Tumor-Associated and Systemic Autoimmunity in Pre-Clinical Breast Cancer among Post-Menopausal Women
Abstract
:1. Introduction
2. Methods
2.1. Sample
2.2. Assays
2.3. Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaenker, P.; Ziman, M.R. Serologic autoantibodies as diagnostic cancer biomarkers—A review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2161–2181. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Casiano, C.A.; Peng, X.X.; Koziol, J.A.; Chan, E.K.; Tan, E.M. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol. Biomark. Prev. 2003, 12, 136–143. [Google Scholar]
- Lu, H.; Goodell, V.; Disis, M.L. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J. Proteome Res. 2008, 7, 1388–1394. [Google Scholar] [CrossRef]
- Tan, H.T.; Low, J.; Lim, S.G.; Chung, M.C. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009, 276, 6880–6904. [Google Scholar] [CrossRef]
- Anderson, K.S.; Sibani, S.; Wallstrom, G.; Qiu, J.; Mendoza, E.A.; Raphael, J.; Hainsworth, E.; Montor, W.R.; Wong, J.; Park, J.G.; et al. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J. Proteome Res. 2011, 10, 85–96. [Google Scholar] [CrossRef]
- Meistere, I.; Werner, S.; Zayakin, P.; Silina, K.; Rulle, U.; Pismennaja, A.; Santare, D.; Kikuste, I.; Isajevs, S.; Leja, M.; et al. The Prevalence of Cancer-Associated Autoantibodies in Patients with Gastric Cancer and Progressive Grades of Premalignant Lesions. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1564–1574. [Google Scholar] [CrossRef]
- Qiu, J.; Keyser, B.; Lin, Z.T.; Wu, T. Autoantibodies as Potential Biomarkers in Breast Cancer. Biosensors 2018, 8, 67. [Google Scholar] [CrossRef]
- Rauf, F.; Anderson, K.S.; LaBaer, J. Autoantibodies in Early Detection of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2475–2485. [Google Scholar] [CrossRef]
- Ladd, J.J.; Chao, T.; Johnson, M.M.; Qiu, J.; Chin, A.; Israel, R.; Pitteri, S.J.; Mao, J.; Wu, M.; Amon, L.M.; et al. Autoantibody signatures involving glycolysis and splicesome proteins precede a diagnosis of breast cancer among postmenopausal women. Cancer Res. 2013, 73, 1502–1513. [Google Scholar] [CrossRef]
- Hanash, S.M.; Baik, C.S.; Kallioniemi, O. Emerging molecular biomarkers—Blood-based strategies to detect and monitor cancer. Nat. Rev. Clin. Oncol. 2011, 8, 142–150. [Google Scholar] [CrossRef]
- Tabernero, M.D.; Lv, L.L.; Anderson, K.S. Autoantibody profiles as biomarkers of breast cancer. Cancer Biomark. 2010, 6, 247–256. [Google Scholar] [CrossRef]
- Satoh, M.; Chan, E.K.; Ho, L.A.; Rose, K.M.; Parks, C.G.; Cohn, R.D.; Jusko, T.A.; Walker, N.J.; Germolec, D.R.; Whitt, I.Z.; et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012, 64, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.G.; Miller, F.W.; Satoh, M.; Chan, E.K.; Andrushchenko, Z.; Birnbaum, L.S.; Jusko, T.A.; Kissling, G.E.; Patel, M.D.; Rose, K.M.; et al. Reproductive and hormonal risk factors for antinuclear antibodies (ANA) in a representative sample of U.S. women. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2492–2502. [Google Scholar] [CrossRef]
- Lleo, A.; Invernizzi, P.; Gao, B.; Podda, M.; Gershwin, M.E. Definition of human autoimmunity—Autoantibodies versus autoimmune disease. Autoimmun. Rev. 2010, 9, A259–A266. [Google Scholar] [CrossRef]
- Chao, T.; Ladd, J.J.; Qiu, J.; Johnson, M.M.; Israel, R.; Chin, A.; Wang, H.; Prentice, R.L.; Feng, Z.; Disis, M.L.; et al. Proteomic profiling of the autoimmune response to breast cancer antigens uncovers a suppressive effect of hormone therapy. Proteom. Clin. Appl. 2013, 7, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Amon, L.M.; Pitteri, S.J.; Li, C.I.; McIntosh, M.; Ladd, J.J.; Disis, M.; Porter, P.; Wong, C.H.; Zhang, Q.; Lampe, P.; et al. Concordant release of glycolysis proteins into the plasma preceding a diagnosis of ER+ breast cancer. Cancer Res. 2012, 72, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, E.E.; Tibben, M.M.; Rosing, H.; Schellens, J.H.; Beijnen, J.H. Determination of ruthenium originating from the investigational anti-cancer drug NAMI-A in human plasma ultrafiltrate, plasma, and urine by inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1521–1530. [Google Scholar] [CrossRef]
- Ladd, J.J.; Busald, T.; Johnson, M.M.; Zhang, Q.; Pitteri, S.J.; Wang, H.; Brenner, D.E.; Lampe, P.D.; Kucherlapati, R.; Feng, Z.; et al. Increased plasma levels of the APC-interacting protein MAPRE1, LRG1, and IGFBP2 preceding a diagnosis of colorectal cancer in women. Cancer Prev. Res. 2012, 5, 655–664. [Google Scholar] [CrossRef]
- Li, C.I.; Mirus, J.E.; Zhang, Y.; Ramirez, A.B.; Ladd, J.J.; Prentice, R.L.; McIntosh, M.W.; Hanash, S.M.; Lampe, P.D. Discovery and preliminary confirmation of novel early detection biomarkers for triple-negative breast cancer using preclinical plasma samples from the Women′s Health Initiative observational study. Breast Cancer Res. Treat. 2012, 135, 611–618. [Google Scholar] [CrossRef]
- Lu, H.; Ladd, J.; Feng, Z.; Wu, M.; Goodell, V.; Pitteri, S.J.; Li, C.I.; Prentice, R.; Hanash, S.M.; Disis, M.L. Evaluation of known oncoantibodies, HER2, p53, and cyclin B1, in prediagnostic breast cancer sera. Cancer Prev. Res. 2012, 5, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Mackey, R.H.; Walitt, B.T.; Deane, K.D.; Holers, V.M.; Robinson, W.H.; Sokolove, J.; Chang, Y.; Liu, S.; Parks, C.G.; et al. Determinants of mortality among postmenopausal women who report rheumatoid arthritis in the Women’s Health Initiative. Arthritis Rheum. 2013, 66, 497. [Google Scholar] [CrossRef]
- Tonino, P.; Kiss, B.; Strom, J.; Methawasin, M.; Smith, J.E.; Kolb, J.; Labeit, S.; Granzier, H. The giant protein titin regulates the length of the striated muscle thick filament. Nat. Commun. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, C.; Rowell, J.; Ferreiro, A. A rising titan: TTN review and mutation update. Hum. Mutat. 2014, 35, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Gohler, S.; Da Silva Filho, M.I.; Johansson, R.; Enquist-Olsson, K.; Henriksson, R.; Hemminki, K.; Lenner, P.; Forsti, A. Functional germline variants in driver genes of breast cancer. Cancer Causes Control 2017, 28, 259–271. [Google Scholar] [CrossRef]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef]
- Yamamoto, A.M.; Gajdos, P.; Eymard, B.; Tranchant, C.; Warter, J.M.; Gomez, L.; Bourquin, C.; Bach, J.F.; Garchon, H.J. Anti-titin antibodies in myasthenia gravis: Tight association with thymoma and heterogeneity of nonthymoma patients. Arch. Neurol. 2001, 58, 885–890. [Google Scholar] [CrossRef]
- Berger, B.; Stich, O.; Labeit, S.; Rauer, S. Screening for anti-titin antibodies in patients with various paraneoplastic neurological syndromes. J. Neuroimmunol. 2016, 295–296, 18–20. [Google Scholar] [CrossRef]
- Stergiou, C.; Lazaridis, K.; Zouvelou, V.; Tzartos, J.; Mantegazza, R.; Antozzi, C.; Andreetta, F.; Evoli, A.; Deymeer, F.; Saruhan-Direskeneli, G.; et al. Titin antibodies in “seronegative” myasthenia gravis—A new role for an old antigen. J. Neuroimmunol. 2016, 292, 108–115. [Google Scholar] [CrossRef]
- Muenks, A.G.; Stiers, K.M.; Beamer, L.J. Sequence-structure relationships, expression profiles, and disease-associated mutations in the paralogs of phosphoglucomutase 1. PLoS ONE 2017, 12, e0183563. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Ichikawa, M.; Lyons, J.J.; Datta, S.; Lamborn, I.T.; Jing, H.; Kim, E.S.; Biancalana, M.; Wolfe, L.A.; et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J. Allergy Clin. Immunol. 2014, 133, 1400–1409.e5. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Doppler, H.; Perez, E.A.; Andorfer, C.A.; Sun, Z.; Anastasiadis, P.Z.; Thompson, E.; Geiger, X.J.; Storz, P. Pharmacologic reversion of epigenetic silencing of the PRKD1 promoter blocks breast tumor cell invasion and metastasis. Breast Cancer Res. 2013, 15, R66. [Google Scholar] [CrossRef] [PubMed]
- Soltys, A.I.; Axford, J.S.; Sutton, B.J. Rheumatoid factors: Where are we now? Ann. Rheum. Dis. 1997, 56, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.J.; Rossen, R.D.; Lewis, V.M.; Laughter, A.H.; Douglass, C.C. Rheumatoid factor and tumor-host interaction. Proc. Natl. Acad. Sci. USA 1976, 73, 2106–2108. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Casciola-Rosen, L.; Rosen, A. Review: Cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol. 2015, 67, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.G.; Darrah, E.; Shah, A.A.; Skora, A.D.; Casciola-Rosen, L.A.; Wigley, F.M.; Boin, F.; Fava, A.; Thoburn, C.; Kinde, I.; et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 2014, 343, 152–157. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity′s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Hardenbergh, D.; Molina, E.; Naik, R.; Geetha, D.; Chaturvedi, S.; Timlin, H. Factors mediating cancer risk in systemic lupus erythematosus. Lupus 2022, 31, 1285–1295. [Google Scholar] [CrossRef]
- Clarke, A.E.; Pooley, N.; Marjenberg, Z.; Langham, J.; Nicholson, L.; Langham, S.; Embleton, N.; Wang, X.; Desta, B.; Barut, V.; et al. Risk of malignancy in patients with systemic lupus erythematosus: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 1230–1241. [Google Scholar] [CrossRef]
- Wadström, H.; Pettersson, A.; Smedby, K.E.; Askling, J. Risk of breast cancer before and after rheumatoid arthritis, and the impact of hormonal factors. Ann. Rheum. Dis. 2020, 79, 581–586. [Google Scholar] [CrossRef]
- Satoh, M.; Vazquez-Del Mercado, M.; Chan, E.K. Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod. Rheumatol. 2009, 19, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; Rober, N.; Andrade, L.E.; Mahler, M. The Clinical Relevance of Anti-DFS70 Autoantibodies. Clin. Rev. Allergy Immunol. 2017, 52, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Nagele, E.P.; Han, M.; Acharya, N.K.; DeMarshall, C.; Kosciuk, M.C.; Nagele, R.G. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS ONE 2013, 8, e60726. [Google Scholar] [CrossRef]
- Walitt, B.; Mackey, R.; Kuller, L.; Deane, K.D.; Robinson, W.; Holers, V.M.; Chang, Y.F.; Moreland, L. Predictive Value of Autoantibody Testing for Validating Self-reported Diagnoses of Rheumatoid Arthritis in the Women’s Health Initiative. Am. J. Epidemiol. 2013, 177, 887–893. [Google Scholar] [CrossRef]
- Walitt, B.T.; Constantinescu, F.; Katz, J.D.; Weinstein, A.; Wang, H.; Hernandez, R.K.; Hsia, J.; Howard, B.V. Validation of self-report of rheumatoid arthritis and systemic lupus erythematosus: The Women’s Health Initiative. J. Rheumatol. 2008, 35, 811–818. [Google Scholar]
- Nisihara, R.; Machoski, M.C.C.; Neppel, A.; Maestri, C.A.; Messias-Reason, I.; Skare, T.L. Anti-nuclear antibodies in patients with breast cancer. Clin. Exp. Immunol. 2018, 193, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Ochs, R.L.; Mahler, M.; Basu, A.; Rios-Colon, L.; Sanchez, T.W.; Andrade, L.E.; Fritzler, M.J.; Casiano, C.A. The significance of autoantibodies to DFS70/LEDGFp75 in health and disease: Integrating basic science with clinical understanding. Clin. Exp. Med. 2016, 16, 273–293. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, T.W.; Casiano, C.A. DFS70/LEDGFp75: An Enigmatic Autoantigen at the Interface between Autoimmunity, AIDS, and Cancer. Front. Immunol. 2015, 6, 116. [Google Scholar] [CrossRef]
- Basu, A.; Rojas, H.; Banerjee, H.; Cabrera, I.B.; Perez, K.Y.; De Leon, M.; Casiano, C.A. Expression of the stress response oncoprotein LEDGF/p75 in human cancer: A study of 21 tumor types. PLoS ONE 2012, 7, e30132. [Google Scholar] [CrossRef]
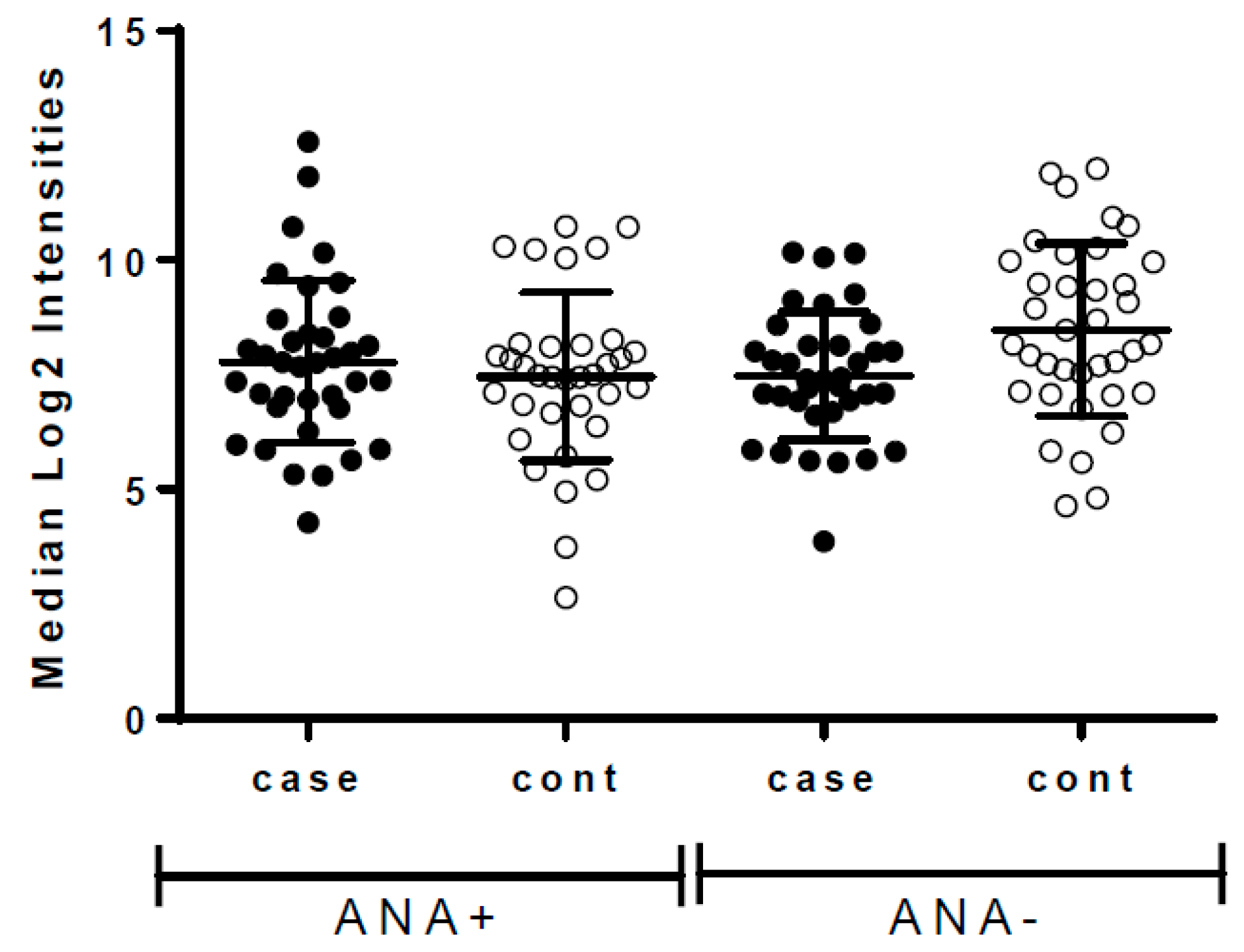
| Incident Breast Cancer | Controls (No Breast Cancer during Follow-Up) | |||||
|---|---|---|---|---|---|---|
| ANA-Pos N = 37 | ANA-Neg N = 36 | p-Value a | ANA-Pos N = 35 | ANA-Neg N = 37 | p-Value a | |
| Matching factors | ||||||
| Age—years (SE) | 64.9 (6.0) | 64.9 (5.7) | --- | 64.7 (6.1) | 64.9 (6.0) | --- |
| Years to breast cancer or follow-up time (SE) | 6.8 (3.9) | 6.9 (3.8) | --- | 13.1 (3.6) | 13.0 (3.0) | --- |
| Other autoantibodies, medications, and hormone use | N (%) | N (%) | N (%) | N (%) | ||
| Anti-CCP positive | 3 (8) | 1 (3) | 0.32 | 4 (11) | 4 (11) | 0.97 |
| Anti-RF positive | 10 (27) | 3 (8) | 0.038 | 7 (19) | 4 (11) | 0.31 |
| Current DMARD use | 4 (11) | 0 (--) | 0.044 | 1 (3) | 3 (8) | 0.32 |
| Current Prednisone | 1 (3) | 2 (6) | 0.54 | 0 (--) | 2 (5) | 0.16 |
| Current estrogen use E only E + P | 11 (30) 4 (12) | 8 (22) 5 (14) | 0.56 | 7 (19) 5 (13) | 10 (27) 3 (8) | 0.64 |
| Other Characteristics | ||||||
| Lupus (self-reported at baseline or follow-up) | 1 (3) | 2 (5) | 0.57 | 6 (17) | 6 (17) | 0.96 |
| Breast cancer tumor ER positive Invasive | 27 (87) b 28 (76) | 27 (75) 31 (86) | 0.22 0.26 | NA NA | NA NA | --- |
| Cases (N = 37 ANA+, 36 ANA−) | Controls (N = 35 ANA+, 37 ANA−) | ||||
|---|---|---|---|---|---|
| Antigen | Median Ratio a | p-Value b | Median Ratio a | p-Value b | Function/Pathways |
| Higher anti-TAA in ANA-positive versus ANA-negative women | |||||
| PGM3 | 1.48 | 0.004 | 1.67 | 0.128 | Phosphoacetylglucosamine mutase: mediates glycogen formation/utilization, defects linked to autoimmunity |
| TTN | 1.41 | 0.005 | 1.79 | 0.129 | Tintin: protein kinase, widespread in muscle; antibodies in scleroderma; somatic mutation in cancer |
| DUSP26 | 1.17 | 0.018 | 1.15 | 0.492 | Dual specificity phosphatase 26: protein-tyrosine-phosphatase |
| PRKD1 | 1.05 | 0.801 | 1.73 | 0.024 | Protein Kinase D1: rap1 signaling pathway. |
| UCHL1 | 0.89 | 0.970 | 1.33 | 0.046 | Truncated calcium binding protein |
| Lower TAA in ANA-positive versus ANA-negative women | |||||
| ACTA2 | 0.86 | 0.021 | 0.88 | 0.629 | Actin Alpha 2, smooth muscle, aorta; interacting with TTN/TNF; exosomal protein of colorectal cancer |
| C5orf45 | 0.79 | 0.032 | 0.81 | 0.397 | Chromosome 5, open reading frame 45; expressed in breast cancer MCF7, chronic B-lymphocytic leukemia |
| C13orf24 | 0.84 | 0.008 | 0.92 | 0.994 | Progesterone-induced blocking factor 1: immunomodulatory, increases TH2 response, NK cells |
| TAA | Log2TAA Mean (SE) | Range | Age in Years (SE) | Days to dx (SE) |
|---|---|---|---|---|
| ANA-positive breast cancer cases (N = 37) | ||||
| 64.9 (6.0) | 2484 (1396) | |||
| EBNA | 13.9 (1.7) | 10–17 | −0.34 | −0.27 |
| PGM3 | 8.5 (0.86) | 6.7–10 | −0.02 | −0.38 |
| TTN | 8.5 (1.4) | 6.0–13 | 0.09 | −0.15 |
| DUSP26 | 9.9 (0.88) | 7.3–12 | 0.13 | −0.30 |
| PRKD1 | 4.5 (2.3) | 0–7.7 | 0.21 | 0.19 |
| UCHL1 | 9.4 (1.2) | 6.2–13 | 0.17 | −0.01 |
| ACTA2 | 12 (0.5) | 11–13 | −0.09 | 0.04 |
| C5orf45 | 11 (0.7) | 9.9–15 | 0.30 | 0.14 |
| C13orf24 | 11 (0.41) | 10–12 | 0.24 | 0.03 |
| ANA-negative breast cancer cases (N = 36) | ||||
| 64.9 (5.7) | 2504 (1404) | |||
| EBNA | 14.1 (1.7) | 11–18 | −0.13 | −0.22 |
| PGM3 | 8.2 (0.7) | 6.8–10 | −0.11 | −0.09 |
| TTN | 8.0 (1.1) | 6.0–11 | −0.14 | 0.30 |
| DUSP26 | 9.6 (1.0) | 7.3–11 | 0.10 | −0.35 |
| PRKD1 | 4.4 (2.4) | 0–7.7 | −0.06 | 0.50 |
| UCHL1 | 9.4 (1.3) | 6.2–13 | −0.20 | 0.16 |
| ACTA2 | 12 (0.4) | 11–13 | −0.21 | −0.04 |
| C5orf45 | 11 (0.8) | 10–15 | −0.01 | 0.22 |
| C13orf24 | 11 (0.7) | 10–13 | 0.14 | −0.01 |
| ANA-positive controls (N = 35) | ||||
| 64.8 (6.0) | NA | |||
| EBNA | 13.9 (1.7) | 11–17 | −0.28 | --- |
| PGM3 | 8.6 (1.5) | 5.0–12 | 0.41 | --- |
| TTN | 8.1 (1.2) | 5.3–12 | −0.00 | --- |
| DUSP26 | 9.7 (1.1) | 7.4–11 | 0.01 | --- |
| PRKD1 | 4.5 (2.5) | 0–8.1 | 0.24 | --- |
| UCHL1 | 9.5 (2.4) | 0–15 | 0.38 | --- |
| ACTA2 | 12 (0.5) | 10–13 | 0.24 | --- |
| C5orf45 | 11 (0.6) | 9.7–12 | 0.24 | --- |
| C13orf24 | 11 (0.6) | 10–14 | 0.14 | |
| ANA-negative controls (N = 37) | ||||
| 64.9 (6.0) | NA | |||
| EBNA | 13.7 (1.8) | 11–16 | −0.04 | --- |
| PGM3 | 8.3 (1.7) | 5.0–12 | 0.00 | --- |
| TTN | 7.9 (1.3) | 5.3–12 | 0.25 | --- |
| DUSP26 | 9.7 (1.2) | 7.4–11 | −0.11 | --- |
| PRKD1 | 3.9 (2.6) | 0–7.2 | −0.02 | --- |
| UCHL1 | 8.9 (2.8) | 0–13 | 0.22 | --- |
| ACTA2 | 12 (0.6) | 10–13 | 0.05 | --- |
| C5orf45 | 11 (0.6) | 9.7–12 | 0.14 | --- |
| C13orf24 | 11 (0.5) | 9.7–12 | −0.09 | |
| Controls | Pre-Clinical Breast Cancer Cases | ||
|---|---|---|---|
| Antigens | No Breast Cancer Diagnosis N = 64 Beta (p-Value) | 7+ Years to Diagnosis N = 30 Beta (p-Value) | <7 Years to Diagnosis N = 35 Beta (p-Value) |
| PGM3 | 0.64 (0.09) | 0.42 (0.12) | 0.56 (0.09) |
| TTN | 0.44 (0.16) | 0.11 (0.84) | 1.63 (0.002) |
| DUSP26 | 0.20 (0.49) | 0.80 (0.07) | 0.37 (0.12) |
| PRKD1 | 1.36 (0.025) | −1.53 (0.044) | 0.85 (0.38) |
| UCHL1 | 1.25 (0.032) | −0.25 (0.58) | −0.02 (0.97) |
| ACTA2 | −0.13 (0.28) | −0.13 (0.48) | −0.26 (0.041) |
| C5orf45 | −0.18 (0.15) | −0.52 (0.14) | −0.30 (0.031) |
| C13orf24 | −0.01 (0.97) | −0.30 (0.14) | −0.32 (0.09) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parks, C.G.; Wilson, L.E.; Capello, M.; Deane, K.D.; Hanash, S.M. Tumor-Associated and Systemic Autoimmunity in Pre-Clinical Breast Cancer among Post-Menopausal Women. Biomolecules 2023, 13, 1566. https://doi.org/10.3390/biom13111566
Parks CG, Wilson LE, Capello M, Deane KD, Hanash SM. Tumor-Associated and Systemic Autoimmunity in Pre-Clinical Breast Cancer among Post-Menopausal Women. Biomolecules. 2023; 13(11):1566. https://doi.org/10.3390/biom13111566
Chicago/Turabian StyleParks, Christine G., Lauren E. Wilson, Michela Capello, Kevin D. Deane, and Samir M. Hanash. 2023. "Tumor-Associated and Systemic Autoimmunity in Pre-Clinical Breast Cancer among Post-Menopausal Women" Biomolecules 13, no. 11: 1566. https://doi.org/10.3390/biom13111566






