulated Proteins in the Opportunistic Pathogen Pseudomonas aeruginosa by Proteomics. Environ. Microbiol. 2003, 5, 1350–1369. [Google Scholar] [CrossRef]
- Multiple Sequence Alignment. Available online: https://www.ebi.ac.uk/Tools/msa/muscle/ (accessed on 10 August 2023).
- Geneious Prime. Available online: http://www.geneious.com/ (accessed on 10 August 2023).
- Porcelli, M.; Peluso, I.; Marabotti, A.; Facchiano, A.; Cacciapuoti, G. Biochemical Characterization and Homology Modeling of a Purine-Specific Ribonucleoside Hydrolase from the Archaeon Sulfolobus solfataricus: Insights into Mechanisms of Protein Stabilization. Arch. Biochem. Biophys. 2009, 483, 55–65. [Google Scholar] [CrossRef]
- Iovane, E.; Giabbai, B.; Muzzolini, L.; Matafora, V.; Fornili, A.; Minici, C.; Giannese, F.; Degano, M. Structural Basis for Substrate Specificity in Group I Nucleoside Hydrolases. Biochemistry 2008, 47, 4418–4426. [Google Scholar] [CrossRef]
- Fornili, A.; Giabbai, B.; Garau, G.; Degano, M. Energy Landscapes Associated with Macromolecular Conformational Changes from Endpoint Structures. J. Am. Chem. Soc. 2010, 132, 17570–17577. [Google Scholar] [CrossRef]
- Giabbai, B.; Degano, M. Crystal Structure to 1.7 Å of the Escherichia coli Pyrimidine Nucleoside Hydrolase YeiK, a Novel Candidate for Cancer Gene Therapy. Structure 2004, 12, 739–749. [Google Scholar] [CrossRef]
- Farone, A.; Farone, M.; Kline, P.; Quinn, T.; Sinkala, Z. A Practical Approach for Computing the Active Site of the Ribonucleoside Hydrolase of E. coli Encoded by RihC. Adv. Exp. Med. Biol. 2010, 680, 437–443. [Google Scholar] [CrossRef]
- Horenstein, B.A.; Schramm, V.L. Electronic Nature of the Transition State for Nucleoside Hydrolase. A Blueprint for Inhibitor Design. Biochemistry 1993, 32, 7089–7097. [Google Scholar] [CrossRef]
- Garrett, E.R.; Mehta, P.J. Solvolysis of Adenine Nucleosides. I. Effects of Sugars and Adenine Substituents on Acid Solvolyses. J. Am. Chem. Soc. 1972, 94, 8532–8541. [Google Scholar] [CrossRef]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The Genome of the African Trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef]
- Berg, M.; Kohl, L.; Van der Veken, P.; Joossens, J.; Al-Salabi, M.I.; Castagna, V.; Giannese, F.; Cos, P.; Versées, W.; Steyaert, J.; et al. Evaluation of Nucleoside Hydrolase Inhibitors for Treatment of African Trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 1900–1908. [Google Scholar] [CrossRef]
- Parkin, A.; Estupinan, D.W.; Schramm, B.; Horenstein, V.L.; Schramm, B.A. Amidrazone Analogues of D-Ribofuranose as Transition-State Inhibitors of Nucleoside Hydrolase. Biochemistry 1994, 33, 7089. [Google Scholar] [CrossRef]
- Evans, G.; Schramm, V.; Tyler, P. The Immucillins: Design, Synthesis and Application of Transition-State Analogues. Curr. Med. Chem. 2015, 22, 3897–3909. [Google Scholar] [CrossRef]
- Nyhan, W.L. Disorders of Purine and Pyrimidine Metabolism. Mol. Genet. Metab. 2005, 86, 25–33. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Leishmune® Vaccine: The Newest Tool for Prevention and Control of Canine Visceral Leishmaniosis and Its Potential as a Transmission-Blocking Vaccine. Vet. Parasitol. 2006, 141, 1–8. [Google Scholar] [CrossRef]
- Huysmans, G.; Ranquin, A.; Wyns, L.; Steyaert, J.; Van Gelder, P. Encapsulation of Therapeutic Nucleoside Hydrolase in Functionalised Nanocapsules. J. Control. Release 2005, 102, 171–179. [Google Scholar] [CrossRef]
- Ranquin, A.; Versées, W.; Meier, W.; Steyaert, J.; Van Gelder, P. Therapeutic Nanoreactors: Combining Chemistry and Biology in a Novel Triblock Copolymer Drug Delivery System. Nano Lett. 2005, 5, 2220–2224. [Google Scholar] [CrossRef]
- Zhao, B.-D.; Lv, J.; Shen, Y.-Y.; Gui, L.; Chen, Z.-X.; Chen, J.; Lu, F.-P.; Li, M. Efficient Transformation of Uridine by Escherichia coli Based on Signal Peptide and Molecular Chaperone Strategy. Biotechnol. Bull. 2022, 38, 238–249. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Uefuji, H.; Ogita, S.; Sano, H. Transgenic Tobacco Plants Producing Caffeine: A Potential New Strategy for Insect Pest Control. Transgenic Res. 2006, 15, 667–672. [Google Scholar] [CrossRef] [PubMed]













| Organism Type | Organism | Rih Type | Short Name | NCBI Code |
|---|---|---|---|---|
| Archaea | Saccharolobus solfataricus | IAG-NH | SsoIAGNH | WP_009991400.1 |
| Archaea | Natrinema salifodinae | IAG-NH | NsaIAGNH | WP_049989006.1 |
| Archaea | Acidianus manzaensis | IAG-NH | AmaIAGNH | ARM75010.1 |
| Archaea | Natrialba swarupiae | RihC | NswRihC | TYT60529.1 |
| Archaea | Haloarcula marismortui | IAG-NH | HmaIAGNH | QCP93065.1 |
| Archaea | Sulfolobus acidocaldarius | IAG-NH | SacIAGNH | AGE70026.1 |
| Bacteria | Intrasporangium chromatireducens | RihC | IchRihC | WP_034716916.1 |
| Bacteria | Nitrospira sp. OLB3 | RihC | NitRihC | KXK05231.1 |
| Bacteria | Chloroflexi bacterium OLB14 | RihC | ChlRihC | KXK12577.1 |
| Bacteria | Bacillus safensis | RihC | BsaRihC | WP_061110295.1 |
| Bacteria | Pseudomonas aeruginosa | RihC | PaeRihC | WP_061189914.1 |
| Bacteria | Brucella anthropi | RihC | BanRihC | WP_061347523.1 |
| Bacteria | Limosilactobacillus reuteri | RihC | LreRihC | MBU5982057.1 |
| Bacteria | Microbacterium resistens | * | MreRih | WP_067247535.1 |
| Bacteria | Nocardiopsis listeri | RihC | NliRihC | WP_067600215.1 |
| Bacteria | Periweissella cryptocerci | RihC | PcrRihC | WP_133363136.1 |
| Bacteria | Weissella uvarum | RihC | WuvRihC | WP_205144808.1 |
| Bacteria | Leuconostoc palmae | RihC | LpaRihC | WP_220742199.1 |
| Bacteria | Oceanobacillus salinisoli | RihC | OsaRihC | WP_156290634.1 |
| Bacteria | Oenococcus sicerae | RihC | OsiRihC | WP_128686110.1 |
| Bacteria | Glycomyces fuscus | RihC | GfuRihC | WP_097633998.1 |
| Bacteria | Bacillus thuringiensis | IAG-NH | BthIAGNH | ABU48602.1 |
| Bacteria | Escherichia coli | RihB | EcoRihB | B6I8L3.1 |
| Bacteria | Citrobacter koseri | RihA | CkoRihA | A8AJF8.1 |
| Bacteria | Klebsiella pneumoniae | RihC | KpnRihC | A6T4G4.1 |
| Bacteria | Metagenomic library | RihC | RK9NH | AYV61512.1 |
| Bacteria | Salmonella enterica | RihC | SenRihC | APQ66417.1 |
| Bacteria | Ochrobactrum quorumnocens | RihC | OquRihC | WP_095444951.1 |
| Bacteria | Lentilactobacillus buchneri | RihC | LbuRihC | AEB72406.1 |
| Bacteria | Xanthomonas campestris | IAG-NH | XcaIAGNH | UAU33856.1 |
| Bacteria | Klebsiella pneumoniae | RihA | KpnRihA | CDQ54287.1 |
| Bacteria | Escherichia coli | RihA | EcoRihA | CAD6019387.1 |
| Bacteria | Serratia marcescens | RihA | SmaRihA | OUI66314.1 |
| Bacteria | Salmonella enterica | RihA | SenRihA | CAH2854758.1 |
| Bacteria | Shewanella hafniensis | RihA | ShaRihA | CAD6364937.1 |
| Bacteria | Enterobacter asburiae | RihA | EasRihA | WP_163330465.1 |
| Bacteria | Aeromonas jandaei | RihA | AjaRihA | WP_156852166.1 |
| Bacteria | Klebsiella spallanzanii | RihB | KspRihB | VUS89946.1 |
| Bacteria | Rhodococcus opacus | RihB | RopRihB | CAG7622013.1 |
| Bacteria | Bacillus altitudinis | RihB | BalRihB | CAI7725891.1 |
| Bacteria | Staphylococcus aureus | RihB | SauRihB | CAG9964796.1 |
| Bacteria | Pseudomonas sp. MM227 | RihB | PseRihB | CAI3787937.1 |
| Bacteria | Staphylococcus hominis | IAG-NH | ShoIAGNH | SUM69402.1 |
| Bacteria | Streptococcus agalactiae | IAG-NH | SagIAGNH | CCO74302.1 |
| Bacteria | Bacillus cereus | IAG-NH | BceIAGNH | AFQ09851.1 |
| Bacteria | Anatilimnocola aggregata | * | AagRih | WP_202921648.1 |
| Bacteria | Escherichia coli | RihC | EcoRihC | CAD6022375.1 |
| Fungi | Daldinia sp. | * | DalRih | KAI8965458.1 |
| Fungi | Hypoxylon rubiginosum | * | HruRih | KAI6091274.1 |
| Fungi | Annulohypoxylon bovei | * | AboRih | KAI2472106.1 |
| Fungi | Poronia punctata | * | PpuRih | KAI1816947.1 |
| Fungi | Daldinia bambusicola | * | DbaRih | KAI1807258.1 |
| Fungi | Biscogniauxia marginata | * | BmaRih | KAI1498832.1 |
| Fungi | Whalleya microplaca | * | WmiRih | KAI1077167.1 |
| Fungi | Purpureocillium lilacinum | * | PliRih | PWI74805.1 |
| Yeast | Cyberlindnera jadinii | RihA | CjaRihA | CEP20255.1 |
| Yeast | Nakaseomyces glabratus | RihC | NglRihC | KAH7604059.1 |
| Yeast | Suhomyces tanzawaensis | RihC | StaRihC | XP_020067128.1 |
| Yeast | Candida parapsilosis | RihC | CpaRihC | KAF6065839.1 |
| Yeast | Yamadazyma tenuis | * | YteRih | EGV65217.1 |
| Reptiles | Platysternon megacephalum | * | PmeRih | TFJ96048.1 |
| Arachnids | Sarcoptes scabiei | * | SscRih | UXI17525.1 |
| Actinopterygii | Anoplopoma fimbria | * | AfiRih | XP_054471125.1 |
| Actinopterygii | Dunckerocampus dactyliophorus | * | DdaRih | XP_054644538.1 |
| Cephalopoda | Octopus bimaculoides | RihC | ObiRihC | XP_014774668.1 |
| Insects | Aethina tumida | RihC | AtuRihC | XP_019871744.1 |
| Actinopterygii | Nibea albiflora | RihC | NalRihC | KAG8004511.1 |
| Actinopterygii | Nothobranchius furzeri | RihC | NfuRihC | XP_015807835.1 |
| Actinopterygii | Pimephales promelas | RihC | PprRihC | KAG1945547.1 |
| Insects | Anastrepha obliqua | RihC | AobRihC | XP_054728927.1 |
| Protozoa | Trypanosoma brucei brucei | IG-NH | TbrIGNH | XP_846080.1 |
| Protozoa | Trypanosoma vivax | IAG-NH | TviIAGNH | AAG38561.2 |
| Protozoa | Trypanosoma cruzi | IAG-NH | TcrIAGNH | EAN96320.1 |
| Protozoa | Trypanosoma congolense | IAG-NH | TcoIAGNH | AAG38560.1 |
| Protozoa | Leishmania donovani | RihC | LdoRihC | AAG02281.1 |
| Protozoa | Leishmania donovani | IG-NH | LdoIGNH | AYU77254.1 |
| Protozoa | Symbiodinium sp. | RihA | SymRihA | CAE7031721.1 |
| Protozoa | Symbiodinium pilosum | RihB | SpiRihB | CAE7496820.1 |
| Eudicots | Arabidopsis thaliana | RihC | AthRihC1 | AED92622.1 |
| Eudicots | Arabidopsis thaliana | RihC | AthRihC2 | AED92625.1 |
| Eudicots | Arabidopsis thaliana | RihC | AthRihC3 | AED92623.1 |
| Monocots | Zea mays | RihA | MaysRihA | NP_001105259.2 |
| Bryopsida | Physcomitrella patens | RihC | PpaRihC1 | AFZ84928.1 |
| Bryopsida | Physcomitrella patens | RihC | PpaRihC2 | AFR46616.1 |
| Bryopsida | Physcomitrella patens | RihC | PpaRihC3 | AFZ84924.1 |
| Monocots | Zea mays | RihC | MaysRihC | AFD54993.1 |
| Chlorophyceae | Dunaliella salina | RihC | DsaRihC | KAF5829699.1 |
| Organism | Hydrolase | PDB Code |
|---|---|---|
| Saccharolobus solfataricus | IAG-NH | 3T8I |
| Saccharolobus solfataricus | RihB | 3T8J |
| Escherichia coli | RihA | 1YOE |
| Escherichia coli K-12 | RihA | 3G5I |
| Shewanella loihica PV-4 | RihA | 4WR2 |
| Escherichia coli | RihB | 1Q8F |
| Escherichia coli K-12 | RihB | 3B9X |
| Escherichia coli K-12 | RihB | 3MKM |
| Gardnerella vaginalis 315-A | RihB | 6BA0 |
| Bacillus anthracis | RihC | 2C40 |
| Gardnerella vaginalis 315-A | RihC | 6BA1 |
| Caenorhabditis elegans | IAG-NH | 5MJ7 |
| Trichomonas vaginalis | IAG-NH | 8DB8 |
| Trypanosoma brucei | IAG-NH | 4I73 |
| Trypanosoma vivax | IAG-NH | 1HOZ |
| Trypanosoma brucei | IG-NH | 3FZ0 |
| Crithidia fasciculata | RihC | 1MAS |
| Leishmania braziliensis | RihC | 5TSQ |
| Leishmania major | RihC | 1EZR |
| Zea mays | RihA | 6ZK1 |
| Physcomitrella patens | RihC | 4KPN |
| Zea mays | RihC | 4KPO |
| Organism | Rih Type | kcaturidine, s−1 | KMuridine, µM | kcatcytidin, s−1 | KMcitidine, µM | kcatguanosine, s−1 | KMguanosine, µM | kcatadenosine, s−1 | KMadenosine, µM | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Trypanosoma vivax | IAG-NH | ND | ND | 0.338 ± 0.005 | 925 ± 39 | 2.3 ± 0.1 | 2.3 ± 0.5 | 2.6 ± 0.2 | 8 ± 2 | [12] |
| Trypanosoma vivax | IAG-NH | 0.022 ± 0.002 | 586 ± 150 | 0.338 ± 0.005 | 925 ± 39 | 1.9 | 3.82 | 1.46 ± 0.06 | 8.54 ± 1.29 | [13] |
| Trypanosoma cruzi | IAG-NH | ND | ND | ND | ND | ND | 13 ± 2 | ND | ND | [2] |
| Sulfolobus solfataricus | IAG-NH | 0.060 ± 0.002 | 3950 ± 500 | NA | NA | 29.1 ± 0.9 | 160 ± 16 | 3.8 ± 0.1 | 60 ± 8 | [52,69] |
| Caenorhabditis elegans | IAG-NH | 0.0022 ± 0.0002 | 2843 ± 813 | 0.50 ± 0.03 | 10004 ± 320 | 0.84 ± 0.09 | 164 ± 56 | 0.78 ± 0.05 | 93 ± 16 | [34] |
| Trypanosoma brucei brucei | IG-NH | 0.0038 ± 0.0008 | 1451 ± 600 | 0.52 ± 0.04 | 271 ± 49 | 37.6 ± 0.5 | 4.5 ± 0.2 | 0.237 ± 0.005 | <1 | [4] |
| Escherichia coli | RihB | 4.7 ± 0.1 | 142 ± 8 | 11.6 ± 1.8 | 532 ± 101 | NA | NA | NA | NA | [72] |
| Escherichia coli | RihB | 5.4 ± 0.2 | 120 ± 10 | ND | ND | ND | ND | ND | ND | [70] |
| Sulfolobus solfataricus | RihB | 7.1 ± 0.2 | 310 ± 20 | 39.4 ± 1.2 | 970 ± 50 | NA | NA | NA | NA | [50] |
| Escherichia coli | RihC | 10.85 ± 0.23 | 408 ± 184 | 1.12 ± 0.53 | 682 ± 298 | ND | ND | 1.15 ± 0.47 | 416 ± 249 | [65] |
| Crithidia fasciculata | RihC | ND | 1220 ± 40 | ND | 4700 ± 500 | ND | 420 ± 10 | ND | 460 ± 30 | [5] |
| Salmonella enterica | RihC | 46 ± 3 | 1060 ± 100 | 7.8 ± 1.3 | 9200 ± 1200 | ND | ND | 2.06 ± 0.07 | 160 ± 20 | [63] |
| Lactobacillus buchneri LBK78 | RihC | 3.6 a | 320 ± 10 a | ND | ND | ND | ND | ND | ND | [59] |
| Agromyces sp. MM-1 | RihC | 9.4 ± 0.5 a | 3600 ± 400 a | ND | ND | ND | ND | ND | ND | [58] |
| Leishmania major | RihC | 32 ± 6 | 234 ± 112 | 0.36 ± 0.05 | 422 ± 175 | 0.59 ± 0.03 | 140 ± 23 | 0.57 ± 0.04 | 185 ± 46 | [8] |
| Organism | Rih Type | kcatinosine (s−1) | KMinosine (µM) | kcatxanthosine (s−1) | KMxanthosine (µM) | Source |
|---|---|---|---|---|---|---|
| Trypanosoma vivax | IAG-NH | 5.19 ± 0.08 | 5.37 ± 0.42 | ND | ND | [13] |
| Trypanosoma cruzi | IAG-NH | ND | 18 ± 0.1 | ND | ND | [2] |
| Sulfolobus solfataricus | IAG-NH | 23.3 ± 0.8 | 340 ± 20 | ND | ND | [69] |
| Caenorhabditis elegans | IAG-NH | 0.79 ± 0.04 | 295 ± 44 | 0.029 ± 0.002 | 1038 ± 274 | [34] |
| Trypanosoma brucei brucei | IG-NH | 28 ± 1 | 1.9 ± 0.4 | 6.2 ± 0.3 | 280 ± 40 | [4] |
| Escherichia coli | RihB | NA | NA | ND | ND | [72] |
| Escherichia coli | RihB | 0.086 ± 0.003 | 2340 ± 320 | ND | ND | [70] |
| Escherichia coli | RihC | 4.31 ± 0.22 | 422 ± 225 | 6.30 ± 0.05 | 454 ± 165 | [65] |
| Crithidia fasciculata | RihC | 44 ± 3 | 150 ± 40 | ND | ND | [15] |
| Salmonella enterica | RihC | 9.0 ± 0.15 a 8.1 ± 0.14 b | 650 ± 60 a 1280 ± 130 b | 62 ± 8 a 26.3 ± 0.3 b | 5900 ± 1000 a 790 ± 50 b | [63] |
| Crithidia fasciculata | RihC | ND | 380 ± 30 | ND | ND | [5] |
| Leishmania major | RihC | 119 ± 34 | 445 ± 209 | ND | ND | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaposhnikov, L.A.; Savin, S.S.; Tishkov, V.I.; Pometun, A.A. Ribonucleoside Hydrolases–Structure, Functions, Physiological Role and Practical Uses. Biomolecules 2023, 13, 1375. https://doi.org/10.3390/biom13091375
Shaposhnikov LA, Savin SS, Tishkov VI, Pometun AA. Ribonucleoside Hydrolases–Structure, Functions, Physiological Role and Practical Uses. Biomolecules. 2023; 13(9):1375. https://doi.org/10.3390/biom13091375
Chicago/Turabian StyleShaposhnikov, Leonid A., Svyatoslav S. Savin, Vladimir I. Tishkov, and Anastasia A. Pometun. 2023. "Ribonucleoside Hydrolases–Structure, Functions, Physiological Role and Practical Uses" Biomolecules 13, no. 9: 1375. https://doi.org/10.3390/biom13091375