Phosphoinositide Signaling in Immune Cell Migration
Abstract
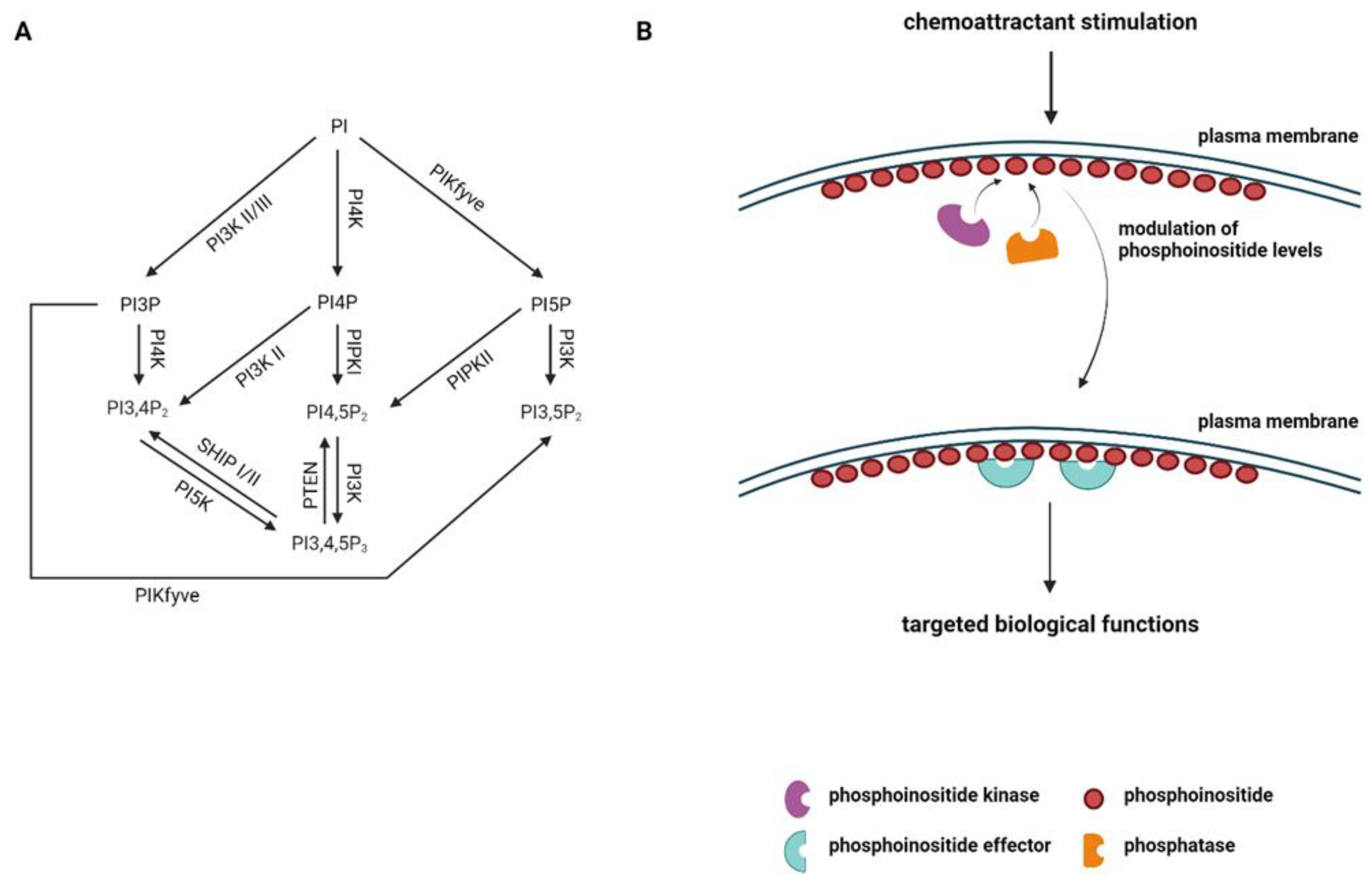
:1. Introduction
2. Role of Phosphoinositide 3-Kinases (PI3Ks) in Immune Cell Migration
2.1. PI3K and Neutrophil Migration
2.2. PI3K and Macrophage Migration
2.3. PI3K and B-Cell Migration
2.4. PI3K and T-Cell Migration
3. PIPKIs and PI4,5P2 in Immune Cell Migration
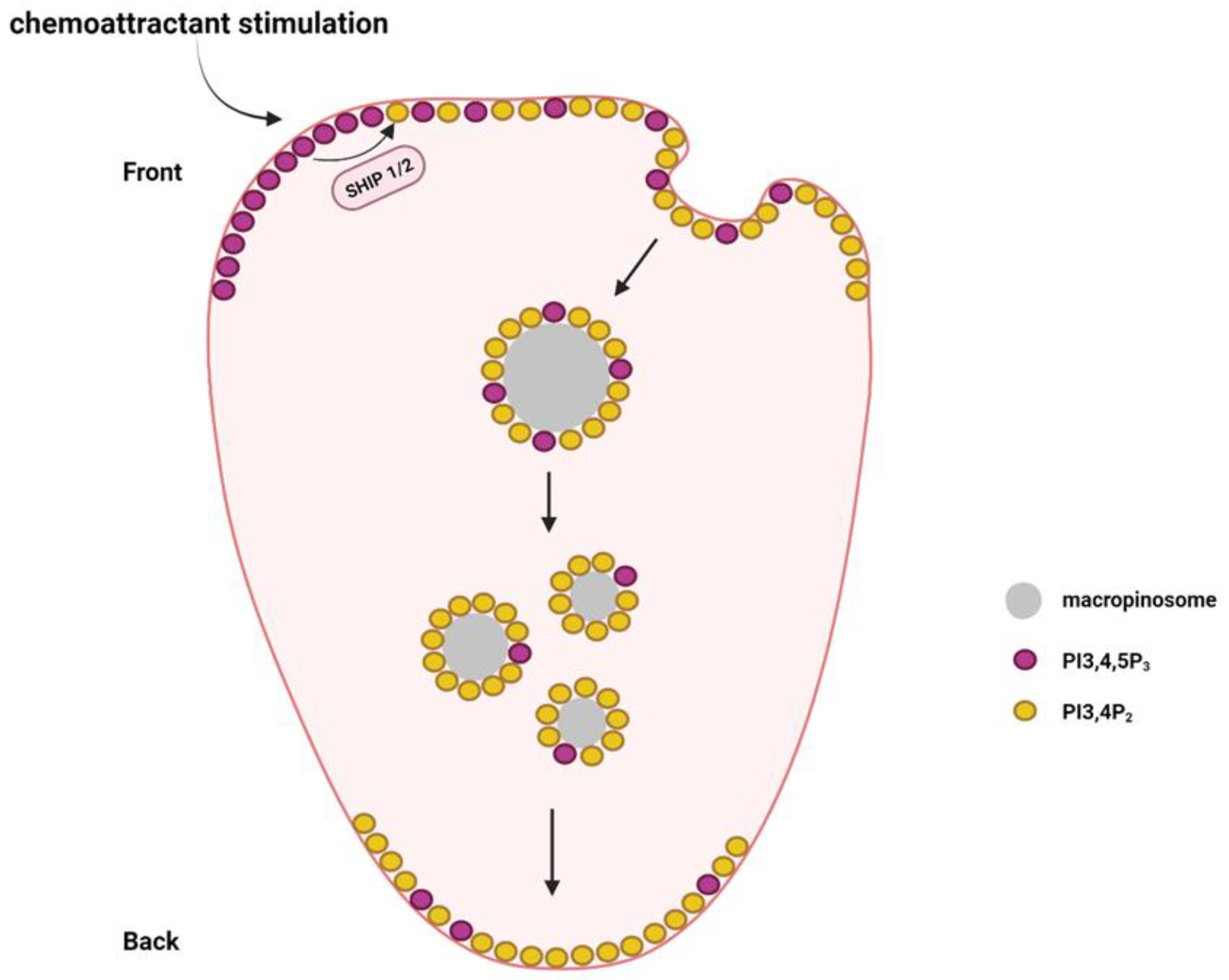
4. Other Phosphoinositide Signaling in Immune Cell Migration
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Le Berre, M.; Lautenschlaeger, F.; Maiuri, P.; Callan-Jones, A.; Heuzé, M.; Takaki, T.; Voituriez, R.; Piel, M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 2015, 160, 659–672. [Google Scholar] [CrossRef]
- Moreau, H.D.; Piel, M.; Voituriez, R.; Lennon-Duménil, A.M. Integrating Physical and Molecular Insights on Immune Cell Migration. Trends Immunol. 2018, 39, 632–643. [Google Scholar] [CrossRef]
- Vesperini, D.; Montalvo, G.; Qu, B.; Lautenschläger, F. Characterization of immune cell migration using microfabrication. Biophys. Rev. 2021, 13, 185–202. [Google Scholar] [CrossRef]
- Renkawitz, J.; Schumann, K.; Weber, M.; Lämmermann, T.; Pflicke, H.; Piel, M.; Polleux, J.; Spatz, J.P.; Sixt, M. Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol. 2009, 11, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Bunney, T.D.; Katan, M. Phosphoinositide signalling in cancer: Beyond PI3K and PTEN. Nat. Rev. Cancer 2010, 10, 342–352. [Google Scholar] [CrossRef]
- Hammond, G.R.V.; Burke, J.E. Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr. Opin. Cell Biol. 2020, 63, 57–67. [Google Scholar] [CrossRef]
- Okkenhaug, K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 2013, 31, 675–704. [Google Scholar] [CrossRef]
- Edwards-Hicks, J.; Apostolova, P.; Buescher, J.M.; Maib, H.; Stanczak, M.A.; Corrado, M.; Klein Geltink, R.I.; Maccari, M.E.; Villa, M.; Carrizo, G.E.; et al. Phosphoinositide acyl chain saturation drives CD8(+) effector T cell signaling and function. Nat. Immunol. 2023, 24, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Gawden-Bone, C.M.; Frazer, G.L.; Richard, A.C.; Ma, C.Y.; Strege, K.; Griffiths, G.M. PIP5 Kinases Regulate Membrane Phosphoinositide and Actin Composition for Targeted Granule Secretion by Cytotoxic Lymphocytes. Immunity 2018, 49, 427–437.e4. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Rossjohn, J.; Wakelam, M.J. Phospholipid signaling in innate immune cells. J. Clin. Investig. 2018, 128, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Schilke, R.M.; Blackburn, C.M.R.; Bamgbose, T.T.; Woolard, M.D. Interface of Phospholipase Activity, Immune Cell Function, and Atherosclerosis. Biomolecules 2020, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Leevers, S.J.; Ahmadi, K.; Timms, J.; Katso, R.; Driscoll, P.C.; Woscholski, R.; Parker, P.J.; Waterfield, M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001, 70, 535–602. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Gulluni, F.; De Santis, M.C.; Margaria, J.P.; Martini, M.; Hirsch, E. Class II PI3K Functions in Cell Biology and Disease. Trends Cell Biol. 2019, 29, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Jaber, N.; Dou, Z.; Chen, J.S.; Catanzaro, J.; Jiang, Y.P.; Ballou, L.M.; Selinger, E.; Ouyang, X.; Lin, R.Z.; Zhang, J.; et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc. Natl. Acad. Sci. USA 2012, 109, 2003–2008. [Google Scholar] [CrossRef]
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Saudemont, A.; Colucci, F. PI3K signaling in lymphocyte migration. Cell Cycle 2009, 8, 3307–3310. [Google Scholar] [CrossRef]
- Galvão, I.; Sousa, L.P.; Teixeira, M.M.; Pinho, V. PI3K Isoforms in Cell Signalling and Innate Immune Cell Responses. Curr. Top. Microbiol. Immunol. 2022, 436, 147–164. [Google Scholar] [CrossRef]
- Cain, R.J.; Ridley, A.J. Phosphoinositide 3-kinases in cell migration. Biol. Cell 2009, 101, 13–29. [Google Scholar] [CrossRef]
- Gambardella, L.; Vermeren, S. Molecular players in neutrophil chemotaxis--focus on PI3K and small GTPases. J. Leukoc. Biol. 2013, 94, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, C.; Masinovsky, B.; Dick, K.; Sowell, C.G.; Staunton, D.E. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J. Immunol. 2003, 170, 2647–2654. [Google Scholar] [CrossRef]
- Yoo, S.K.; Deng, Q.; Cavnar, P.J.; Wu, Y.I.; Hahn, K.M.; Huttenlocher, A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 2010, 18, 226–236. [Google Scholar] [CrossRef]
- Servant, G.; Weiner, O.D.; Herzmark, P.; Balla, T.; Sedat, J.W.; Bourne, H.R. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000, 287, 1037–1040. [Google Scholar] [CrossRef]
- Li, Z.; Dong, X.; Wang, Z.; Liu, W.; Deng, N.; Ding, Y.; Tang, L.; Hla, T.; Zeng, R.; Li, L.; et al. Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 2005, 7, 399–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yang, H.; Liu, H.; Lu, Y.; Han, L.; Liu, G. Kinase AKT controls innate immune cell development and function. Immunology 2013, 140, 143–152. [Google Scholar] [CrossRef]
- Glogauer, M.; Marchal, C.C.; Zhu, F.; Worku, A.; Clausen, B.E.; Foerster, I.; Marks, P.; Downey, G.P.; Dinauer, M.; Kwiatkowski, D.J. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J. Immunol. 2003, 170, 5652–5657. [Google Scholar] [CrossRef]
- Pestonjamasp, K.N.; Forster, C.; Sun, C.; Gardiner, E.M.; Bohl, B.; Weiner, O.; Bokoch, G.M.; Glogauer, M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood 2006, 108, 2814–2820. [Google Scholar] [CrossRef]
- Kumar, S.; Xu, J.; Perkins, C.; Guo, F.; Snapper, S.; Finkelman, F.D.; Zheng, Y.; Filippi, M.D. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood 2012, 120, 3563–3574. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, M.; Zhan, L.; Li, Z.; Ai, Y.; Wu, D.; Huang, C.K. Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc. Natl. Acad. Sci. USA 2002, 99, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Irie-Sasaki, J.; Jones, R.G.; Oliveira-dos-Santos, A.J.; Stanford, W.L.; Bolon, B.; Wakeham, A.; Itie, A.; Bouchard, D.; Kozieradzki, I.; et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 2000, 287, 1040–1046. [Google Scholar] [CrossRef]
- Heit, B.; Liu, L.; Colarusso, P.; Puri, K.D.; Kubes, P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J. Cell Sci. 2008, 121, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.J.; Milne, L.; Kulkarni, S.; Sasaki, T.; Walker, S.; Andrews, S.; Crabbe, T.; Finan, P.; Jones, G.; Jackson, S.; et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 2007, 9, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Reutershan, J.; Saprito, M.S.; Wu, D.; Rückle, T.; Ley, K. Phosphoinositide 3-kinase gamma required for lipopolysaccharide-induced transepithelial neutrophil trafficking in the lung. Eur. Respir. J. 2010, 35, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Lee, S.; Briscoe, C.; Ellsworth, C.; Firtel, R.A. Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells. Proc. Natl. Acad. Sci. USA 2000, 97, 5225–5230. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Ladwein, M.; Dimchev, G.A.; Hein, A.; Schwenkmezger, L.; Arens, S.; Ladwein, K.I.; Margit Holleboom, J.; Schur, F.; Victor Small, J.; et al. Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J. Cell Sci. 2013, 126, 4572–4588. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Filippi, M.D.; Cancelas, J.A.; Siefring, J.E.; Williams, E.P.; Jasti, A.C.; Harris, C.E.; Lee, A.W.; Prabhakar, R.; Atkinson, S.J.; et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 2003, 302, 445–449. [Google Scholar] [CrossRef]
- Sun, C.X.; Downey, G.P.; Zhu, F.; Koh, A.L.; Thang, H.; Glogauer, M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 2004, 104, 3758–3765. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Nishikimi, A.; Tanaka, Y.; Takii, R.; Noda, M.; Inayoshi, A.; Watanabe, K.; Sanematsu, F.; Sasazuki, T.; Sasaki, T.; et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 2006, 174, 647–652. [Google Scholar] [CrossRef]
- Nishikimi, A.; Fukuhara, H.; Su, W.; Hongu, T.; Takasuga, S.; Mihara, H.; Cao, Q.; Sanematsu, F.; Kanai, M.; Hasegawa, H.; et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 2009, 324, 384–387. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Pixley, F.J. Macrophage Migration and Its Regulation by CSF-1. Int. J. Cell Biol. 2012, 2012, 501962. [Google Scholar] [CrossRef] [PubMed]
- Maridonneau-Parini, I. Control of macrophage 3D migration: A therapeutic challenge to limit tissue infiltration. Immunol. Rev. 2014, 262, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.E.; Prigmore, E.; Calvez, R.; Hogan, C.; Dunn, G.A.; Hirsch, E.; Wymann, M.P.; Ridley, A.J. Requirement for PI 3-kinase gamma in macrophage migration to MCP-1 and CSF-1. Exp. Cell Res. 2003, 290, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.; Gay, R.E.; Koopman, W.J. Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: Two cellular mechanisms explain joint destruction? Ann. Rheum. Dis. 1993, 52 (Suppl. 1), S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.; Rose, C.; König, C.; Gajda, M.; Wetzker, R.; Bräuer, R. Loss of phosphoinositide 3-kinase gamma decreases migration and activation of phagocytes but not T cell activation in antigen-induced arthritis. BMC Musculoskelet. Disord. 2010, 11, 63. [Google Scholar] [CrossRef]
- Munugalavadla, V.; Borneo, J.; Ingram, D.A.; Kapur, R. p85alpha subunit of class IA PI-3 kinase is crucial for macrophage growth and migration. Blood 2005, 106, 103–109. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D.C. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B Cells, Antibodies, and More. Clin. J. Am. Soc. Nephrol. 2016, 11, 137–154. [Google Scholar] [CrossRef]
- Rumsey, L.M.; Teague, R.M.; Benedict, S.H.; Chan, M.A. MIP-1alpha induces activation of phosphatidylinositol-3 kinase that associates with Pyk-2 and is necessary for B-cell migration. Exp. Cell Res. 2001, 268, 77–83. [Google Scholar] [CrossRef]
- Ali, A.Y.; Wu, X.; Eissa, N.; Hou, S.; Ghia, J.E.; Murooka, T.T.; Banerji, V.; Johnston, J.B.; Lin, F.; Gibson, S.B.; et al. Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration. Leukemia 2018, 32, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Reif, K.; Okkenhaug, K.; Sasaki, T.; Penninger, J.M.; Vanhaesebroeck, B.; Cyster, J.G. Cutting edge: Differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J. Immunol. 2004, 173, 2236–2240. [Google Scholar] [CrossRef]
- Wissmann, S.; Stolp, B.; Jímenez, A.M.; Stein, J.V. DOCK2 and phosphoinositide-3 kinase δ mediate two complementary signaling pathways for CXCR5-dependent B cell migration. Front. Immunol. 2022, 13, 982383. [Google Scholar] [CrossRef]
- Finlay, D.; Cantrell, D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann. N. Y. Acad. Sci. 2010, 1183, 149–157. [Google Scholar] [CrossRef]
- Asperti-Boursin, F.; Real, E.; Bismuth, G.; Trautmann, A.; Donnadieu, E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J. Exp. Med. 2007, 204, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.J.; Verdijk, P.; van der Raaij-Helmer, E.M.; Navis, M.; Hensbergen, P.J.; Leurs, R.; Tensen, C.P. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood 2003, 102, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Cronshaw, D.G.; Owen, C.; Brown, Z.; Ward, S.G. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J. Immunol. 2004, 172, 7761–7770. [Google Scholar] [CrossRef]
- Jarmin, S.J.; David, R.; Ma, L.; Chai, J.G.; Dewchand, H.; Takesono, A.; Ridley, A.J.; Okkenhaug, K.; Marelli-Berg, F.M. T cell receptor-induced phosphoinositide-3-kinase p110delta activity is required for T cell localization to antigenic tissue in mice. J. Clin. Investig. 2008, 118, 1154–1164. [Google Scholar] [CrossRef]
- Auger, K.R.; Serunian, L.A.; Soltoff, S.P.; Libby, P.; Cantley, L.C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 1989, 57, 167–175. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem. J. 1984, 220, 345–360. [Google Scholar] [CrossRef]
- Sun, Y.; Thapa, N.; Hedman, A.C.; Anderson, R.A. Phosphatidylinositol 4,5-bisphosphate: Targeted production and signaling. Bioessays 2013, 35, 513–522. [Google Scholar] [CrossRef]
- Heck, J.N.; Mellman, D.L.; Ling, K.; Sun, Y.; Wagoner, M.P.; Schill, N.J.; Anderson, R.A. A conspicuous connection: Structure defines function for the phosphatidylinositol-phosphate kinase family. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 15–39. [Google Scholar] [CrossRef]
- van den Bout, I.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef]
- Lokuta, M.A.; Senetar, M.A.; Bennin, D.A.; Nuzzi, P.A.; Chan, K.T.; Ott, V.L.; Huttenlocher, A. Type Igamma PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol. Biol. Cell 2007, 18, 5069–5080. [Google Scholar] [CrossRef]
- Xu, W.; Wang, P.; Petri, B.; Zhang, Y.; Tang, W.; Sun, L.; Kress, H.; Mann, T.; Shi, Y.; Kubes, P.; et al. Integrin-induced PIP5K1C kinase polarization regulates neutrophil polarization, directionality, and in vivo infiltration. Immunity 2010, 33, 340–350. [Google Scholar] [CrossRef]
- Lacalle, R.A.; Peregil, R.M.; Albar, J.P.; Merino, E.; Martínez, A.C.; Mérida, I.; Mañes, S. Type I phosphatidylinositol 4-phosphate 5-kinase controls neutrophil polarity and directional movement. J. Cell Biol. 2007, 179, 1539–1553. [Google Scholar] [CrossRef]
- Lam, P.Y.; Yoo, S.K.; Green, J.M.; Huttenlocher, A. The SH2-domain-containing inositol 5-phosphatase (SHIP) limits the motility of neutrophils and their recruitment to wounds in zebrafish. J. Cell Sci. 2012, 125, 4973–4978. [Google Scholar] [CrossRef]
- Li, X.; Pal, D.S.; Biswas, D.; Iglesias, P.A.; Devreotes, P.N. Reverse fountain flow of phosphatidylinositol-3,4-bisphosphate polarizes migrating cells. EMBO J. 2021, 40, e105094. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Ghosh, S.; Erneux, C. The impact of phosphoinositide 5-phosphatases on phosphoinositides in cell function and human disease. J. Lipid Res. 2019, 60, 276–286. [Google Scholar] [CrossRef]
- Liu, Q.; Shalaby, F.; Jones, J.; Bouchard, D.; Dumont, D.J. The SH2-containing inositol polyphosphate 5-phosphatase, ship, is expressed during hematopoiesis and spermatogenesis. Blood 1998, 91, 2753–2759. [Google Scholar] [CrossRef]
- Pesesse, X.; Deleu, S.; De Smedt, F.; Drayer, L.; Erneux, C. Identification of a second SH2-domain-containing protein closely related to the phosphatidylinositol polyphosphate 5-phosphatase SHIP. Biochem. Biophys. Res. Commun. 1997, 239, 697–700. [Google Scholar] [CrossRef]
- Nishio, M.; Watanabe, K.; Sasaki, J.; Taya, C.; Takasuga, S.; Iizuka, R.; Balla, T.; Yamazaki, M.; Watanabe, H.; Itoh, R.; et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat. Cell Biol. 2007, 9, 36–44. [Google Scholar] [CrossRef]
- Mondal, S.; Subramanian, K.K.; Sakai, J.; Bajrami, B.; Luo, H.R. Phosphoinositide lipid phosphatase SHIP1 and PTEN coordinate to regulate cell migration and adhesion. Mol. Biol. Cell 2012, 23, 1219–1230. [Google Scholar] [CrossRef]
- Michael, M.; McCormick, B.; Anderson, K.E.; Karmakar, U.; Vermeren, M.; Schurmans, S.; Amour, A.; Vermeren, S. The 5-Phosphatase SHIP2 Promotes Neutrophil Chemotaxis and Recruitment. Front. Immunol. 2021, 12, 671756. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Hou, S.; Malek, M.; Kielkowska, A.; Noh, E.; Makondo, K.J.; Du, Q.; Wilkins, J.A.; Johnston, J.B.; et al. Phosphatidylinositol-3,4-Bisphosphate and Its Binding Protein Lamellipodin Regulate Chemotaxis of Malignant B Lymphocytes. J. Immunol. 2016, 196, 586–595. [Google Scholar] [CrossRef]
- Bae, Y.H.; Ding, Z.; Das, T.; Wells, A.; Gertler, F.; Roy, P. Profilin1 regulates PI(3,4)P2 and lamellipodin accumulation at the leading edge thus influencing motility of MDA-MB-231 cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21547–21552. [Google Scholar] [CrossRef]
- Dimchev, G.; Amiri, B.; Humphries, A.C.; Schaks, M.; Dimchev, V.; Stradal, T.E.B.; Faix, J.; Krause, M.; Way, M.; Falcke, M.; et al. Lamellipodin tunes cell migration by stabilizing protrusions and promoting adhesion formation. J. Cell Sci. 2020, 133, jcs239020. [Google Scholar] [CrossRef]
- Gajardo, T.; Bernard, M.; Lô, M.; Turck, E.; Leveau, C.; El-Daher, M.T.; Deslys, A.; Panikulam, P.; Menche, C.; Kurowska, M.; et al. Actin dynamics regulation by TTC7A/PI4KIIIα limits DNA damage and cell death under confinement. J. Allergy Clin. Immunol. 2023, 152, 949–960. [Google Scholar] [CrossRef]
- Ren, C.; Yuan, Q.; Braun, M.; Zhang, X.; Petri, B.; Zhang, J.; Kim, D.; Guez-Haddad, J.; Xue, W.; Pan, W.; et al. Leukocyte Cytoskeleton Polarization Is Initiated by Plasma Membrane Curvature from Cell Attachment. Dev. Cell 2019, 49, 206–219.e7. [Google Scholar] [CrossRef]
- Dayam, R.M.; Sun, C.X.; Choy, C.H.; Mancuso, G.; Glogauer, M.; Botelho, R.J. The Lipid Kinase PIKfyve Coordinates the Neutrophil Immune Response through the Activation of the Rac GTPase. J. Immunol. 2017, 199, 2096–2105. [Google Scholar] [CrossRef]
- Oppelt, A.; Lobert, V.H.; Haglund, K.; Mackey, A.M.; Rameh, L.E.; Liestøl, K.; Schink, K.O.; Pedersen, N.M.; Wenzel, E.M.; Haugsten, E.M.; et al. Production of phosphatidylinositol 5-phosphate via PIKfyve and MTMR3 regulates cell migration. EMBO Rep. 2013, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-derived suppressor cells: An emerging target for anticancer immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakar, R.; Ghosh, C.; Sun, Y. Phosphoinositide Signaling in Immune Cell Migration. Biomolecules 2023, 13, 1705. https://doi.org/10.3390/biom13121705
Kakar R, Ghosh C, Sun Y. Phosphoinositide Signaling in Immune Cell Migration. Biomolecules. 2023; 13(12):1705. https://doi.org/10.3390/biom13121705
Chicago/Turabian StyleKakar, Ruchi, Chinmoy Ghosh, and Yue Sun. 2023. "Phosphoinositide Signaling in Immune Cell Migration" Biomolecules 13, no. 12: 1705. https://doi.org/10.3390/biom13121705





