GABRG2 Variants Associated with Febrile Seizures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Complementary DNA Constructs
2.2. Cell Culture and Transfection of Human GABAA Receptors
2.3. Determination of GABA-Elicited Responses by Automated Patch Clamp
2.4. Western Blot and Surface Biotinylation
2.5. Structural Simulation of GABAA Receptor γ2 Subunit Mutations
2.6. Mutation Tolerance of the GABAA Receptor γ2 Subunit
2.7. Statistical Analysis
3. Results
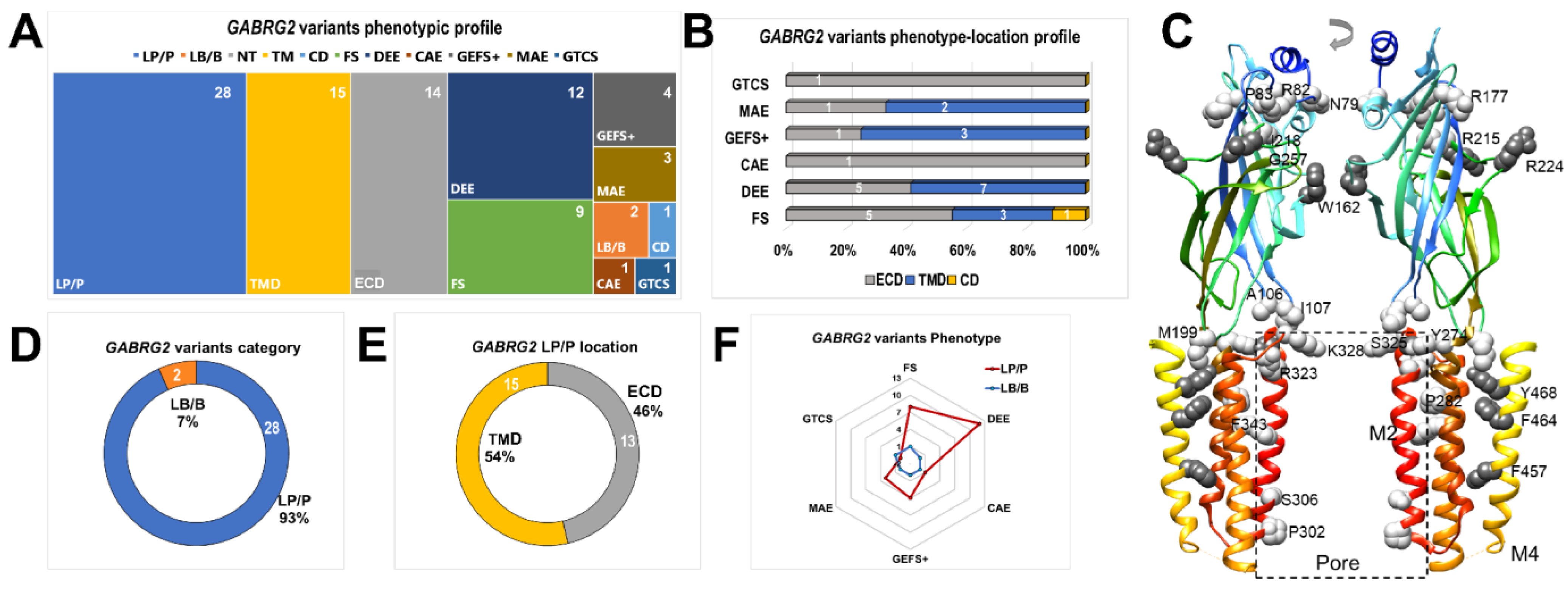
3.1. GABRG2 Variants and Proband Phenotypes
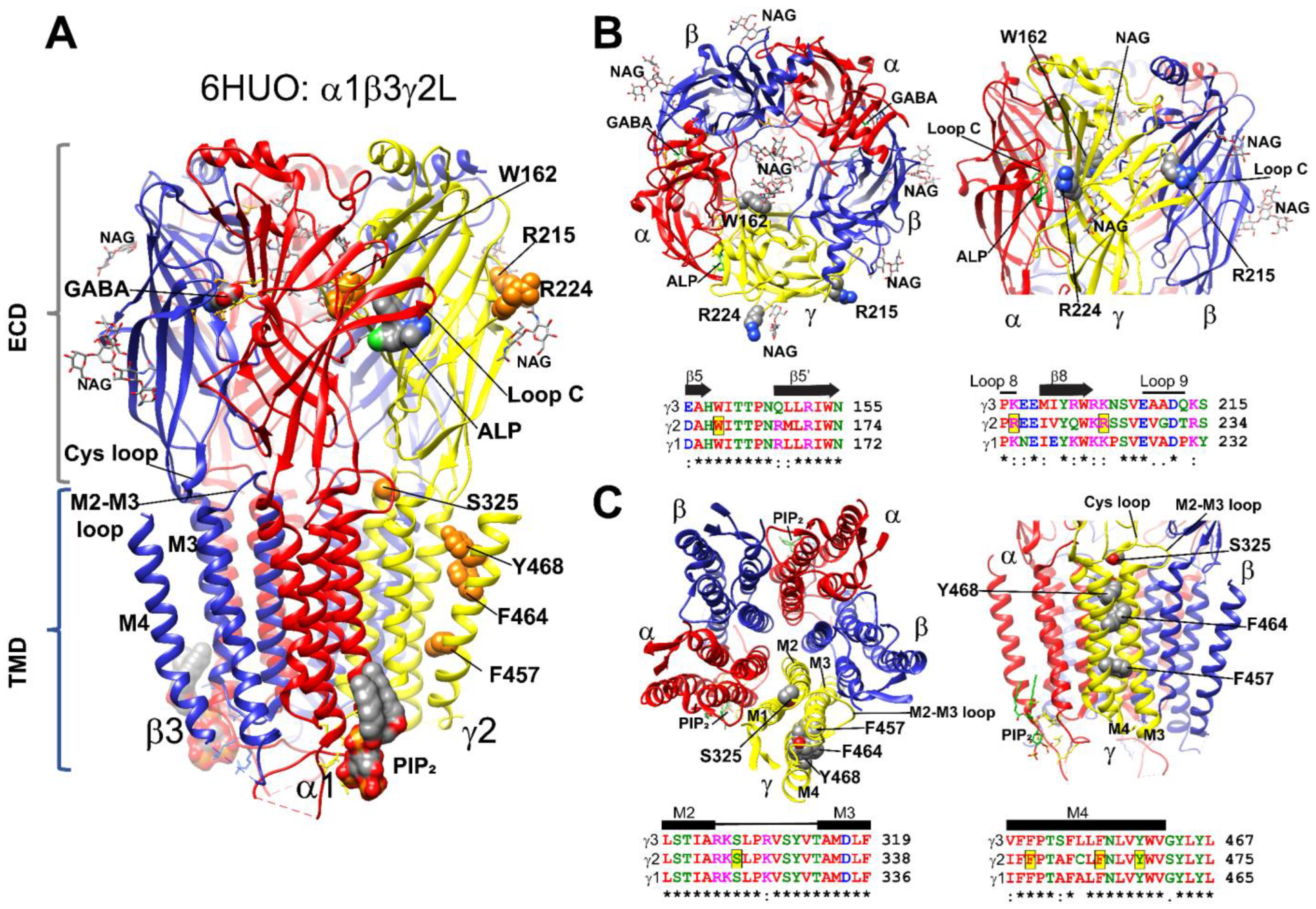
3.2. GABRG2 Variants Were Mapped to Structural Cassettes of the γ2 Subunits That Govern the GABAA Receptor Function
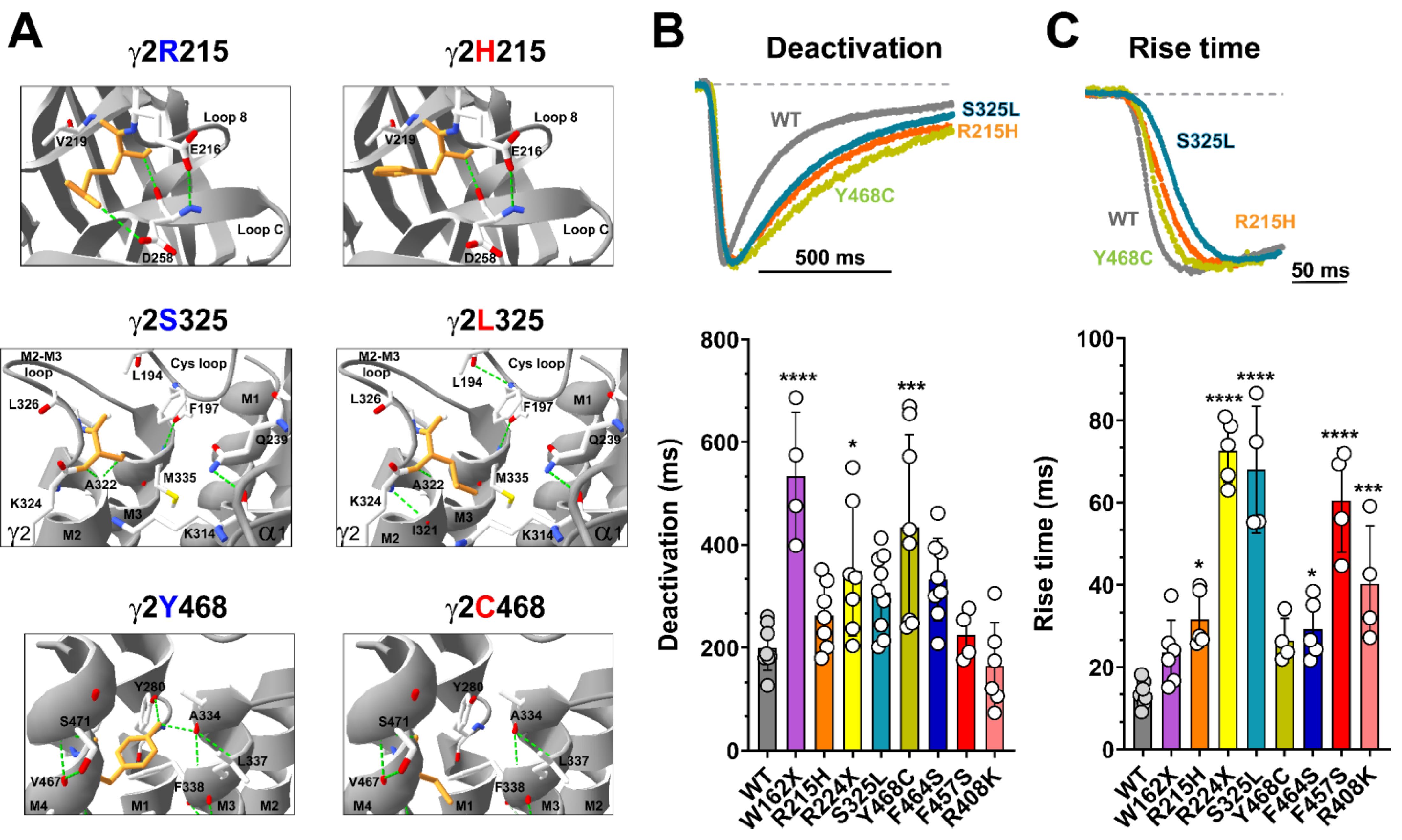
3.3. Mutant γ2 Subunits Altered Structural Domains Related to GABAA Receptor Gating
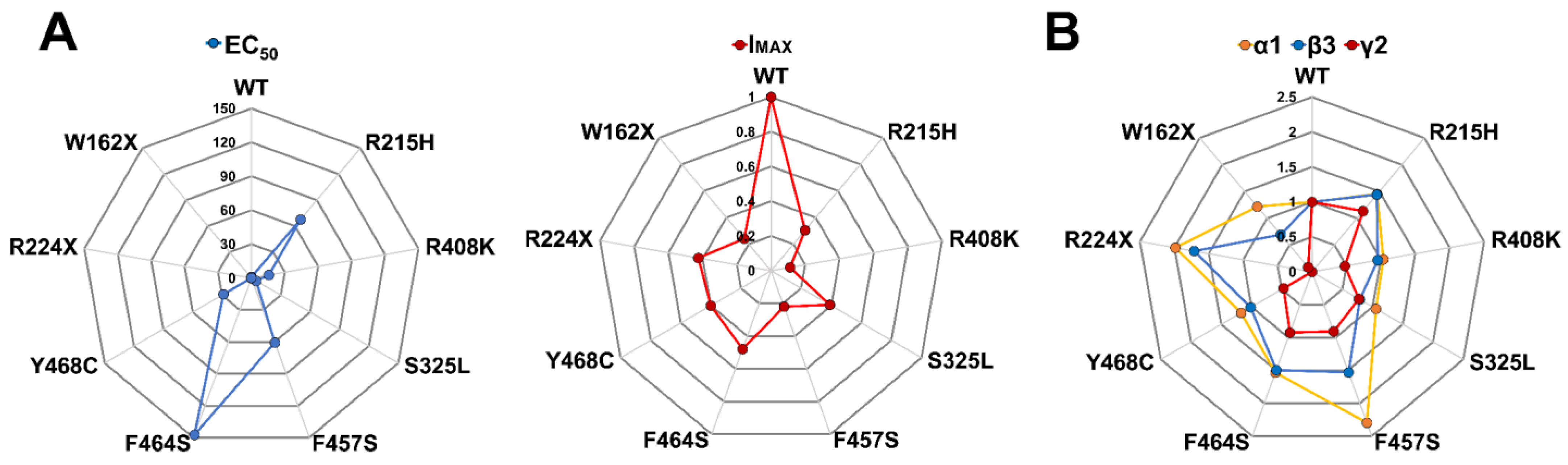
3.4. Mutant γ2 Subunits Alter GABAA Receptor Kinetics
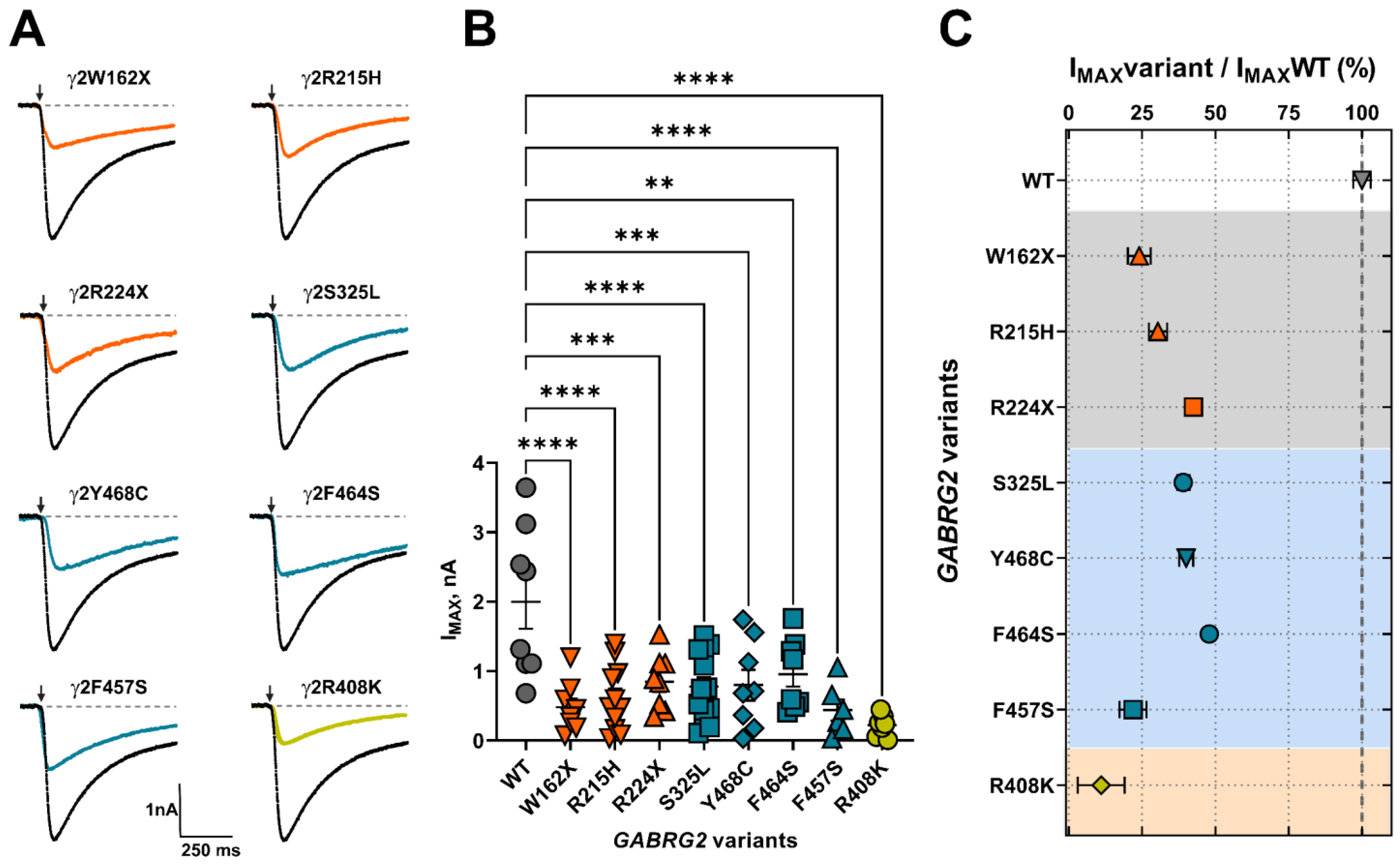
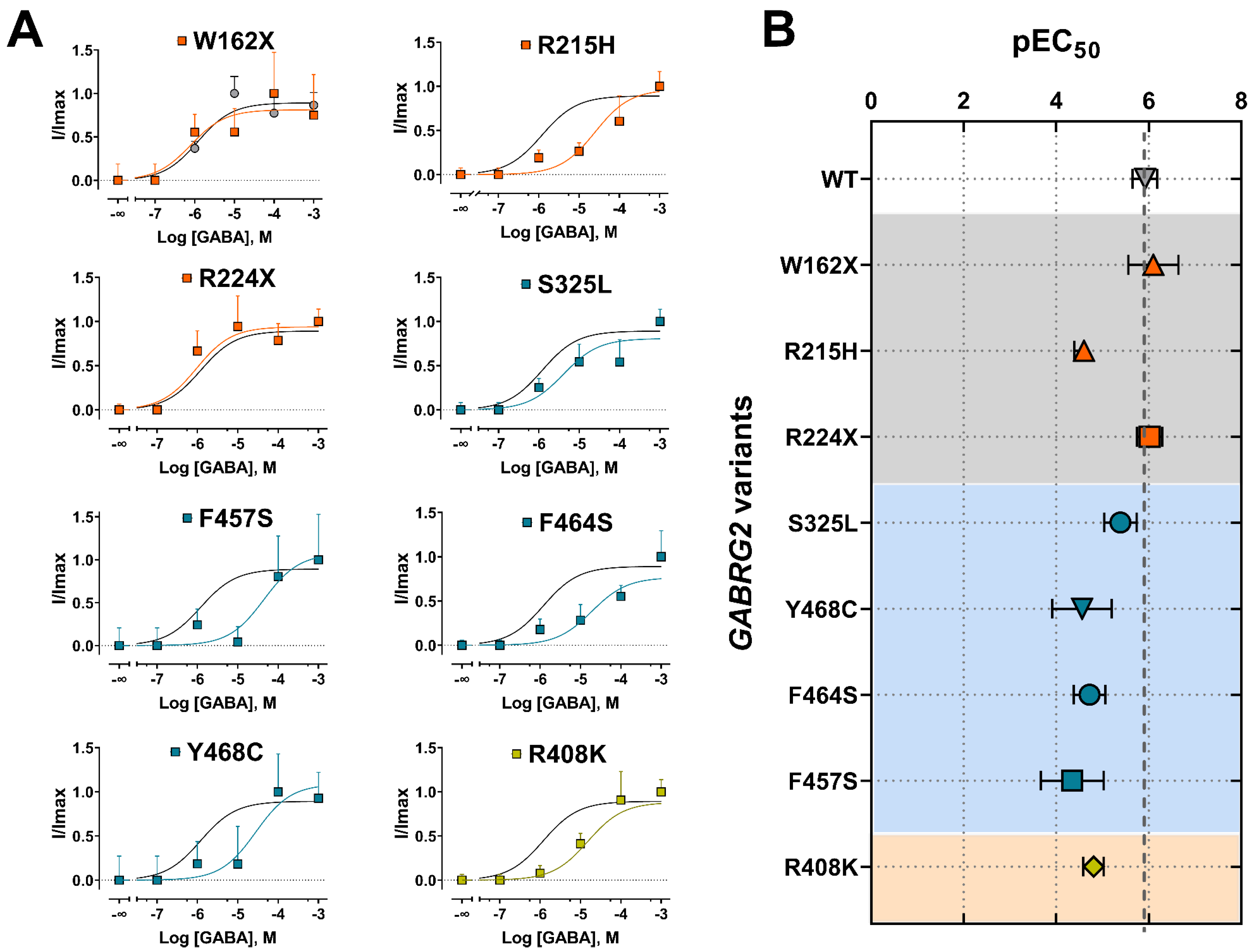
3.5. Mutant γ2 Subunits Decreased GABAA Receptor Function
3.6. Mutant γ2 Subunits Changed GABAA Receptor Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrant, M.; Nusser, Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005, 6, 215–229. [Google Scholar] [CrossRef]
- Essrich, C.; Lorez, M.; Benson, J.A.; Fritschy, J.M.; Luscher, B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998, 1, 563–571. [Google Scholar] [CrossRef]
- Schweizer, C.; Balsiger, S.; Bluethmann, H.; Mansuy, I.M.; Fritschy, J.M.; Mohler, H.; Luscher, B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol. Cell. Neurosci. 2003, 24, 442–450. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M., Jr.; Lindahl, E.; Hibbs, R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- Laverty, D.; Desai, R.; Uchanski, T.; Masiulis, S.; Stec, W.J.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Steyaert, J.; Miller, K.W.; et al. Cryo-EM structure of the human alpha1beta3gamma2 GABA(A) receptor in a lipid bilayer. Nature 2019, 565, 516–520. [Google Scholar] [CrossRef]
- Masiulis, S.; Desai, R.; Uchanski, T.; Serna Martin, I.; Laverty, D.; Karia, D.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Kotecha, A.; et al. GABA(A) receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef]
- Haas, K.F.; Macdonald, R.L. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J. Physiol. 1999, 514 Pt 1, 27–45. [Google Scholar] [CrossRef]
- Chandra, D.; Korpi, E.R.; Miralles, C.P.; De Blas, A.L.; Homanics, G.E. GABAA receptor gamma 2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 2005, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.O.; Reid, C.A.; Single, F.N.; Davies, P.J.; Chiu, C.; Murphy, S.; Clarke, A.L.; Dibbens, L.; Krestel, H.; Mulley, J.C.; et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc. Natl. Acad. Sci. USA 2007, 104, 17536–17541. [Google Scholar] [CrossRef] [Green Version]
- Reid, C.A.; Kim, T.; Phillips, A.M.; Low, J.; Berkovic, S.F.; Luscher, B.; Petrou, S. Multiple molecular mechanisms for a single GABAA mutation in epilepsy. Neurology 2013, 80, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Q.; Shen, W.; Zhou, C.; Xu, D.; Macdonald, R.L. The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nat. Neurosci. 2015, 18, 988–996. [Google Scholar] [CrossRef] [Green Version]
- Warner, T.A.; Shen, W.; Huang, X.; Liu, Z.; Macdonald, R.L.; Kang, J.Q. Differential molecular and behavioural alterations in mouse models of GABRG2 haploinsufficiency versus dominant negative mutations associated with human epilepsy. Hum. Mol. Genet. 2016, 25, 3192–3207. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Sahir, N.; Murakami, S.; Luellen, B.A.; Earnheart, J.C.; Lal, R.; Kim, J.Y.; Song, H.; Luscher, B. Defects in dendrite and spine maturation and synaptogenesis associated with an anxious-depressive-like phenotype of GABAA receptor-deficient mice. Neuropharmacology 2015, 88, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Guo, S.; Xu, S.; Chen, Z.; Wang, L.; Ding, J.; Huo, J.; Xiao, L.; He, Z.; Jin, Z.; et al. Neocortex- and hippocampus-specific deletion of Gabrg2 causes temperature-dependent seizures in mice. Cell Death Dis. 2021, 12, 553. [Google Scholar] [CrossRef]
- Huang, X.; Hernandez, C.C.; Hu, N.; Macdonald, R.L. Three epilepsy-associated GABRG2 missense mutations at the gamma+/beta- interface disrupt GABAA receptor assembly and trafficking by similar mechanisms but to different extents. Neurobiol. Dis. 2014, 68, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Q.; Macdonald, R.L. Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome. JAMA Neurol. 2016, 73, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, R.L.; Kang, J.Q. Molecular pathology of genetic epilepsies associated with GABAA receptor subunit mutations. Epilepsy Curr./Am. Epilepsy Soc. 2009, 9, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, R.L.; Kang, J.Q. mRNA surveillance and endoplasmic reticulum quality control processes alter biogenesis of mutant GABAA receptor subunits associated with genetic epilepsies. Epilepsia 2012, 53 (Suppl. 9), 59–70. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, R.L.; Kang, J.-Q.; Gallagher, M.J.; Feng, H.-J. GABAA-Receptor Mutations Associated With Idiopathic Generalized Epilepsies and Febrile Seizures. In The GABA Receptors; Enna, S.J., Möhler, H., Eds.; The Receptors; Humana Press: Totowa, NJ, USA, 2007; pp. 111–142. [Google Scholar]
- Carvill, G.L.; Heavin, S.B.; Yendle, S.C.; McMahon, J.M.; O’Roak, B.J.; Cook, J.; Khan, A.; Dorschner, M.O.; Weaver, M.; Calvert, S.; et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat. Genet. 2013, 45, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Reinthaler, E.M.; Dejanovic, B.; Lal, D.; Semtner, M.; Merkler, Y.; Reinhold, A.; Pittrich, D.A.; Hotzy, C.; Feucht, M.; Steinbock, H.; et al. Rare variants in gamma-aminobutyric acid type A receptor genes in rolandic epilepsy and related syndromes. Ann. Neurol. 2015, 77, 972–986. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Kong, W.; Hu, N.; Zhang, Y.; Shen, W.; Jackson, L.; Liu, X.; Jiang, Y.; Macdonald, R.L. Altered Channel Conductance States and Gating of GABA(A) Receptors by a Pore Mutation Linked to Dravet Syndrome. eNeuro 2017, 4, 0251-1. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, C.C.; Tian, X.; Hu, N.; Shen, W.; Catron, M.A.; Yang, Y.; Chen, J.; Jiang, Y.; Zhang, Y.; Macdonald, R.L. Dravet syndrome-associated mutations in GABRA1, GABRB2 and GABRG2 define the genetic landscape of defects of GABA(A) receptors. Brain Commun. 2021, 3, fcab033. [Google Scholar] [CrossRef]
- Shen, D.; Hernandez, C.C.; Shen, W.; Hu, N.; Poduri, A.; Shiedley, B.; Rotenberg, A.; Datta, A.N.; Leiz, S.; Patzer, S.; et al. De novo GABRG2 mutations associated with epileptic encephalopathies. Brain 2017, 140, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Boillot, M.; Morin-Brureau, M.; Picard, F.; Weckhuysen, S.; Lambrecq, V.; Minetti, C.; Striano, P.; Zara, F.; Iacomino, M.; Ishida, S.; et al. Novel GABRG2 mutations cause familial febrile seizures. Neurol. Genet. 2015, 1, e35. [Google Scholar] [CrossRef] [Green Version]
- Todd, E.; Gurba, K.N.; Botzolakis, E.J.; Stanic, A.K.; Macdonald, R.L. GABAA receptor biogenesis is impaired by the gamma2 subunit febrile seizure-associated mutation, GABRG2(R177G). Neurobiol. Dis. 2014, 69, 215–224. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, X.; Cheng, M.; Zeng, Q.; Deng, J.; Tian, X.; Wang, Y.; Yu, J.; Shi, W.; Wu, W.; et al. Phenotypic Spectrum and Prognosis of Epilepsy Patients With GABRG2 Variants. Front. Mol. Neurosci. 2022, 15, 809163. [Google Scholar] [CrossRef]
- Skotte, L.; Fadista, J.; Bybjerg-Grauholm, J.; Appadurai, V.; Hildebrand, M.S.; Hansen, T.F.; Banasik, K.; Grove, J.; Albinana, C.; Geller, F.; et al. Genome-wide association study of febrile seizures implicates fever response and neuronal excitability genes. Brain 2022, 145, 555–568. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Macdonald, R.L. A structural look at GABA(A) receptor mutations linked to epilepsy syndromes. Brain Res. 2019, 1714, 234–247. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Klassen, T.L.; Jackson, L.G.; Gurba, K.; Hu, N.; Noebels, J.L.; Macdonald, R.L. Deleterious Rare Variants Reveal Risk for Loss of GABAA Receptor Function in Patients with Genetic Epilepsy and in the General Population. PLoS ONE 2016, 11, e0162883. [Google Scholar] [CrossRef] [Green Version]
- Obergrussberger, A.; Bruggemann, A.; Goetze, T.A.; Rapedius, M.; Haarmann, C.; Rinke, I.; Becker, N.; Oka, T.; Ohtsuki, A.; Stengel, T.; et al. Automated Patch Clamp Meets High-Throughput Screening: 384 Cells Recorded in Parallel on a Planar Patch Clamp Module. J. Lab Autom. 2016, 21, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Lyskov, S.; Chou, F.C.; Conchúir, S.; Der, B.S.; Drew, K.; Kuroda, D.; Xu, J.; Weitzner, B.D.; Renfrew, P.D.; Sripakdeevong, P.; et al. Serverification of molecular modeling applications: The Rosetta Online Server that Includes Everyone (ROSIE). PLoS ONE 2013, 8, e63906. [Google Scholar] [CrossRef] [Green Version]
- Thieker, D.F.; Maguire, J.B.; Kudlacek, S.T.; Leaver-Fay, A.; Lyskov, S.; Kuhlman, B. Stabilizing proteins, simplified: A Rosetta-based webtool for predicting favorable mutations. Protein Sci. 2022, 31, e4428. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30 (Suppl. 1), S162–S173. [Google Scholar] [CrossRef]
- Wiel, L.; Baakman, C.; Gilissen, D.; Veltman, J.A.; Vriend, G.; Gilissen, C. MetaDome: Pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum. Mutat. 2019, 40, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Stephenson, J.D.; Sillitoe, I.; Orengo, C.A.; Thornton, J.M. VarSite: Disease variants and protein structure. Protein Sci. 2020, 29, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [Green Version]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Wiel, L.; Venselaar, H.; Veltman, J.A.; Vriend, G.; Gilissen, C. Aggregation of population-based genetic variation over protein domain homologues and its potential use in genetic diagnostics. Hum. Mutat. 2017, 38, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, R.L.; Olsen, R.W. GABAA receptor channels. Annu. Rev. Neurosci. 1994, 17, 569–602. [Google Scholar] [CrossRef]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Klausberger, T.; Fuchs, K.; Mayer, B.; Ehya, N.; Sieghart, W. GABA(A) receptor assembly. Identification and structure of gamma(2) sequences forming the intersubunit contacts with alpha(1) and beta(3) subunits. J. Biol. Chem. 2000, 275, 8921–8928. [Google Scholar] [CrossRef] [Green Version]
- Sarto, I.; Wabnegger, L.; Dogl, E.; Sieghart, W. Homologous sites of GABA(A) receptor alpha(1), beta(3) and gamma(2) subunits are important for assembly. Neuropharmacology 2002, 43, 482–491. [Google Scholar] [CrossRef]
- Bianchi, M.T.; Macdonald, R.L. Slow phases of GABA(A) receptor desensitization: Structural determinants and possible relevance for synaptic function. J. Physiol. 2002, 544, 3–18. [Google Scholar] [CrossRef]
- Bianchi, M.T.; Haas, K.F.; Macdonald, R.L. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalan, S.P.; Czajkowski, C. Structural link between gamma-aminobutyric acid type A (GABAA) receptor agonist binding site and inner beta-sheet governs channel activation and allosteric drug modulation. J. Biol. Chem. 2012, 287, 6714–6724. [Google Scholar] [CrossRef] [Green Version]
- Althoff, T.; Hibbs, R.E.; Banerjee, S.; Gouaux, E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 2014, 512, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Lo, W.Y.; Lagrange, A.H.; Hernandez, C.C.; Gurba, K.N.; Macdonald, R.L. Co-expression of gamma2 subunits hinders processing of N-linked glycans attached to the N104 glycosylation sites of GABAA receptor beta2 subunits. Neurochem. Res. 2014, 39, 1088–1103. [Google Scholar] [CrossRef] [Green Version]
- Kasaragod, V.B.; Mortensen, M.; Hardwick, S.W.; Wahid, A.A.; Dorovykh, V.; Chirgadze, D.Y.; Smart, T.G.; Miller, P.S. Mechanisms of inhibition and activation of extrasynaptic αβ GABA(A) receptors. Nature 2022, 602, 529–533. [Google Scholar] [CrossRef]
- Marini, C.; Harkin, L.A.; Wallace, R.H.; Mulley, J.C.; Scheffer, I.E.; Berkovic, S.F. Childhood absence epilepsy and febrile seizures: A family with a GABA(A) receptor mutation. Brain 2003, 126, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Parrini, E.; Marini, C.; Mei, D.; Galuppi, A.; Cellini, E.; Pucatti, D.; Chiti, L.; Rutigliano, D.; Bianchini, C.; Virdo, S.; et al. Diagnostic Targeted Resequencing in 349 Patients with Drug-Resistant Pediatric Epilepsies Identifies Causative Mutations in 30 Different Genes. Hum. Mutat. 2017, 38, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Wallace, R.H.; Marini, C.; Petrou, S.; Harkin, L.A.; Bowser, D.N.; Panchal, R.G.; Williams, D.A.; Sutherland, G.R.; Mulley, J.C.; Scheffer, I.E.; et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001, 28, 49–52. [Google Scholar] [CrossRef]
- Kang, J.Q.; Macdonald, R.L. The GABAA receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 8672–8677. [Google Scholar] [CrossRef] [Green Version]
- Baulac, S.; Huberfeld, G.; Gourfinkel-An, I.; Mitropoulou, G.; Beranger, A.; Prud’homme, J.F.; Baulac, M.; Brice, A.; Bruzzone, R.; LeGuern, E. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: A mutation in the gamma2-subunit gene. Nat. Genet. 2001, 28, 46–48. [Google Scholar] [CrossRef]
- Audenaert, D.; Schwartz, E.; Claeys, K.G.; Claes, L.; Deprez, L.; Suls, A.; Van Dyck, T.; Lagae, L.; Van Broeckhoven, C.; Macdonald, R.L.; et al. A novel GABRG2 mutation associated with febrile seizures. Neurology 2006, 67, 687–690. [Google Scholar] [CrossRef]
- Shi, X.; Huang, M.C.; Ishii, A.; Yoshida, S.; Okada, M.; Morita, K.; Nagafuji, H.; Yasumoto, S.; Kaneko, S.; Kojima, T.; et al. Mutational analysis of GABRG2 in a Japanese cohort with childhood epilepsies. J. Hum. Genet. 2010, 55, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Migita, K.; Yamada, J.; Nikaido, Y.; Shi, X.; Kaneko, S.; Hirose, S.; Ueno, S. Properties of a novel GABAA receptor gamma2 subunit mutation associated with seizures. J. Pharm. Sci. 2013, 121, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Lachance-Touchette, P.; Brown, P.; Meloche, C.; Kinirons, P.; Lapointe, L.; Lacasse, H.; Lortie, A.; Carmant, L.; Bedford, F.; Bowie, D.; et al. Novel alpha1 and gamma2 GABAA receptor subunit mutations in families with idiopathic generalized epilepsy. Eur. J. Neurosci. 2011, 34, 237–249. [Google Scholar] [CrossRef]
- Komulainen-Ebrahim, J.; Schreiber, J.M.; Kangas, S.M.; Pylkas, K.; Suo-Palosaari, M.; Rahikkala, E.; Liinamaa, J.; Immonen, E.V.; Hassinen, I.; Myllynen, P.; et al. Novel variants and phenotypes widen the phenotypic spectrum of GABRG2-related disorders. Seizure 2019, 69, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Zou, F.; McWalter, K.; Schmidt, L.; Decker, A.; Picker, J.D.; Lincoln, S.; Sweetser, D.A.; Briere, L.C.; Harini, C.; Members of the Undiagnosed Diseases, N.; et al. Expanding the phenotypic spectrum of GABRG2 variants: A recurrent GABRG2 missense variant associated with a severe phenotype. J. Neurogenet. 2017, 31, 30–36. [Google Scholar] [CrossRef]
- Perucca, P.; Scheffer, I.E.; Harvey, A.S.; James, P.A.; Lunke, S.; Thorne, N.; Gaff, C.; Regan, B.M.; Damiano, J.A.; Hildebrand, M.S.; et al. Real-world utility of whole exome sequencing with targeted gene analysis for focal epilepsy. Epilepsy Res. 2017, 131, 1–8. [Google Scholar] [CrossRef]
- Hannan, S.; Affandi, A.H.B.; Minere, M.; Jones, C.; Goh, P.; Warnes, G.; Popp, B.; Trollmann, R.; Nizetic, D.; Smart, T.G. Differential Coassembly of alpha1-GABA(A)Rs Associated with Epileptic Encephalopathy. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 5518–5530. [Google Scholar] [CrossRef]
- Ishii, A.; Kanaumi, T.; Sohda, M.; Misumi, Y.; Zhang, B.; Kakinuma, N.; Haga, Y.; Watanabe, K.; Takeda, S.; Okada, M.; et al. Association of nonsense mutation in GABRG2 with abnormal trafficking of GABAA receptors in severe epilepsy. Epilepsy Res. 2014, 108, 420–432. [Google Scholar] [CrossRef]
- Shi, Y.W.; Zhang, Q.; Cai, K.; Poliquin, S.; Shen, W.; Winters, N.; Yi, Y.H.; Wang, J.; Hu, N.; Macdonald, R.L.; et al. Synaptic clustering differences due to different GABRB3 mutations cause variable epilepsy syndromes. Brain 2019, 142, 3028–3044. [Google Scholar] [CrossRef]
- Cogliati, F.; Giorgini, V.; Masciadri, M.; Bonati, M.T.; Marchi, M.; Cracco, I.; Gentilini, D.; Peron, A.; Savini, M.N.; Spaccini, L.; et al. Pathogenic Variants in STXBP1 and in Genes for GABAa Receptor Subunities Cause Atypical Rett/Rett-like Phenotypes. Int. J. Mol. Sci. 2019, 20, 3621. [Google Scholar] [CrossRef] [Green Version]
- Klassen, T.; Davis, C.; Goldman, A.; Burgess, D.; Chen, T.; Wheeler, D.; McPherson, J.; Bourquin, T.; Lewis, L.; Villasana, D.; et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 2011, 145, 1036–1048. [Google Scholar] [CrossRef] [Green Version]
- Helbig, I.; Tayoun, A.A. Understanding Genotypes and Phenotypes in Epileptic Encephalopathies. Mol. Syndr. 2016, 7, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Johannesen, K.M.; Iqbal, S.; Guazzi, M.; Mohammadi, N.A.; Perez-Palma, E.; Schaefer, E.; De Saint Martin, A.; Abiwarde, M.T.; McTague, A.; Pons, R.; et al. Structural mapping of GABRB3 variants reveals genotype-phenotype correlations. Genet. Med. 2022, 24, 681–693. [Google Scholar] [CrossRef]
- Kaczor, P.T.; Michalowski, M.A.; Mozrzymas, J.W. alpha(1) Proline 277 Residues Regulate GABA(A)R Gating through M2-M3 Loop Interaction in the Interface Region. ACS Chem. Neurosci. 2022, 13, 3044–3056. [Google Scholar] [CrossRef]
- Kaczor, P.T.; Wolska, A.D.; Mozrzymas, J.W. alpha(1) Subunit Histidine 55 at the Interface between Extracellular and Transmembrane Domains Affects Preactivation and Desensitization of the GABA(A) Receptor. ACS Chem. Neurosci. 2021, 12, 562–572. [Google Scholar] [CrossRef]
- Syed, P.; Durisic, N.; Harvey, R.J.; Sah, P.; Lynch, J.W. Effects of GABA(A) Receptor alpha3 Subunit Epilepsy Mutations on Inhibitory Synaptic Signaling. Front. Mol. Neurosci. 2020, 13, 602559. [Google Scholar] [CrossRef]
- Terejko, K.; Michalowski, M.A.; Izykowska, I.; Dominik, A.; Brzostowicz, A.; Mozrzymas, J.W. Mutations at the M2 and M3 Transmembrane Helices of the GABA(A)Rs alpha(1) and beta(2) Subunits Affect Primarily Late Gating Transitions Including Opening/Closing and Desensitization. ACS Chem. Neurosci. 2021, 12, 2421–2436. [Google Scholar] [CrossRef]
- Gielen, M.; Barilone, N.; Corringer, P.J. The desensitization pathway of GABA(A) receptors, one subunit at a time. Nat. Commun. 2020, 11, 5369. [Google Scholar] [CrossRef]
- Brnich, S.E.; Abou Tayoun, A.N.; Couch, F.J.; Cutting, G.R.; Greenblatt, M.S.; Heinen, C.D.; Kanavy, D.M.; Luo, X.; McNulty, S.M.; Starita, L.M.; et al. Recommendations for application of the functional evidence PS3/BS3 criterion using the ACMG/AMP sequence variant interpretation framework. Genome Med. 2019, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Kanavy, D.M.; McNulty, S.M.; Jairath, M.K.; Brnich, S.E.; Bizon, C.; Powell, B.C.; Berg, J.S. Comparative analysis of functional assay evidence use by ClinGen Variant Curation Expert Panels. Genome Med. 2019, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.H.; Hernandez, C.C.; Wu, J.; Zhou, N.; Hsu, H.Y.; Shen, M.L.; Wang, Y.C.; Macdonald, R.L.; Wu, D.C. A Missense Mutation A384P Associated with Human Hyperekplexia Reveals a Desensitization Site of Glycine Receptors. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 2818–2831. [Google Scholar] [CrossRef] [Green Version]
- Dash, B.; Li, M.D. Analysis of rare variations reveals roles of amino acid residues in the N-terminal extracellular domain of nicotinic acetylcholine receptor (nAChR) alpha6 subunit in the functional expression of human alpha6*-nAChRs. Mol. Brain 2014, 7, 35. [Google Scholar] [CrossRef] [Green Version]


| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Inheritance | Father | De Novo | Father | Mother | De Novo | Mother | De Novo | Mother | Father |
| Genomic position * | Chr5:161580337 | Chr5:161580316 | Chr5:161530907 | Chr5:161580349 | Chr5:161580349 | Chr5:161580169 | Chr5:161576165 | Chr5:161524801 | Chr5:161530933 |
| Gene | GABRG2 | GABRG2 | GABRG2 | GABRG2 | GABRG2 | GABRG2 | GABRG2 | GABRG2 | GABRG2 |
| Nucleotide change | c.1391T > C | c.1370T > C | c.644G > A | c.1403A > G | c.1403A > G | c.1223G > A | c.974C > T | c.485G > A | c.670C > T |
| Amino acid change | p.Phe464 Ser | p.Phe457 Ser | p.Arg215 His | p.Tyr468 Cys | p.Tyr468 Cys | p.Arg408Lys | p.Ser325 Leu | p.Trp162 X | p.Arg224 X |
| Predictions Polyphen2 | Damaging | Damaging | Damaging | Damaging | Damaging | Benign | Damaging | Damaging | Damaging |
| Predictions SIFT | Deleterious | Deleterious | Deleterious | Deleterious | Deleterious | Benign | Deleterious | Deleterious | Deleterious |
| Sex | Male | Female | Male | Male | Male | Female | Female | Female | Female |
| Diagnosis | FS | FS | FS | GEFS+/FS | FS | FS | FS | FS | MAE |
| Current age | Two years one month | Five years nine months | One year one month | Four years seven months | Three years three months | Four years three months | One year five months | Two years seven months | Seven years |
| Age at onset | One year three months | One year three months | One year | Eleven months | One year six months | One year six months | One year five months | One year | N/D |
| EEG | The bilateral posterior has a slightly higher quantity of slow waves in the waking stage; bilateral central area episodic spikes and wave complex during sleep. | Background activity lower frequency. Right central vertex spikes and slow-wave complex discharges; widespread slow-wave paroxysm during sleep. | Normal | A few diffuse spike-wave and slow spike-wave discharges during sleep. Posterior head (three years); normal EEG (three years nine months). | Normal | Normal | N/D | Normal | Epileptiform discharges: synchronous bilateral spike–slow wave and bifrontal maximum consistent (3.8 yrs.); generalized 2.5 Hz spike-wave complex discharge associated with epileptic spasms and slow rhythms (four years); generalized spike-wave discharges in stage II sleep (seven years). |
| Seizure types | Tonic-clonic seizures | Tonic-clonic seizures | Tonic seizures | Tonic seizures | Tonic seizures | Tonic-clonic seizures | Tonic-clonic seizures | Tonic seizures | Complex partial seizures; frontal lobe epilepsy; absence and myoclonic seizures. |
| AED response | N/A | N/A | N/A | LEV was only used as a prophylactic drug in febrile conditions. | CZP was only used as a prophylactic drug in febrile conditions. | N/A | N/A | N/A | Responded to ketogenic diet therapy; and TPM. |
| Seizure outcome | Six times per year | Twice and seizure-free for three years | Two times total, within two months | Seizure-free for two years and nine months | Two times per year | Three times per year | Two times total, within one month | Five times per year | At 4 years old, 3 times per mon.; at 6.5 years old, seizure-free for a while, then returned. |
| MRI findings | CT: normal | N/D | Normal | Normal | Normal | Normal | CT: normal | Normal | Within normal limits. |
| Neurological exam | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Shaking extremities; epileptic spasms showed spells consisting of a head nod, arms abduction, and recovery within 3–4 s associated with spike-wave discharges on EEG (4 years). |
| Motor development | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | N/D |
| Cognitive outcome | Intellectual disability | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Mild intellectual developmental delay. |
| Others | Sleep disorders | Neonatal hypoxic-ischemic encephalopathy | Has family history, brother. | Has family history, six cases out of ten family members, including three males and three females. | N/A | N/A | Neonatal amniotic fluid choking cough. | Has family history, mother. | Headaches (3–4 times a month) in the last 2.5 years. |
| Deactivation (ms) | Rise Time (ms) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | n | Summary | Adjusted p Value 1 | Mean | S.D. | n | Summary | Adjusted p Value 1 | |
| WT | 199.9 | 43.88 | 8 | 13.67 | 3.065 | 7 | ||||
| W162X | 533.8 | 124.6 | 4 | **** | <0.0001 | 23.51 | 7.964 | 6 | ns | 0.3086 |
| R215H | 263 | 64.65 | 7 | ns | 0.7903 | 31.68 | 6.894 | 5 | * | 0.0123 |
| R224X | 350.1 | 126.4 | 7 | * | 0.0414 | 72.64 | 7.629 | 5 | **** | <0.0001 |
| S325L | 307.7 | 75.67 | 9 | ns | 0.1888 | 68.01 | 15.44 | 4 | **** | <0.0001 |
| Y468C | 433.9 | 180.7 | 8 | *** | 0.0002 | 26.52 | 5.408 | 4 | ns | 0.1744 |
| F464S | 332.6 | 79.92 | 8 | ns | 0.0748 | 29.22 | 7.173 | 5 | * | 0.0398 |
| F457S | 224.8 | 48.26 | 4 | ns | 0.9994 | 60.51 | 12.64 | 4 | **** | <0.0001 |
| R408K | 165.4 | 84.56 | 6 | ns | 0.9924 | 40.23 | 14.13 | 4 | *** | 0.0003 |
| IMAX (nA) | IMAX variant/IMAX WT (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.E.M. | n | Summary | Adjusted p Value 1 | Mean | S.D. | n | Summary | Adjusted p Value 1 | |
| WT | 2.00 | 0.38 | 8 | 100.00 | 2.95 | 8 | ||||
| W162X | 0.48 | 0.13 | 8 | **** | <0.0001 | 24.07 | 3.87 | 8 | **** | <0.0001 |
| R215H | 0.61 | 0.14 | 11 | **** | <0.0001 | 30.44 | 3.10 | 11 | **** | <0.0001 |
| R224X | 0.85 | 0.14 | 8 | *** | 0.0007 | 42.52 | 1.70 | 8 | **** | <0.0001 |
| S325L | 0.78 | 0.14 | 13 | **** | <0.0001 | 39.07 | 2.13 | 13 | **** | <0.0001 |
| Y468C | 0.80 | 0.22 | 8 | *** | 0.0004 | 40.06 | 2.39 | 8 | **** | <0.0001 |
| F464S | 0.96 | 0.18 | 8 | ** | 0.0025 | 47.93 | 1.58 | 8 | **** | <0.0001 |
| F457S | 0.44 | 0.15 | 6 | **** | <0.0001 | 21.96 | 4.60 | 6 | **** | <0.0001 |
| R408K | 0.22 | 0.06 | 7 | **** | <0.0001 | 11.10 | 7.98 | 7 | **** | <0.0001 |
| pEC50 | S.D. | n | Summary | Adjusted p Value 1 | |
|---|---|---|---|---|---|
| WT | 5.92 | 0.27 | 5–10 | ||
| W162X | 6.10 | 0.54 | 5–14 | ns | 0.2353 |
| R215H | 4.60 | 0.21 | 5–16 | **** | <0.0001 |
| R224X | 6.02 | 0.27 | 5–11 | ns | 0.8233 |
| S325L | 5.39 | 0.35 | 5–13 | **** | <0.0001 |
| Y468C | 4.56 | 0.64 | 5–12 | **** | <0.0001 |
| F464S | 4.72 | 0.34 | 5–10 | **** | <0.0001 |
| F457S | 4.35 | 0.68 | 6–13 | **** | <0.0001 |
| R408K | 4.81 | 0.22 | 5–10 | **** | <0.0001 |
| Surface | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1 | β3 | γ2 | |||||||||||||
| Mean | S.E.M. | n | Summary | Adjusted p Value 1 | Mean | S.E.M. | n | Summary | Adjusted p Value 1 | Mean | S.E.M. | n | Summary | Adjusted p Value 1 | |
| WT | 0.998 | 0.003 | 4 | 0.998 | 0.003 | 4 | 0.997 | 0.002 | 4 | ||||||
| W162X | 1.373 | 0.110 | 4 | * | 0.0249 | 1.481 | 0.232 | 4 | ** | 0.0018 | 0.177 | 0.049 | 4 | **** | <0.0001 |
| R215H | 0.901 | 0.030 | 4 | ns | 0.9688 | 0.809 | 0.044 | 4 | ns | 0.5488 | 0.701 | 0.044 | 4 | ns | 0.1179 |
| R224X | 1.992 | 0.158 | 4 | **** | <0.0001 | 1.528 | 0.076 | 4 | *** | 0.0005 | 0.000 | 0.000 | 4 | **** | <0.0001 |
| S325L | 0.987 | 0.047 | 4 | ns | 0.9999 | 0.652 | 0.127 | 4 | * | 0.0464 | 0.513 | 0.066 | 4 | ** | 0.0018 |
| Y468C | 0.871 | 0.004 | 4 | ns | 0.8831 | 0.680 | 0.060 | 4 | ns | 0.0805 | 0.118 | 0.003 | 4 | **** | <0.0001 |
| F464S | 1.416 | 0.114 | 4 | ** | 0.0094 | 1.107 | 0.094 | 4 | ns | 0.9413 | 0.152 | 0.018 | 4 | **** | <0.0001 |
| F457S | 1.669 | 0.163 | 4 | **** | <0.0001 | 1.110 | 0.162 | 4 | ns | 0.9331 | 0.200 | 0.038 | 4 | **** | <0.0001 |
| R408K | 0.936 | 0.043 | 4 | ns | 0.9972 | 0.841 | 0.079 | 4 | ns | 0.7358 | 0.198 | 0.017 | 4 | **** | <0.0001 |
| Total | |||||||||||||||
| α1 | β3 | γ2 | |||||||||||||
| Mean | S.E.M. | n | Summary | Adjusted p Value 1 | Mean | S.E.M. | n | Summary | Adjusted p Value 1 | Mean | S.E.M. | n | Summary | Adjusted p Value 1 | |
| WT | 0.997 | 0.002 | 4 | 0.995 | 0.003 | 4 | 0.998 | 0.003 | 4 | ||||||
| W162X | 1.143 | 0.056 | 4 | ns | 0.1496 | 2.099 | 0.100 | 4 | **** | <0.0001 | 0.152 | 0.019 | 4 | **** | <0.0001 |
| R215H | 0.630 | 0.038 | 4 | **** | <0.0001 | 0.561 | 0.029 | 4 | **** | <0.0001 | 0.622 | 0.018 | 4 | **** | <0.0001 |
| R224X | 1.012 | 0.108 | 4 | ns | 0.9997 | 0.923 | 0.083 | 4 | ns | 0.8255 | 0.417 | 0.018 | 4 | **** | <0.0001 |
| S325L | 0.934 | 0.011 | 4 | ns | 0.8971 | 0.855 | 0.052 | 4 | ns | 0.1798 | 0.661 | 0.011 | 4 | **** | <0.0001 |
| Y468C | 0.759 | 0.068 | 4 | ** | 0.0031 | 0.669 | 0.033 | 4 | **** | <0.0001 | 0.251 | 0.008 | 4 | **** | <0.0001 |
| F464S | 0.932 | 0.040 | 4 | ns | 0.8822 | 0.763 | 0.071 | 4 | ** | 0.0042 | 0.164 | 0.002 | 4 | **** | <0.0001 |
| F457S | 0.728 | 0.013 | 4 | *** | 0.0006 | 0.757 | 0.054 | 4 | ** | 0.0031 | 0.226 | 0.012 | 4 | **** | <0.0001 |
| R408K | 0.902 | 0.015 | 4 | ns | 0.5654 | 0.904 | 0.053 | 4 | ns | 0.6213 | 0.416 | 0.019 | 4 | **** | <0.0001 |
| Surface/Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1 | β3 | γ2 | |||||||||||||
| Mean | S.E.M. | n | Summary | Adjusted p Value 1 | Mean | S.E.M. | n | Summary | Adjusted p Value 1 | Mean | S.E.M. | n | Summary | Adjusted p Value 1 | |
| WT | 0.9997 | 0.0002 | 4 | 0.9995 | 0.0003 | 4 | 0.9997 | 0.0002 | 4 | ||||||
| W162X | 1.2186 | 0.1474 | 4 | ns | 0.7537 | 0.6958 | 0.0822 | 4 | ns | 0.4171 | 0.0861 | 0.0861 | 4 | **** | <0.0001 |
| R215H | 1.4511 | 0.1230 | 4 | ns | 0.0832 | 1.4422 | 0.0371 | 4 | ns | 0.0931 | 1.1348 | 0.0998 | 4 | ns | 0.9731 |
| R224X | 1.9858 | 0.0804 | 4 | **** | <0.0001 | 1.7130 | 0.2173 | 4 | ** | 0.0012 | 0.0000 | 0.0000 | 4 | **** | <0.0001 |
| S325L | 1.0567 | 0.0504 | 4 | ns | 0.9996 | 0.7890 | 0.1730 | 4 | ns | 0.7855 | 0.7743 | 0.0963 | 4 | ns | 0.7279 |
| Y468C | 1.1723 | 0.0907 | 4 | ns | 0.9051 | 1.0146 | 0.0715 | 4 | ns | 0.9999 | 0.4699 | 0.0037 | 4 | * | 0.0277 |
| F464S | 1.5324 | 0.1621 | 4 | * | 0.0265 | 1.4955 | 0.1905 | 4 | * | 0.0453 | 0.9243 | 0.1031 | 4 | ns | 0.9994 |
| F457S | 2.2951 | 0.2244 | 4 | **** | <0.0001 | 1.5292 | 0.2986 | 4 | * | 0.0277 | 0.9053 | 0.1843 | 4 | ns | 0.9968 |
| R408K | 1.0362 | 0.0309 | 4 | ns | 0.9997 | 0.9543 | 0.1379 | 4 | ns | 0.9996 | 0.4772 | 0.0377 | 4 | * | 0.0308 |
| GABRG2 Variant | Variant Category 1 | Phenotype 2 | Location 3 | Reference | gnomAD 4 | Other SNPs 4 |
|---|---|---|---|---|---|---|
| R82Q | LP/P | CAE | ECD | [54,56,57] | yes | R82W 4 |
| R82Q | LP/P | FS | ECD | [54,56,57] | yes | - |
| K328M | LP/P | GEFS+ | TMD | [58] | no | - |
| R177G | LP/P | FS | ECD | [59] | no | R177Q 5 |
| N79S | LB/B | GTCS | ECD | [15,60,61] | yes | - |
| P83S | LP/P | FS | ECD | [15,62] | no | P83L 6 |
| P83S | LP/P | DEE | ECD | [63] | no | - |
| Y274C | LP/P | MAE | TMD | [55] | no | - |
| R323Q | LP/P | DEE | TMD | [21,24] | yes | - |
| R323Q | LP/P | MAE | TMD | [20] | yes | - |
| A106T | LP/P | DEE | ECD | [24,64] | yes | A106P 5 |
| I107T | LP/P | DEE | ECD | [24] | no | - |
| P282S | LP/P | DEE | TMD | [24] | no | - |
| R323W | LP/P | DEE | TMD | [24] | yes | - |
| F343L | LP/P | DEE | TMD | [24] | no | - |
| P282T | LP/P | DEE | TMD | [63] | no | - |
| S306F | LP/P | DEE | TMD | [63] | no | - |
| G257R | LP/P | DEE | ECD | [21] | no | - |
| I218S | LP/P | DEE | ECD | [65] | no | - |
| M199V | LP/P | GEFS+ | ECD | [25] | yes | M199K/T 7 |
| P302L | LP/P | DEE | TMD | [22] | no | - |
| F464S | LP/P | FS | TMD | Current study | no | - |
| F457S | LP/P | FS | TMD | Current study | no | F457L 5 |
| R215H | LP/P | FS | ECD | Current study | no | R215P/L 4 |
| Y468C | LP/P | GEFS+ | TMD | Current study | yes | - |
| Y468C | LP/P | GEFS+ | TMD | Current study | yes | -- |
| R408K | LB/B | FS | CD | Current study | no | R408T 5 |
| S325L | LP/P | FS | TMD | Current study | no | - |
| W162X | LP/P | FS | ECD | Current study | no | - |
| R224X | LP/P | MAE | ECD | Current study | no | R224Q 5,7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, C.C.; Shen, Y.; Hu, N.; Shen, W.; Narayanan, V.; Ramsey, K.; He, W.; Zou, L.; Macdonald, R.L. GABRG2 Variants Associated with Febrile Seizures. Biomolecules 2023, 13, 414. https://doi.org/10.3390/biom13030414
Hernandez CC, Shen Y, Hu N, Shen W, Narayanan V, Ramsey K, He W, Zou L, Macdonald RL. GABRG2 Variants Associated with Febrile Seizures. Biomolecules. 2023; 13(3):414. https://doi.org/10.3390/biom13030414
Chicago/Turabian StyleHernandez, Ciria C., Yanwen Shen, Ningning Hu, Wangzhen Shen, Vinodh Narayanan, Keri Ramsey, Wen He, Liping Zou, and Robert L. Macdonald. 2023. "GABRG2 Variants Associated with Febrile Seizures" Biomolecules 13, no. 3: 414. https://doi.org/10.3390/biom13030414





