Inhibition of Cell Proliferation and Cell Death by Apigetrin through Death Receptor-Mediated Pathway in Hepatocellular Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Chemicals
2.2. Culture of Human Liver Cancer Cells
2.3. Cell Viability Assay through MTT
2.4. Colony Formation Assay
2.5. Wound Healing Assay
2.6. Flow Cytometry Analysis of Cell Cycle
2.7. Determination of Apoptosis by Annexin V
2.8. Protein Extraction and Western Blot Analysis
2.9. Statistical Analysis
3. Results
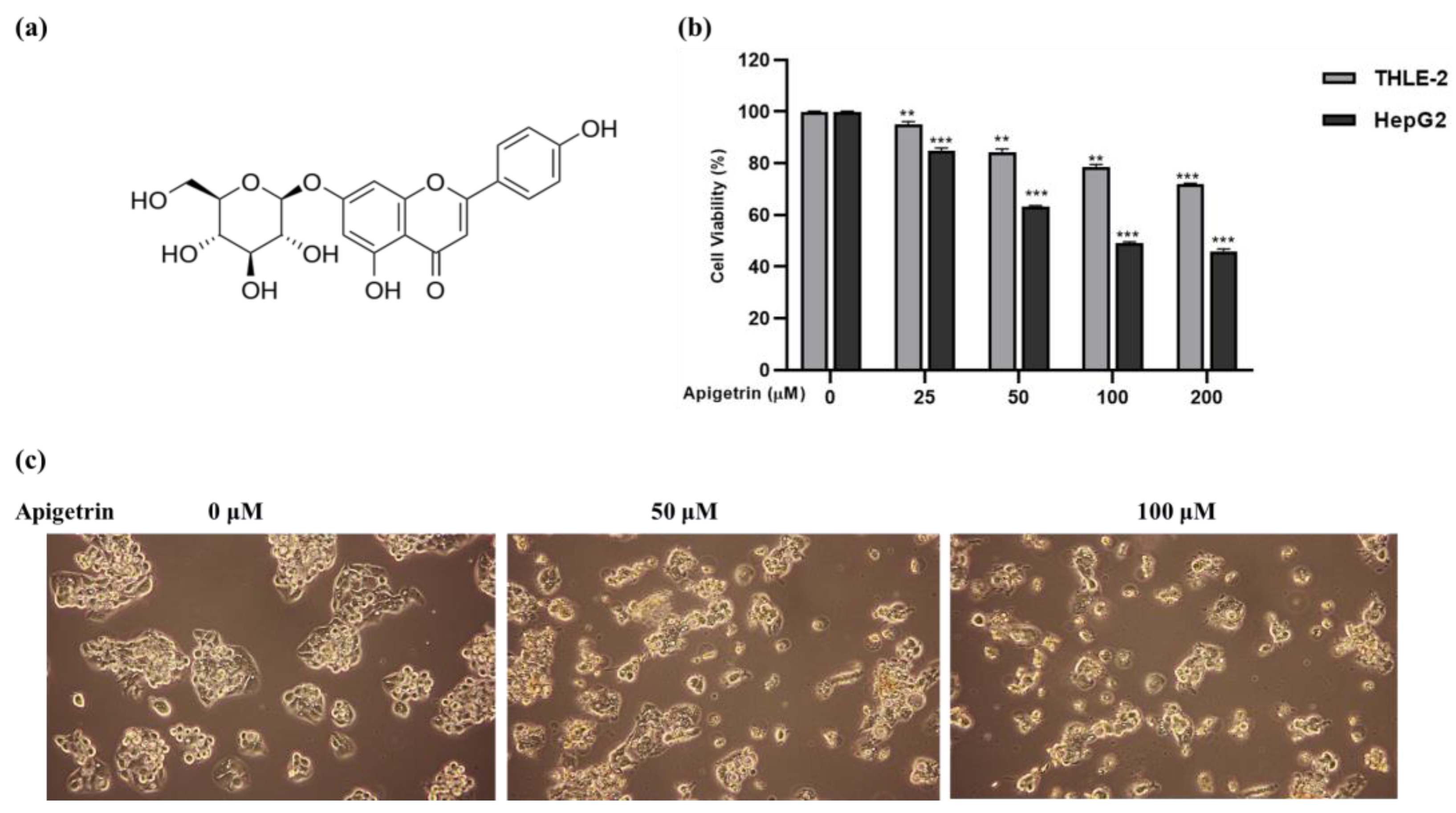
3.1. Apigetrin Suppresses Cell Growth and Causes Cell Death in HepG2 Cells
3.2. Effect of Apigetrin on Cell Proliferation and Cell Migration in HepG2 Cells
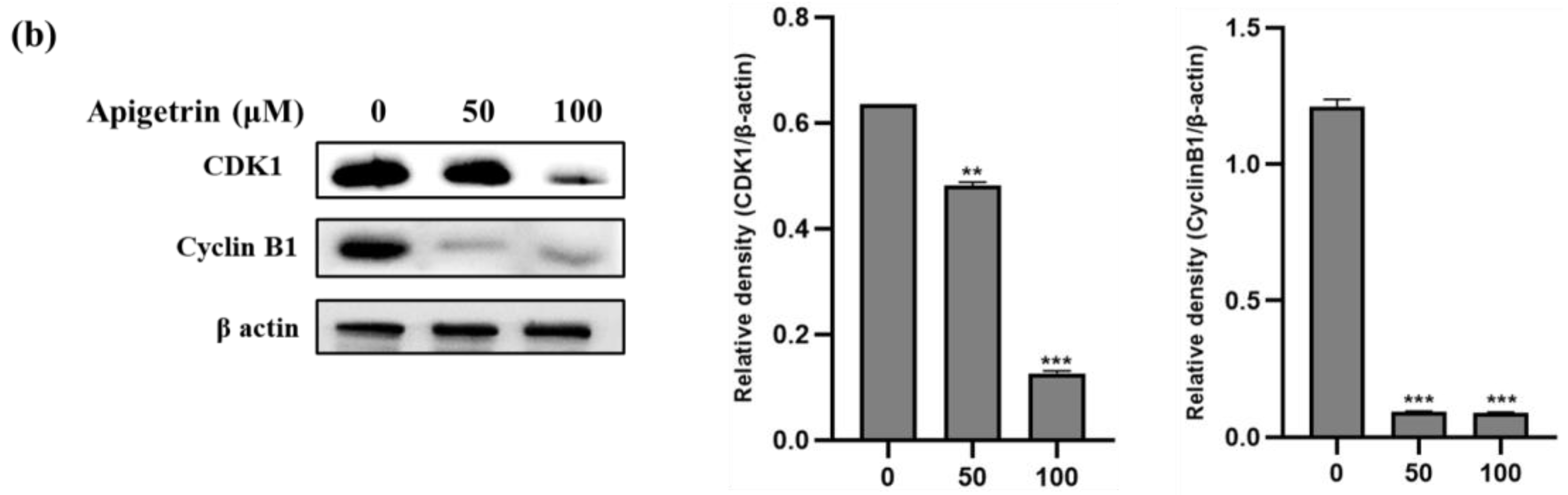
3.3. Apigetrin-Induced G2/M Phase Cell Cycle Arrest in HepG2 Cells
3.4. Apigetrin Caused Apoptosis and Chromatin Condensation in HepG2 Cells
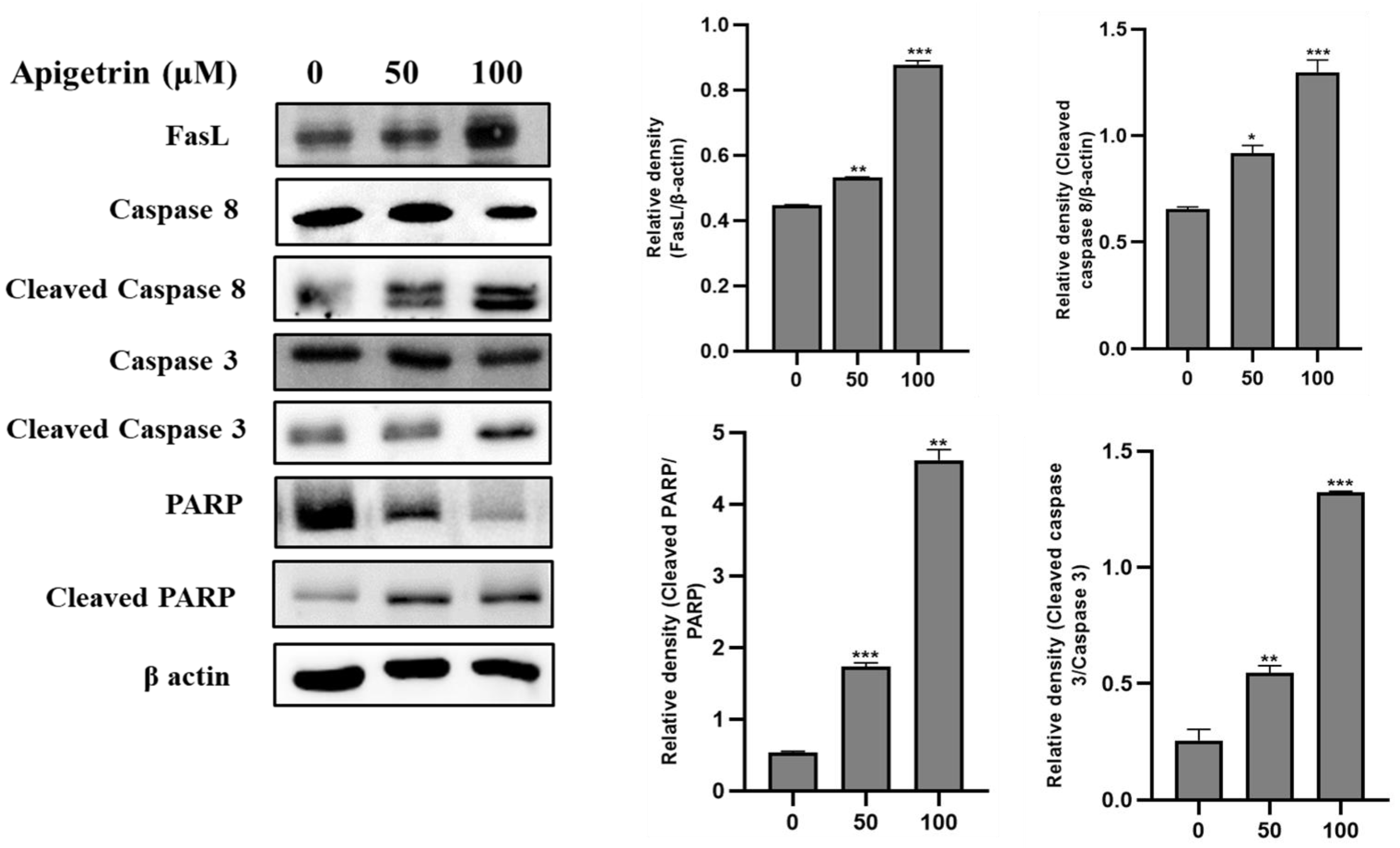
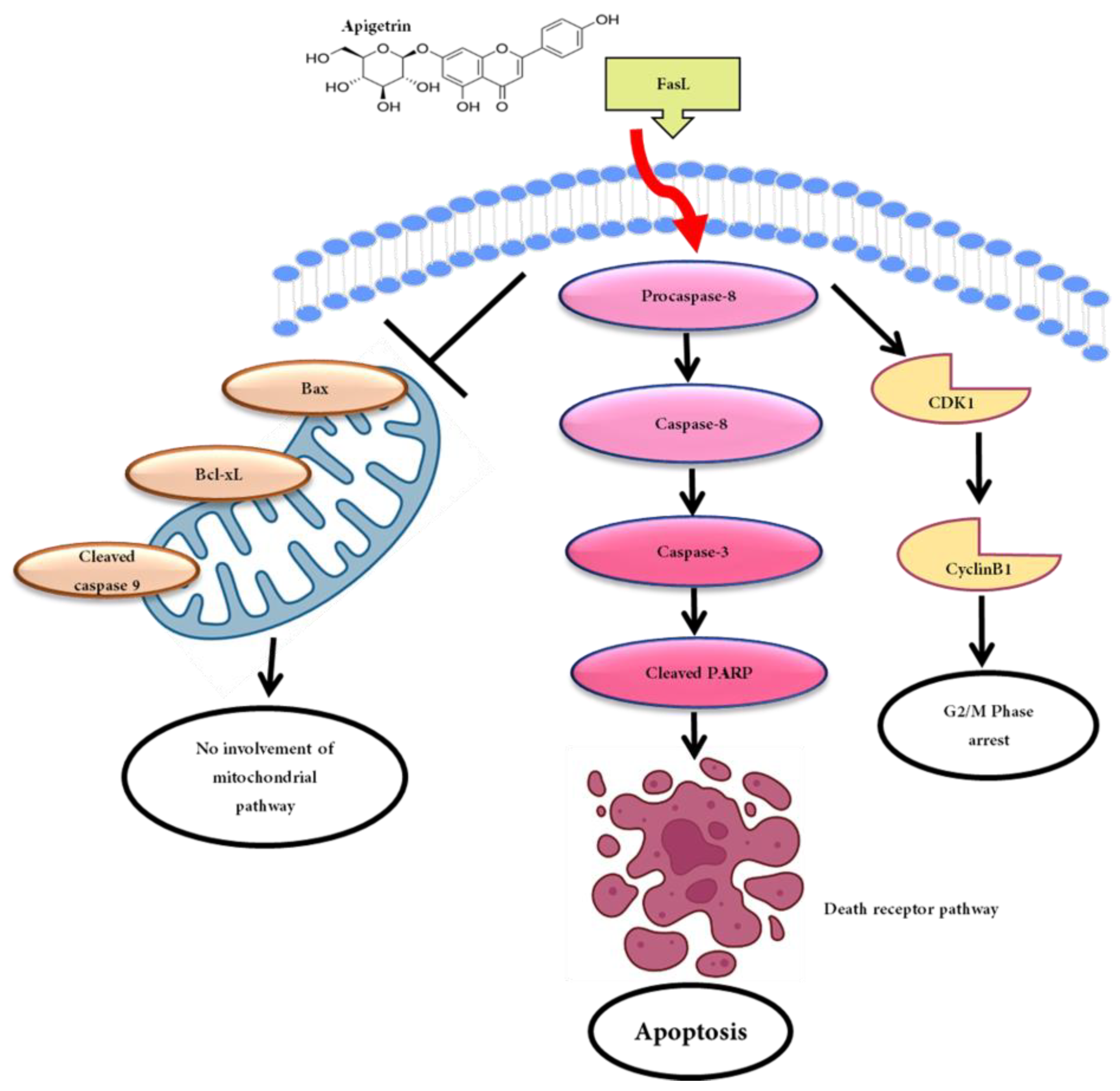
3.5. Apigetrin-Induced Death Receptor-Mediated Apoptosis in HepG2 Cells
3.6. Effects of Apigetrin on Mitochondrial-Mediated Apoptosis in HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Orcutt, S.T.; Anaya, D.A. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control 2018, 25, 1073274817744621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Martin, R.C. Herbal medicine and hepatocellular carcinoma: Applications and challenges. Evid.-Based Complement. Altern. Med. 2011, 2011, 541209. [Google Scholar] [CrossRef] [Green Version]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Gok Yavuz, B.; Hasanov, E.; Lee, S.S.; Mohamed, Y.I.; Curran, M.A.; Koay, E.J.; Cristini, V.; Kaseb, A.O. Current landscape and future directions of biomarkers for immunotherapy in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2021, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The hallmarks of flavonoids in cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Sak, K. Intake of individual flavonoids and risk of carcinogenesis: Overview of epidemiological evidence. Nutr. Cancer 2017, 69, 1119–1150. [Google Scholar] [CrossRef]
- Lim, H.-S.; Kim, O.-S.; Kim, B.-Y.; Jeong, S.-J. Apigetrin from Scutellaria baicalensis Georgi inhibits neuroinflammation in BV-2 microglia and exerts neuroprotective effect in HT22 hippocampal cells. J. Med. Food 2016, 19, 1032–1040. [Google Scholar] [CrossRef]
- Nakazaki, E.; Tsolmon, S.; Han, J.; Isoda, H. Proteomic study of granulocytic differentiation induced by apigenin 7-glucoside in human promyelocytic leukemia HL-60 cells. Eur. J. Nutr. 2013, 52, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tsolmon, S.; Nakazaki, E.; Han, J.; Isoda, H. Apigetrin induces erythroid differentiation of human leukemia cells K562: Proteomics approach. Mol. Nutr. Food Res. 2011, 55, S93–S102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.-S.; Sun, X.-L.; Xu, B.; Li, G.; Song, M. Mechanisms of apigenin-7-glucoside as a hepatoprotective agent. Biomed. Environ. Sci. 2005, 18, 65–70. [Google Scholar] [PubMed]
- Bouzaiene, N.N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Schafer, K. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Anand, P.; Padwad, Y.S.; Dogra, V.; Acharya, V. DNA damage response proteins synergistically affect the cancer prognosis and resistance. Free Radic. Biol. Med. 2022, 178, 174–188. [Google Scholar] [CrossRef]
- Dalton, S. Linking the cell cycle to cell fate decisions. Trends Cell Biol. 2015, 25, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- D’arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, J.-A.; Park, K.-I.; Kim, S.M.; Kim, G.S. Flavonoid-induced apoptotic cell death in human cancer cells and its mechanisms. J. Biomed. Transl. Res. 2020, 21, 50–58. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Zafar, S.; Sarfraz, I.; Rasul, A.; Shah, M.A.; Hussain, G.; Zahoor, M.K.; Shafiq, N.; Riaz, A.; Selamoglu, Z.; Sarker, S.D. Osthole: A multifunctional natural compound with potential anticancer, antioxidant and anti-inflammatory Activities. Mini Rev. Med. Chem. 2021, 21, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yue, R.-F.; Jin, Z.; He, L.-M.; Shen, R.; Du, D.; Tang, Y.-Z. Efficiency comparison of apigenin-7-O-glucoside and trolox in antioxidative stress and anti-inflammatory properties. J. Pharm. Pharmacol. 2020, 72, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lu, N.-N.; Feng, L. Apigetrin inhibits gastric cancer progression through inducing apoptosis and regulating ROS-modulated STAT3/JAK2 pathway. Biochem. Biophys. Res. Commun. 2018, 498, 164–170. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Abusaliya, A.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Jeong, S.H.; Vetrivel, P.; Heo, J.D.; Kim, J.-A.; Won, C.k. Apigetrin Promotes TNFα-Induced Apoptosis, Necroptosis, G2/M Phase Cell Cycle Arrest, and ROS Generation through Inhibition of NF-κB Pathway in Hep3B Liver Cancer Cells. Cells 2022, 11, 2734. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lv, X.; Gao, L.; Liu, J.; Wang, W.; Guo, L.; Frasinyuk, M.S.; Zhang, W.; Watt, D.S.; Liu, C. Chalcone Derivative CX258 Suppresses Colorectal Cancer via Inhibiting the TOP2A/Wnt/β-Catenin Signaling. Cells 2023, 12, 1066. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, L.; Chen, X.; Zhao, Y.; Yang, A.; Huang, H.; Ouyang, L.; Pang, D.; Xie, J.; Liu, D. DHMMF, a natural flavonoid from Resina Draconis, inhibits hepatocellular carcinoma progression via inducing apoptosis and G2/M phase arrest mediated by DNA damage-driven upregulation of p21. Biochem. Pharmacol. 2023, 211, 115518. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.-B.; Vetrivel, P.; Ha, S.-E.; Kim, H.-H.; Heo, J.-D.; Won, C.-K.; Kim, S.-M.; Kim, G.-S. Iridin induces G2/M phase cell cycle arrest and extrinsic apoptotic cell death through PI3K/AKT signaling pathway in AGS gastric cancer cells. Molecules 2021, 26, 2802. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef]
- Ha, S.E.; Kim, S.M.; Lee, H.J.; Vetrivel, P.; Venkatarame Gowda Saralamma, V.; Heo, J.D.; Kim, E.H.; Lee, S.J.; Kim, G.S. Scutellarein induces fas-mediated extrinsic apoptosis and G2/M cell cycle arrest in Hep3B hepatocellular carcinoma cells. Nutrients 2019, 11, 263. [Google Scholar]
- Sharifi, S.; Barar, J.; Hejazi, M.S.; Samadi, N. Doxorubicin changes Bax/Bcl-xL ratio, caspase-8 and 9 in breast cancer cells. Adv. Pharm. Bull. 2015, 5, 351. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, P.B.; Kim, H.H.; Abusaliya, A.; Jeong, S.H.; Park, M.Y.; Kim, H.-W.; Seong, J.K.; Ahn, M.; Park, K.I.; Heo, J.D.; et al. Inhibition of Cell Proliferation and Cell Death by Apigetrin through Death Receptor-Mediated Pathway in Hepatocellular Cancer Cells. Biomolecules 2023, 13, 1131. https://doi.org/10.3390/biom13071131
Bhosale PB, Kim HH, Abusaliya A, Jeong SH, Park MY, Kim H-W, Seong JK, Ahn M, Park KI, Heo JD, et al. Inhibition of Cell Proliferation and Cell Death by Apigetrin through Death Receptor-Mediated Pathway in Hepatocellular Cancer Cells. Biomolecules. 2023; 13(7):1131. https://doi.org/10.3390/biom13071131
Chicago/Turabian StyleBhosale, Pritam Bhagwan, Hun Hwan Kim, Abuyaseer Abusaliya, Se Hyo Jeong, Min Yeong Park, Hyun-Wook Kim, Je Kyung Seong, Meejung Ahn, Kwang Il Park, Jeong Doo Heo, and et al. 2023. "Inhibition of Cell Proliferation and Cell Death by Apigetrin through Death Receptor-Mediated Pathway in Hepatocellular Cancer Cells" Biomolecules 13, no. 7: 1131. https://doi.org/10.3390/biom13071131





