The Role of Intrinsically Disordered Proteins in Liquid–Liquid Phase Separation during Calcium Carbonate Biomineralization
Abstract
:1. Introduction
2. Role of Proteins and Divalent Ions in LLPS
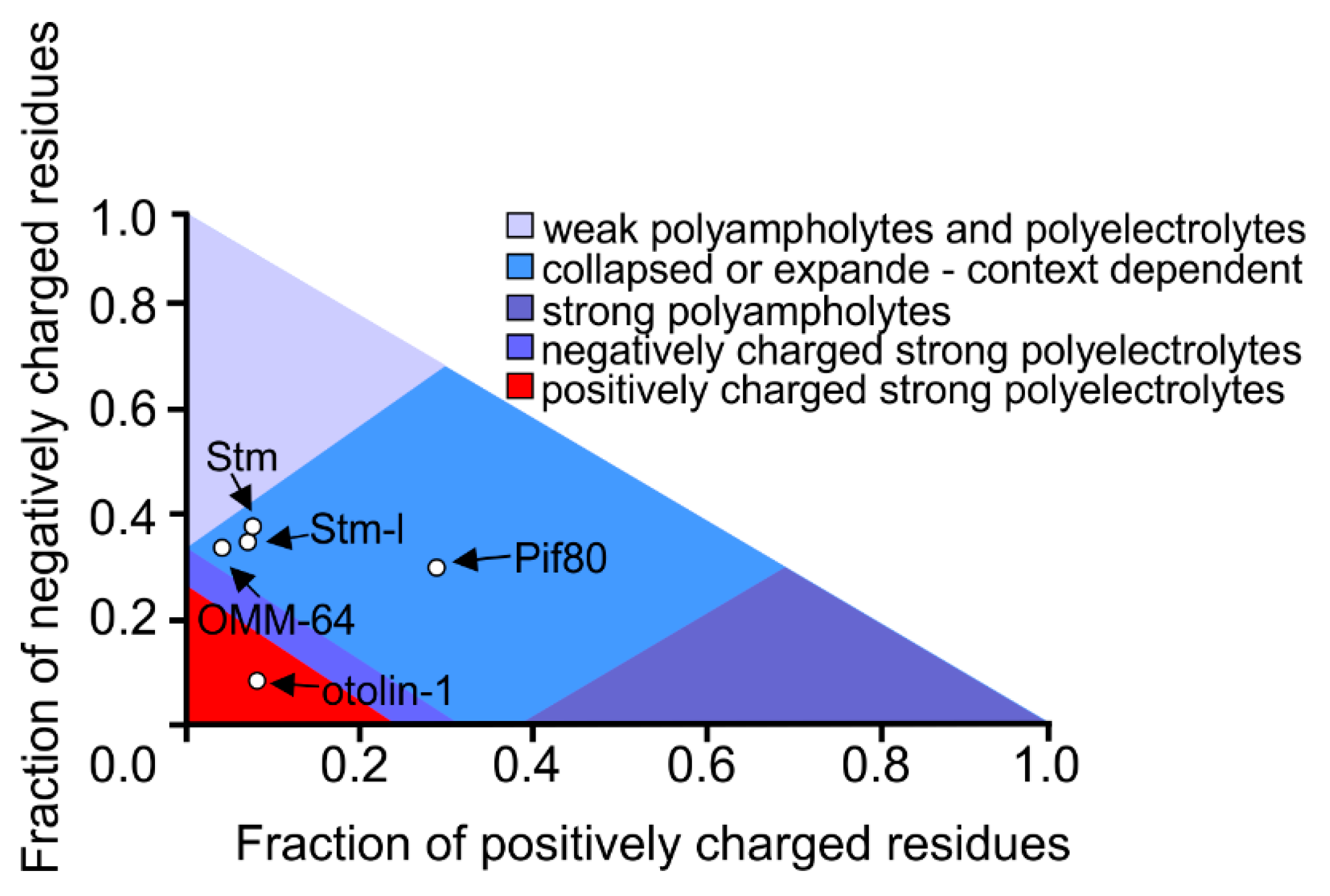
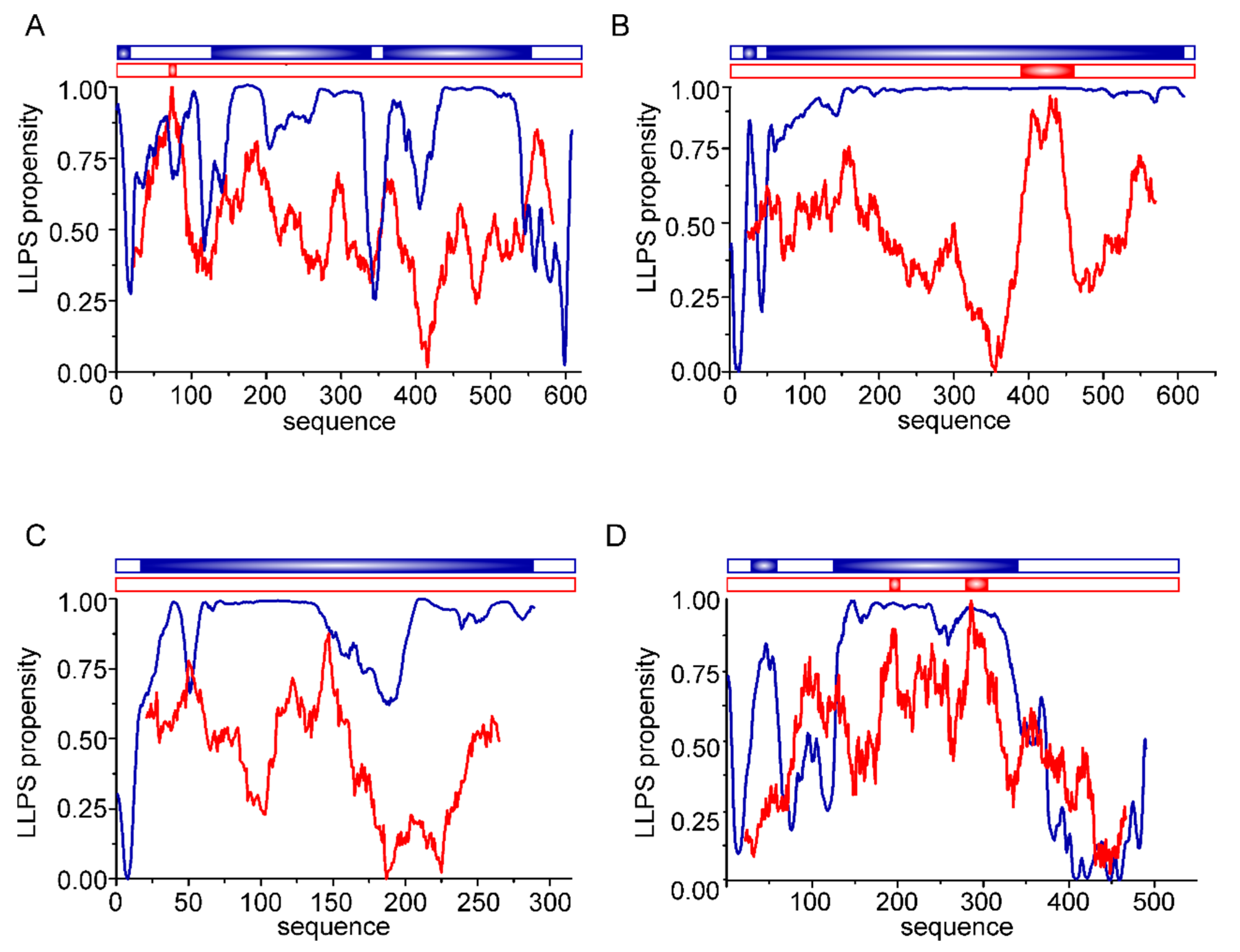
3. Intrinsically Disordered Proteins in Biomineralization
4. The Role of Proteins in Formation of Calcium Carbonate
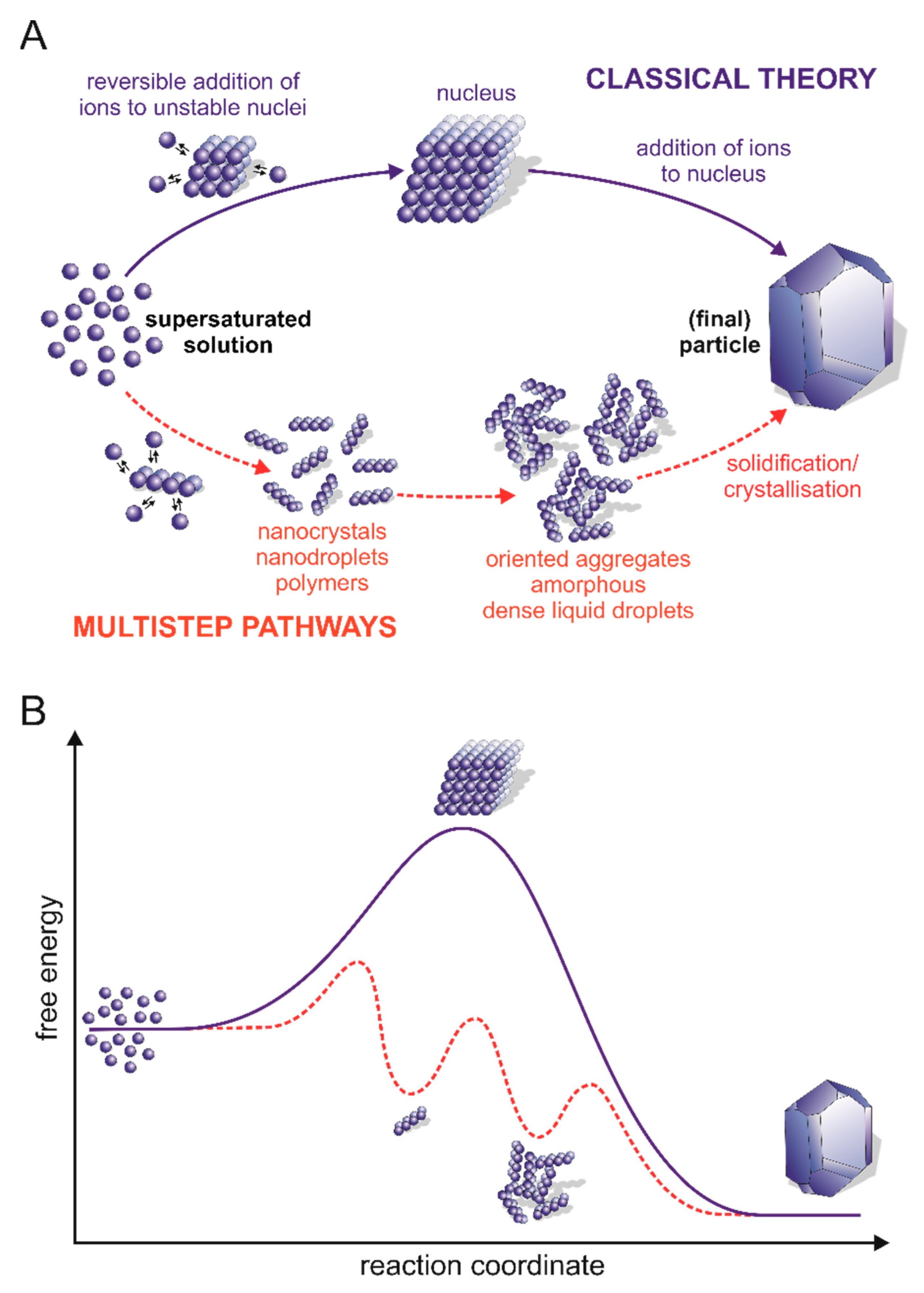
5. Calcium Carbonate Nucleation Process
6. Liquid–Liquid Phase Separation in the Formation of Hard Tissue
7. Can LLPS Impact the Formation of Otoliths?
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomes, E.; Shorter, J. The Molecular Language of Membraneless Organelles. J. Biol. Chem. 2019, 294, 7115–7127. [Google Scholar] [CrossRef] [PubMed]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid Phase Condensation in Cell Physiology and Disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Tompa, P.; Pappu, R.V. Polymer Physics of Intracellular Phase Transitions. Nat. Phys. 2015, 11, 899–904. [Google Scholar] [CrossRef]
- Posey, A.E.; Holehouse, A.S.; Pappu, R.V. Phase Separation of Intrinsically Disordered Proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2018; Volume 611, pp. 1–30. ISBN 9780128156490. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Ditlev, J.A.; Case, L.B.; Rosen, M.K. Who’s In and Who’s Out—Compositional Control of Biomolecular Condensates. J. Mol. Biol. 2018, 430, 4666–4684. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A.A. Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Azaldegui, C.A.; Vecchiarelli, A.G.; Biteen, J.S. The Emergence of Phase Separation as an Organizing Principle in Bacteria. Biophys. J. 2021, 120, 1123–1138. [Google Scholar] [CrossRef]
- Sołtys, K.; Tarczewska, A.; Bystranowska, D.; Sozańska, N. Getting Closer to Decrypting the Phase Transitions of Bacterial Biomolecules. Biomolecules 2022, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Brocca, S.; Grandori, R.; Longhi, S.; Uversky, V. Liquid-Liquid Phase Separation by Intrinsically Disordered Protein Regions of Viruses: Roles in Viral Life Cycle and Control of Virus-Host Interactions. Int. J. Mol. Sci. 2020, 21, 9045. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.P.; Sun, Y.C.; Qiu, D.C.; Lin, Y.H.; Chen, Y.Q.; Kuo, J.C.; Huang, J.R. Liquid-Liquid Phase Separation and Extracellular Multivalent Interactions in the Tale of Galectin-3. Nat. Commun. 2020, 11, 1229. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L. Biomineralization: An Overview. Connect. Tissue Res. 2003, 44 (Suppl. 1), 5–9. [Google Scholar] [CrossRef] [PubMed]
- Hołubowicz, R.; Porębska, A.; Poznar, M.; Różycka, M.; Dobryszycki, P. Biomineralization--Precision of Shape, Structure and Properties Controlled by Proteins. Postepy Biochem. 2015, 61, 364–380. [Google Scholar]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Quigley, D.; Gebauer, D. Stable Prenucleation Mineral Clusters Are Liquid-like Ionic Polymers. Nat. Commun. 2011, 2, 590. [Google Scholar] [CrossRef]
- Gebauer, D.; Cölfen, H. Prenucleation Clusters and Non-Classical Nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of Calcium Carbonate Films by a Polymer-Induced Liquid-Precursor (PILP) Process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Gebauer, D.; Volkel, A.; Colfen, H. Stable Prenucleation Calcium Carbonate Clusters. Science 2008, 332, 1819–1822. [Google Scholar] [CrossRef]
- Qin, D.; He, Z.; Li, P.; Zhang, S. Liquid-Liquid Phase Separation in Nucleation Process of Biomineralization. Front. Chem. 2022, 10, 834503. [Google Scholar] [CrossRef]
- Avaro, J.T.; Wolf, S.L.P.; Hauser, K.; Gebauer, D. Stable Prenucleation Calcium Carbonate Clusters Define Liquid–Liquid Phase Separation. Angew. Chem. Int. Ed. 2020, 59, 6155–6159. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Villarreal-Ramirez, E. Intrinsically Disordered Proteins and Biomineralization. Matrix Biol. 2016, 52–54, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Mitchison, T.J.; Hyman, A.A. Active Liquid-like Behavior of Nucleoli Determines Their Size and Shape in Xenopus Laevis Oocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 4334–4339. [Google Scholar] [CrossRef]
- Zaslavsky, B.Y.; Uversky, V.N. In Aqua Veritas: The Indispensable yet Mostly Ignored Role of Water in Phase Separation and Membrane-Less Organelles. Biochemistry 2018, 57, 2437–2451. [Google Scholar] [CrossRef]
- Alberti, S. Phase Separation in Biology. Curr. Biol. 2017, 27, 1097–1102. [Google Scholar] [CrossRef]
- Martin, E.W.; Mittag, T. Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions. Biochemistry 2018, 57, 2478–2487. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Kriwacki, R.W. Phase Separation in Biology; Functional Organization of a Higher Order. Cell Commun. Signal. 2016, 14, 1. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Dai, T.; Qin, Z.; Lu, H.; Zhang, L.; Zhou, F. Liquid–Liquid Phase Separation in Human Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 290. [Google Scholar] [CrossRef]
- Franzmann, T.M.; Alberti, S.; Morimoto, R.I.; Hartl, F.U.; Kelly, J.W. Protein Phase Separation as a Stress Survival Strategy. Cold Spring Harb. Perspect. Biol. 2019, 11, a034058. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically Disordered Proteins in Overcrowded Milieu: Membrane-Less Organelles, Phase Separation, and Intrinsic Disorder. Curr. Opin. Struct. Biol. 2017, 44, 18–30. [Google Scholar] [CrossRef]
- Chen, J.; Kriwacki, R.W. Intrinsically Disordered Proteins: Structure, Function and Therapeutics. J. Mol. Biol. 2018, 430, 2275–2277. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Emerson, I.A. Dynamic Conformational Flexibility and Molecular Interactions of Intrinsically Disordered Proteins. J. Biosci. 2020, 45, 29. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Dormann, D. Liquid–Liquid Phase Separation in Disease. Annu. Rev. Genet. 2019, 53, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S. The Dynamism of Intrinsically Disordered Proteins: Binding-Induced Folding, Amyloid Formation, and Phase Separation. J. Phys. Chem. B 2020, 124, 11541–11560. [Google Scholar] [CrossRef]
- Singh, V.; Xu, L.; Boyko, S.; Surewicz, K.; Surewicz, W.K. Zinc Promotes Liquid-Liquid Phase Separation of Tau Protein. J. Biol. Chem. 2020, 295, 5850–5856. [Google Scholar] [CrossRef]
- Vega, I.E.; Traverso, E.E.; Ferrer-Acosta, Y.; Matos, E.; Colon, M.; Gonzalez, J.; Dickson, D.; Hutton, M.; Lewis, J.; Yen, S.H. A Novel Calcium-Binding Protein Is Associated with Tau Proteins in Tauopathy. J. Neurochem. 2008, 106, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Acosta, Y.; Rodríguez Cruz, E.N.; Vaquer, A.d.C.; Vega, I.E. Functional and Structural Analysis of the Conserved EFhd2 Protein. Protein Pept. Lett. 2013, 20, 573–583. [Google Scholar] [CrossRef]
- Ferrer-Acosta, Y.; Rodríguez-Cruz, E.N.; Orange, F.; De Jesús-Cortés, H.; Madera, B.; Vaquer-Alicea, J.; Ballester, J.; Guinel, M.J.-F.; Bloom, G.S.; Vega, I.E. EFhd2 Is a Novel Amyloid Protein Associated with Pathological Tau in Alzheimer’s Disease. J. Neurochem. 2013, 125, 921–931. [Google Scholar] [CrossRef]
- Vega, I.E.; Umstead, A.; Kanaan, N.M. EFhd2 Affects Tau Liquid-Liquid Phase Separation. Front. Neurosci. 2019, 13, 845. [Google Scholar] [CrossRef]
- Więch, A.; Tarczewska, A.; Ożyhar, A.; Orłowski, M. Metal Ions Induce Liquid Condensate Formation by the F Domain of Aedes Aegypti Ecdysteroid Receptor. New Perspectives of Nuclear Receptor Studies. Cells 2021, 10, 571. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.-M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Dutagaci, B.; Nawrocki, G.; Goodluck, J.; Ashkarran, A.A.; Hoogstraten, C.G.; Lapidus, L.J.; Feig, M. Charge-Driven Condensation of RNA and Proteins Suggests Broad Role of Phase Separation in Cytoplasmic Environments. Elife 2021, 10, e64004. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.E.; Pollak, A.J.; Worby, C.A.; Xu, J.C.; Tandon, V.; Newton, A.C.; Dixon, J.E. Ca2+-Dependent Liquid-Liquid Phase Separation Underlies Intracellular Ca2+ Stores. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wojtas, M.; Dobryszycki, P.; Ożyhar, A. Intrinsically Disordered Proteins in Biomineralization, Advanced Topics in Biomineralization; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Press, A.I.N.; Marin, F.; Pokroy, B.; Luquet, G.; Layrolle, P.; De Groot, K. Protein Mapping of Calcium Carbonate Biominerals by Immunogold. Biomaterials 2007, 28, 2368–2377. [Google Scholar] [CrossRef]
- Gilbert, P.U.P.A.; Abrecht, M.; Frazer, B.H. The Organic-Mineral Interface in Biominerals. Rev. Mineral. Geochem. 2005, 59, 157–185. [Google Scholar] [CrossRef]
- Kalmar, L.; Homola, D.; Varga, G.; Tompa, P. Structural Disorder in Proteins Brings Order to Crystal Growth in Biomineralization. Bone 2012, 51, 528–534. [Google Scholar] [CrossRef]
- Thomas, O.R.B.B.; Swearer, S.E.; Kapp, E.A.; Peng, P.; Tonkin-Hill, G.Q.; Papenfuss, A.; Roberts, A.; Bernard, P.; Roberts, B.R. The Inner Ear Proteome of Fish. FEBS J. 2019, 286, 66–81. [Google Scholar] [CrossRef]
- Kalka, M.; Markiewicz, N.; Ptak, M.; Sone, E.D.; Ożyhar, A.; Dobryszycki, P.; Wojtas, M. In Vivo and in Vitro Analysis of Starmaker Activity in Zebrafish Otolith Biomineralization. FASEB J. 2019, 33, 6877–6886. [Google Scholar] [CrossRef]
- George, M. Rigid Biological Systems as Models for Synthetic Composites. Science 2005, 310, 1144–1147. [Google Scholar] [CrossRef]
- Poznar, M.; Stolarski, J.; Sikora, A.; Mazur, M.; Olesiak-Bańska, J.; Brach, K.; Ożyhar, A.; Dobryszycki, P. Fish Otolith Matrix Macromolecule-64 (OMM-64) and Its Role in Calcium Carbonate Biomineralization. Cryst. Growth Des. 2020, 20, 5808–5819. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Carloni, J.D.; Demarchi, B.; Sparks, D.; Reid, D.G.; Kunitake, M.E.; Tang, C.C.; Duer, M.J.; Freeman, C.L.; Pokroy, B.; et al. Tuning Hardness in Calcite by Incorporation of Amino Acids. Nat. Mater. 2016, 15, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Koutsoumpeli, E.; Johnson, S.; Evans, J.S. Glycosylation Fosters Interactions between Model Sea Urchin Spicule Matrix Proteins. Implications for Embryonic Spiculogenesis and Biomineralization. Biochemistry 2018, 57, 3032–3035. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S. Glycosylation: A “Last Word” in the Protein-Mediated Biomineralization Process. Crystals 2020, 10, 818. [Google Scholar] [CrossRef]
- Alvares, K. The Role of Acidic Phosphoproteins in Biomineralization. Connect. Tissue Res. 2014, 55, 34–40. [Google Scholar] [CrossRef]
- Wojtas, M.; Wo, M.; Andrzej, O.; Dobryszycki, P.; Wołcyrz, M.; Ożyhar, A.; Dobryszycki, P. Phosphorylation of Intrinsically Disordered Starmaker Protein Increases Its Ability To Control the Formation of Calcium Carbonate Crystals. Cryst. Growth Des. 2012, 12, 158–168. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wen, J.; Wiley, S.E.; Worby, C.A.; Kinch, L.N.; Xiao, J.; Grishin, N.V.; Dixon, J.E. Secreted Kinase Phosphorylates Extracellular Proteins That Regulate Biomineralization. Science 2012, 336, 1150–1153. [Google Scholar] [CrossRef]
- Du, J.; Liu, C.; Xu, G.; Xie, J.; Xie, L.; Zhang, R. Fam20C Participates in the Shell Formation in the Pearl Oyster, Pinctada Fucata. Sci. Rep. 2018, 8, 3563. [Google Scholar] [CrossRef]
- Chang, E.P.; Perovic, I.; Rao, A.; Cölfen, H.; Evans, J.S. Insect Cell Glycosylation and Its Impact on the Functionality of a Recombinant Intracrystalline Nacre Protein, AP24. Biochemistry 2016, 55, 1024–1035. [Google Scholar] [CrossRef]
- Jain, G.; Pendola, M.; Rao, A.; Cölfen, H.; Evans, J.S. A Model Sea Urchin Spicule Matrix Protein Self-Associates To Form Mineral-Modifying Protein Hydrogels. Biochemistry 2016, 55, 4410–4421. [Google Scholar] [CrossRef]
- Takeuchi, T.; Sarashina, I.; Iijima, M.; Endo, K. In Vitro Regulation of CaCO3 Crystal Polymorphism by the Highly Acidic Molluscan Shell Protein Aspein. FEBS Lett. 2008, 582, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, D.; Sarashina, I.; Endo, K. Structure and Expression of an Unusually Acidic Matrix Protein of Pearl Oyster Shells. Biochem. Biophys. Res. Commun. 2004, 320, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Isowa, Y.; Sarashina, I.; Setiamarga, D.H.; Endo, K. A Comparative Study of the Shell Matrix Protein Aspein in Pterioid Bivalves. J. Mol. Evol. 2012, 75, 11–18. [Google Scholar] [CrossRef]
- Inoue, H.; Ohira, T.; Nagasawa, H. Significance of the N- and C-Terminal Regions of CAP-1, a Cuticle Calcification-Associated Peptide from the Exoskeleton of the Crayfish, for Calcification. Peptides 2007, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Shimizu, K.; Oda, M.; Sakamoto, T.; Nishimura, T.; Kato, T. Biomineralization-Inspired Synthesis of Functional Organic/Inorganic Hybrid Materials: Organic Molecular Control of Self-Organization of Hybrids. Org. Biomol. Chem. 2015, 13, 974–989. [Google Scholar] [CrossRef]
- Mass, T.; Drake, J.L.; Haramaty, L.; Kim, J.D.; Zelzion, E.; Bhattacharya, D.; Falkowski, P.G. Cloning and Characterization of Four Novel Coral Acid-Rich Proteins That Precipitate Carbonates In Vitro. Curr. Biol. 2013, 23, 1126–1131. [Google Scholar] [CrossRef]
- Laipnik, R.; Bissi, V.; Sun, C.Y.; Falini, G.; Gilbert, P.U.P.A.; Mass, T. Coral Acid Rich Protein Selects Vaterite Polymorph in Vitro. J. Struct. Biol. 2020, 209, 107431. [Google Scholar] [CrossRef]
- Wilt, F.H. Biomineralization of the Spicules of Sea Urchin Embryos. Zoolog. Sci. 2002, 19, 253–261. [Google Scholar] [CrossRef]
- Illies, M.R.; Peeler, M.T.; Dechtiaruk, A.M.; Ettensohn, C.A. Identification and Developmental Expression of New Biomineralization Proteins in the Sea Urchin Strongylocentrotus Purpuratus. Dev. Genes Evol. 2002, 212, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.N.; Ramcharitar, J.; Campana, S.E. Why Otoliths? Insights from Inner Ear Physiology and Fisheries Biology. Mar. Freshw. Res. 2005, 56, 497–504. [Google Scholar] [CrossRef]
- Ross, M.D.; Pote, K.G. Some Properties of Otoconia. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, Y.W.; Xu, Y.; Thiessen, K.D.; Kramer, K.L. Mechanisms of Otoconia and Otolith Development. Dev. Dyn. 2015, 244, 239–253. [Google Scholar] [CrossRef]
- Thomas, O.R.B.; Swearer, S.E. Otolith Biochemistry—A Review. Rev. Fish. Sci. Aquac. 2019, 27, 458–489. [Google Scholar] [CrossRef]
- Payan, P.; De Pontual, H.; Bœuf, G.; Mayer-gostan, N. Endolymph Chemistry and Otolith Growth in Fish. Comptes Rendus Palevol 2004, 3, 535–547. [Google Scholar] [CrossRef]
- Walther, L.E.; Wenzel, A.; Buder, J.; Bloching, M.B.; Kniep, R.; Blödow, A. Detection of Human Utricular Otoconia Degeneration in Vital Specimen and Implications for Benign Paroxysmal Positional Vertigo. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.J.; Stevenson, A.K.; Yau, P.M.; Kollmar, R. Sparc Protein Is Required for Normal Growth of Zebrafish Otoliths. J. Assoc. Res. Otolaryngol. 2008, 9, 436–451. [Google Scholar] [CrossRef]
- Murayama, E.; Herbomel, P.; Kawakami, A.; Takeda, H.; Nagasawa, H. Otolith Matrix Proteins OMP-1 and Otolin-1 Are Necessary for Normal Otolith Growth and Their Correct Anchoring onto the Sensory Maculae. Mech. Dev. 2005, 122, 791–803. [Google Scholar] [CrossRef]
- Tohse, H.; Takagi, Y.; Nagasawa, H. Identification of a Novel Matrix Protein Contained in a Protein Aggregate Associated with Collagen in Fish Otoliths. FEBS J. 2008, 275, 2512–2523. [Google Scholar] [CrossRef]
- Poznar, M.; Hołubowicz, R.; Wojtas, M.; Gapiński, J.; Banachowicz, E.; Patkowski, A.; Ożyhar, A.; Dobryszycki, P. Structural Properties of the Intrinsically Disordered, Multiple Calcium Ion-Binding Otolith Matrix Macromolecule-64 (OMM-64). Biochim. Biophys. Acta-Proteins Proteom. 2017, 1865, 1358–1371. [Google Scholar] [CrossRef]
- Różycka, M.; Wojtas, M.; Jakób, M.; Stigloher, C.; Grzeszkowiak, M.; Mazur, M.; Ożyhar, A. Intrinsically Disordered and Pliable Starmaker-Like Protein from Medaka (Oryzias Latipes) Controls the Formation of Calcium Carbonate Crystals. PLoS ONE 2014, 9, e114308. [Google Scholar] [CrossRef] [Green Version]
- Petko, J.A.; Millimaki, B.B.; Canfield, V.A.; Riley, B.B.; Levenson, R. Otoc1: A Novel Otoconin-90 Ortholog Required for Otolith Mineralization in Zebrafish. Dev. Neurobiol. 2008, 68, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Sollner, C.; Burghammer, M.; Busch-Nentwich, E.; Berger, J.; Schwarz, H.; Riekel, C.; Nicolson, T.; Söllner, C.; Burghammer, M.; Busch-Nentwich, E.; et al. Control of Crystal Size and Lattice Formation by Starmaker in Otolith Biomineralization. Science 2003, 302, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Różycka, M.; Coronado, I.; Brach, K.; Olesiak-Bańska, J.; Samoć, M.; Zarębski, M.; Dobrucki, J.; Ptak, M.; Weber, E.; Polishchuk, I.; et al. Lattice Shrinkage by Incorporation of Recombinant Starmaker-Like Protein within Bioinspired Calcium Carbonate Crystals. Chemistry 2019, 25, 12740–12750. [Google Scholar] [CrossRef]
- Murayama, E.; Takagi, Y.; Ohira, T.; Davis, J.G.; Greene, M.I.; Nagasawa, H. Fish Otolith Contains a Unique Structural Protein, Otolin-1. Eur. J. Biochem. 2002, 269, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Murayama, E.; Takagi, Y.; Nagasawa, H. Immunohistochemical Localization of Two Otolith Matrix Proteins in the Otolith and Inner Ear of the Rainbow Trout, Oncorhynchus Mykiss: Comparative Aspects between the Adult Inner Ear and Embryonic Otocysts. Histochem. Cell Biol. 2004, 121, 155–166. [Google Scholar] [CrossRef]
- Andrade, L.R.; Lins, U.; Farina, M.; Kachar, B.; Thalmann, R. Immunogold TEM of Otoconin 90 and Otolin-Relevance to Mineralization of Otoconia, and Pathogenesis of Benign Positional Vertigo. Hear. Res. 2012, 292, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.G.; Oberholtzer, J.C.; Burns, F.R.; Greene, M.I. Molecular Cloning and Characterization of an Inner Ear-Specific Structural Protein. Science 1995, 267, 1031–1034. [Google Scholar] [CrossRef]
- Dobryszycki, P.; Hołubowicz, R.; Wojtas, M.; Taube, M.; Kozak, M.; Ożyhar, A.; Dobryszycki, P. Effect of Calcium Ions on Structure and Stability of the C1q-like Domain of Otolin-1 from Human and Zebrafish. FEBS J. 2017, 284, 4278–4297. [Google Scholar] [CrossRef]
- Bielak, K.; Hołubowicz, R.; Zoglowek, A.; Żak, A.; Kędzierski, P.; Ożyhar, A.; Dobryszycki, P. N′-Terminal- and Ca2+-Induced Stabilization of High-Order Oligomers of Full-Length Danio Rerio and Homo Sapiens Otolin-1. Int. J. Biol. Macromol. 2022, 209, 1032–1047. [Google Scholar] [CrossRef]
- Moreland, K.T.; Hong, M.; Lu, W.; Rowley, C.W.; Ornitz, D.M.; De Yoreo, J.J.; Thalmann, R. In Vitro Calcite Crystal Morphology Is Modulated by Otoconial Proteins Otolin-1 and Otoconin-90. PLoS ONE 2014, 9, e95333. [Google Scholar] [CrossRef] [Green Version]
- Silvent, J.; Sire, J.-Y.; Delgado, S. The Dentin Matrix Acidic Phosphoprotein 1 (DMP1) in the Light of Mammalian Evolution. J. Mol. Evol. 2013, 76, 59–70. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Yang, H.; Zhao, X.; Lovas, S.; Lundberg, Y.W. Expression, Functional, and Structural Analysis of Proteins Critical for Otoconia Development. Dev. Dyn. 2010, 239, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Brunn, J.C.; Cook, R.G.; Orkiszewski, R.S.; Malone, J.P.; Veis, A.; Butler, W.T. Evidence for the Proteolytic Processing of Dentin Matrix Protein 1: Identification and characterization of processed fragments and cleavage sites. J. Biol. Chem. 2003, 278, 34700–34708. [Google Scholar] [CrossRef] [PubMed]
- Porębska, A.; Różycka, M.; Hołubowicz, R.; Szewczuk, Z.; Ożyhar, A.; Dobryszycki, P. Functional Derivatives of Human Dentin Matrix Protein 1 Modulate Morphology of Calcium Carbonate Crystals. FASEB J. 2020, 34, 6147–6165. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Vekilov, P.G.; De Yoreo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef]
- Debenedetti, P.G. Metastable Liquids; Princeton University Press: Princeton, NJ, USA, 1996; Volume 1, ISBN 9780691085951. [Google Scholar]
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. Am. J. Sci. 1878, s3–16, 441–458. [Google Scholar] [CrossRef]
- Becker, R.; Döring, W. Kinetische Behandlung Der Keimbildung in Übersättigten Dämpfen. Ann. Phys. 1935, 416, 719–752. [Google Scholar] [CrossRef]
- Farkas, L. Keimbildungsgeschwindigkeit in Übersättigten Dämpfen. Z. Phys. Chem. 1927, 125U, 236–242. [Google Scholar] [CrossRef]
- Volmer, M.; Weber, A. Keimbildung in Übersättigten Gebilden. Z. Phys. Chem. 1926, 119U, 277–301. [Google Scholar] [CrossRef]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in Solutions Revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Gebauer, D.; Raiteri, P.; Gale, J.D.; Cölfen, H. On classical and non-classical views on nucleation. Am. J. Sci. 2018, 318, 969–988. [Google Scholar] [CrossRef]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Glenn, W.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic Evidence for Liquid-Liquid Separation in Supersaturated CaCO3 Solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Koishi, A. Carbonate Mineral Nucleation Pathways; Université Grenoble Alpes: Grenoble, France, 2017. [Google Scholar]
- Evans, J.S. “Liquid-like” Biomineralization Protein Assemblies: A Key to the Regulation of Non-Classical Nucleation. CrystEngComm 2013, 15, 8388–8394. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Sear, R.P. Materials Science. Now You See Them. Science 2008, 322, 1802–1803. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D. How Can Additives Control the Early Stages of Mineralisation? Minerals 2018, 8, 179. [Google Scholar] [CrossRef]
- Bewernitz, M.A.; Gebauer, D.; Long, J.; Cölfen, H.; Gower, L.B. A Metastable Liquid Precursor Phase of Calcium Carbonate and Its Interactions with Polyaspartate. Faraday Discuss. 2012, 159, 291. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Wolf, S.L.P.; Caballero, L.; Melo, F.; Cölfen, H. Gel-Like Calcium Carbonate Precursors Observed by in Situ AFM. Langmuir 2017, 33, 158–163. [Google Scholar] [CrossRef]
- Pouget, E.M.; Bomans, P.H.; Goos, J.A.; Frederik, P.M.; de With, G.; Sommerdijk, N.A. The Initial Stages of Template-Controlled CaCO3 Formation Revealed by Cryo-TEM. Science 2009, 323, 1455–1458. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Weiner, S.; Mahamid, J.; Politi, Y.; Ma, Y.; Addadi, L. Overview of the Amorphous Precursor Phase Strategy in Biomineralization. Front. Mater. Sci. China 2009, 3, 104–108. [Google Scholar] [CrossRef]
- Radha, A.V.; Forbes, T.Z.; Killian, C.E.; Gilbert, P.U.; Navrotsky, A. Transformation and Crystallization Energetics of Synthetic and Biogenic Amorphous Calcium Carbonate. Proc. Natl. Acad. Sci. USA 2010, 107, 16438–16443. [Google Scholar] [CrossRef] [PubMed]
- Alexandra, N. Energetic Clues to Pathways to Biomineralization: Precursors, Clusters, and Nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101. [Google Scholar] [CrossRef]
- Quigley, D.; Freeman, C.L.; Harding, J.H.; Rodger, P.M. Sampling the Structure of Calcium Carbonate Nanoparticles with Metadynamics. J. Chem. Phys. 2011, 134, 44703. [Google Scholar] [CrossRef]
- Tribello, G.A.; Bruneval, F.; Liew, C.; Parrinello, M. A Molecular Dynamics Study of the Early Stages of Calcium Carbonate Growth. J. Phys. Chem. B 2009, 113, 11680–11687. [Google Scholar] [CrossRef]
- Wang, J.; Hou, T. Application of Molecular Dynamics Simulations in Molecular Property Prediction II: Diffusion Coefficient. J. Comput. Chem. 2011, 32, 3505–3519. [Google Scholar] [CrossRef]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-Nucleation Clusters as Solute Precursors in Crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef]
- Faatz, M.; Gröhn, F.; Wegner, G. Amorphous Calcium Carbonate: Synthesis and Potential Intermediate in Biomineralization. Adv. Mater. 2004, 16, 996–1000. [Google Scholar] [CrossRef]
- Wolf, S.E.; Leiterer, J.; Kappl, M.; Emmerling, F.; Tremel, W. Early Homogenous Amorphous Precursor Stages of Calcium Carbonate and Subsequent Crystal Growth in Levitated Droplets. J. Am. Chem. Soc. 2008, 130, 12342–12347. [Google Scholar] [CrossRef]
- Wang, X.; Chou, I.M.; Hu, W.; Burruss, R.C. In Situ Observations of Liquid-Liquid Phase Separation in Aqueous MgSO4 Solutions: Geological and Geochemical Implications. Geochim. Cosmochim. Acta 2013, 103, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Wan, Y.; Hu, W.; Chou, I.M.; Cao, J.; Wang, X.; Wang, M.; Li, Z. In Situ Observations of Liquid–Liquid Phase Separation in Aqueous ZnSO4 Solutions at Temperatures up to 400 °C: Implications for Zn2+–SO42− Association and Evolution of Submarine Hydrothermal Fluids. Geochim. Cosmochim. Acta 2016, 181, 126–143. [Google Scholar] [CrossRef]
- Suzuki, M.; Saruwatari, K.; Kogure, T.; Yamamoto, Y.; Nishimura, T.; Kato, T.; Nagasawa, H. An Acidic Matrix Protein, Pif, Is a Key Macromolecule for Nacre Formation. Science 2009, 325, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S. Aragonite-Associated Biomineralization Proteins Are Disordered and Contain Interactive Motifs. Bioinformatics 2012, 28, 3182–3185. [Google Scholar] [CrossRef] [PubMed]
- Bahn, S.Y.; Jo, B.H.; Choi, Y.S.; Cha, H.J. Control of Nacre Biomineralization by Pif80 in Pearl Oyster. Sci. Adv. 2017, 3, e1700765. [Google Scholar] [CrossRef]
- Farahi, N.; Lazar, T.; Wodak, S.J.; Tompa, P.; Pancsa, R. Integration of Data from Liquid-Liquid Phase Separation Databases Highlights Concentration and Dosage Sensitivity of LLPS Drivers. Int. J. Mol. Sci. 2021, 22, 3017. [Google Scholar] [CrossRef]
- Lin, Y.; Protter, D.S.W.; Rosen, M.K.; Parker, R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 2015, 60, 208–219. [Google Scholar] [CrossRef]
- Belcher, A.M.; Wu, X.H.; Christensen, R.J.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 1996, 381, 56–58. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Unusually Acidic Proteins in Biomineralization. In Handbook of Biomineralization; Bäuerlein, E., Behrens, P., Epple, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; Volume 1, pp. 273–290. [Google Scholar] [CrossRef]
- Mummadisetti, M.P.; Drake, J.L.; Falkowski, P.G. The Spatial Network of Skeletal Proteins in a Stony Coral. J. R. Soc. Interface 2021, 18, 20200859. [Google Scholar] [CrossRef]
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [Green Version]
- Drickamer, K.; Taylor, M.E. Evolving Views of Protein Glycosylation. Trends Biochem. Sci. 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Holehouse, A.S.; Das, R.K.; Ahad, J.N.; Richardson, M.O.G.; Pappu, R.V. CIDER: Resources to Analyze Sequence-Ensemble Relationships of Intrinsically Disordered Proteins. Biophys. J. 2017, 112, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Pancsa, R.; Vranken, W.; Mészáros, B. Computational Resources for Identifying and Describing Proteins Driving Liquid–Liquid Phase Separation. Brief. Bioinform. 2021, 22, bbaa408. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, M.; Fuxreiter, M. Sequence Determinants of the Aggregation of Proteins Within Condensates Generated by Liquid-Liquid Phase Separation. J. Mol. Biol. 2022, 434, 167201. [Google Scholar] [CrossRef]
- Hardenberg, M.; Horvath, A.; Ambrus, V.; Fuxreiter, M.; Vendruscolo, M. Widespread Occurrence of the Droplet State of Proteins in the Human Proteome. Proc. Natl. Acad. Sci. USA 2020, 117, 33254–33262. [Google Scholar] [CrossRef]
- Hatos, A.; Tosatto, S.C.E.; Vendruscolo, M.; Fuxreiter, M. FuzDrop on AlphaFold: Visualizing the Sequence-Dependent Propensity of Liquid–Liquid Phase Separation and Aggregation of Proteins. Nucleic Acids Res. 2022, 50, 337–344. [Google Scholar] [CrossRef]
- Vernon, R.M.C.; Chong, P.A.; Tsang, B.; Kim, T.H.; Bah, A.; Farber, P.; Lin, H.; Forman-Kay, J.D. Pi-Pi Contacts Are an Overlooked Protein Feature Relevant to Phase Separation. Elife 2018, 7, e31486. [Google Scholar] [CrossRef]
- Kapłon, T.M.; Michnik, A.; Drzazga, Z.; Richter, K.; Kochman, M.; Ozyhar, A. The Rod-Shaped Conformation of Starmaker. Biochim. Biophys. Acta 2009, 1794, 1616–1624. [Google Scholar] [CrossRef]
- Horvath, A.; Miskei, M.; Ambrus, V.; Vendruscolo, M.; Fuxreiter, M. Sequence-Based Prediction of Protein Binding Mode Landscapes. PLoS Comput. Biol. 2020, 16, e1007864. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-Inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Redden, G.D.; Ingram, J.C.; Cortez, M.M.; Ferris, F.G.; Smith, R.W. Strontium Incorporation into Calcite Generated by Bacterial Ureolysis. Geochim. Cosmochim. Acta 2004, 68, 3261–3270. [Google Scholar] [CrossRef]
- Clark, M.S. Molecular Mechanisms of Biomineralization in Marine Invertebrates. J. Exp. Biol. 2020, 223, jeb206961. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarczewska, A.; Bielak, K.; Zoglowek, A.; Sołtys, K.; Dobryszycki, P.; Ożyhar, A.; Różycka, M. The Role of Intrinsically Disordered Proteins in Liquid–Liquid Phase Separation during Calcium Carbonate Biomineralization. Biomolecules 2022, 12, 1266. https://doi.org/10.3390/biom12091266
Tarczewska A, Bielak K, Zoglowek A, Sołtys K, Dobryszycki P, Ożyhar A, Różycka M. The Role of Intrinsically Disordered Proteins in Liquid–Liquid Phase Separation during Calcium Carbonate Biomineralization. Biomolecules. 2022; 12(9):1266. https://doi.org/10.3390/biom12091266
Chicago/Turabian StyleTarczewska, Aneta, Klaudia Bielak, Anna Zoglowek, Katarzyna Sołtys, Piotr Dobryszycki, Andrzej Ożyhar, and Mirosława Różycka. 2022. "The Role of Intrinsically Disordered Proteins in Liquid–Liquid Phase Separation during Calcium Carbonate Biomineralization" Biomolecules 12, no. 9: 1266. https://doi.org/10.3390/biom12091266








