Prognostic Signature of Chronic Kidney Disease in Advanced Age: Secondary Analysis from the InGAH Study with One-Year Follow-Up
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients and Methods
2.2. Data for Present Secondary Analysis
2.3. Statistical Analysis
3. Results
3.1. CKD, Frailty and Long-Term Prognosis According to KDIGO
3.2. RRT Group: HD vs. PD
3.3. KTR vs. RRT
3.4. CKD KDIGO G4-5 Patients: No-RRT vs. HD-RRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A.; Mu, W.; Price, K.L.; Johnson, R.J. Blood vessels and the aging kidney. Nephron Exp. Nephrol. 2005, 101, e95–e99. [Google Scholar] [CrossRef] [PubMed]
- Braun, F.; Brinkkötter, P.T. Rückgang der Nierenfunktion im Alter. Z. Gerontol. Geriatr. 2016, 49, 469–476. [Google Scholar] [CrossRef] [PubMed]
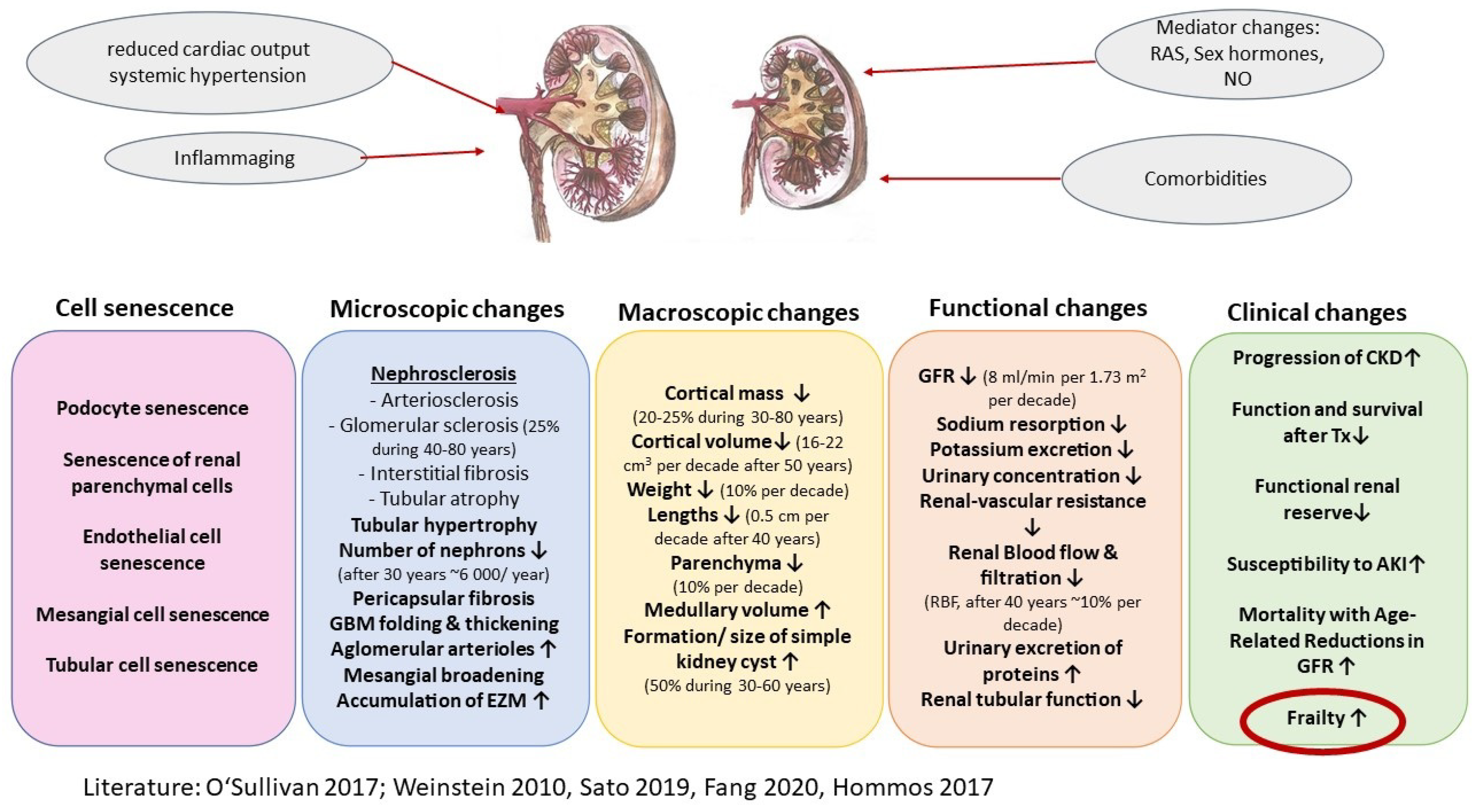
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Benzing, T.; Salant, D. Insights into Glomerular Filtration and Albuminuria. N. Engl. J. Med. 2021, 384, 1437–1446. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2018, 71, A7. [Google Scholar] [CrossRef]
- St Peter, W.L.; Khan, S.S.; Ebben, J.P.; Pereira, B.J.; Collins, A.J. Chronic kidney disease: The distribution of health care dollars. Kidney Int. 2004, 66, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Hallan, S.I.; Matsushita, K.; Sang, Y.; Mahmoodi, B.K.; Black, C.; Ishani, A.; Kleefstra, N.; Naimark, D.; Roderick, P.; Tonelli, M.; et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 2012, 308, 2349–2360. [Google Scholar] [CrossRef]
- Manjunath, G.; Tighiouart, H.; Coresh, J.; Macleod, B.; Salem, D.N.; Griffith, J.L.; Levey, A.S.; Sarnak, M.J. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003, 63, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Shlipak, M.G.; Sarnak, M.J.; Katz, R.; Fried, L.F.; Seliger, S.L.; Newman, A.B.; Siscovick, D.S.; Stehman-Breen, C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005, 352, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Bowling, C.B.; Gao, L.; Rizk, D.; Judd, S.; Tanner, R.M.; McClellan, W.; Warnock, D.G. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin. J. Am. Soc. Nephrol. 2011, 6, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Sancarlo, D.; Franceschi, M.; Aucella, F.; D’Ambrosio, P.; Scarcelli, C.; Ferrucci, L. A multidimensional approach to the geriatric patient with chronic kidney disease. J. Nephrol. 2010, 23 (Suppl. 15), S5–S10. [Google Scholar] [PubMed]
- Pilotto, A.; Panza, F.; Sancarlo, D.; Paroni, G.; Maggi, S.; Ferrucci, L. Usefulness of the multidimensional prognostic index (MPI) in the management of older patients with chronic kidney disease. J. Nephrol. 2012, 25 (Suppl. 19), S79–S84. [Google Scholar] [CrossRef]
- Chowdhury, R.; Peel, N.M.; Krosch, M.; Hubbard, R.E. Frailty and chronic kidney disease: A systematic review. Arch. Gerontol. Geriatr. 2017, 68, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic. Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Yanagita, M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 2019, 15, 625–640. [Google Scholar] [CrossRef]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef] [Green Version]
- Mani, M.K. Prevention of chronic renal failure at the community level. Kidney Int. Suppl. 2003, 63, S86–S89. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, K.; Nangaku, M.; Saito, A.; Inagi, R.; Miyata, T. Current issues and future perspectives of chronic renal failure. J. Am. Soc. Nephrol. 2002, 13 (Suppl. 1), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Hemmelgarn, B.R.; Tonelli, M. Early recognition and prevention of chronic kidney disease. Lancet 2010, 375, 1296–1309. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Wyld, M.; Morton, R.L.; Hayen, A.; Howard, K.; Webster, A.C. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012, 9, e1001307. [Google Scholar] [CrossRef] [PubMed]
- Gwyther, H.; Bobrowicz-Campos, E.; Luis Alves Apostolo, J.; Marcucci, M.; Cano, A.; Holland, C. A realist review to understand the efficacy and outcomes of interventions designed to minimise, reverse or prevent the progression of frailty. Health Psychol. Rev. 2018, 12, 382–404. [Google Scholar] [CrossRef]
- Cook, W.L. The intersection of geriatrics and chronic kidney disease: Frailty and disability among older adults with kidney disease. Adv. Chronic. Kidney Dis. 2009, 16, 420–429. [Google Scholar] [CrossRef]
- Meyer, A.M.; Becker, I.; Siri, G.; Brinkkotter, P.T.; Benzing, T.; Pilotto, A.; Polidori, M.C. The prognostic significance of geriatric syndromes and resources. Aging Clin. Exp. Res. 2020, 32, 115–124. [Google Scholar] [CrossRef]
- Veronese, N.; Custodero, C.; Cella, A.; Demurtas, J.; Zora, S.; Maggi, S.; Barbagallo, M.; Sabba, C.; Ferrucci, L.; Pilotto, A. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 72, 101498. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Pilotto, A.; Ferrucci, L.; Franceschi, M.; D’Ambrosio, L.P.; Scarcelli, C.; Cascavilla, L.; Paris, F.; Placentino, G.; Seripa, D.; Dallapiccola, B.; et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008, 11, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.M.; Becker, I.; Siri, G.; Brinkkotter, P.T.; Benzing, T.; Pilotto, A.; Polidori, M.C. New associations of the Multidimensional Prognostic Index. Z. Gerontol. Geriatr. 2019, 52, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Pickert, L.; Meyer, A.M.; Becker, I.; Heess, A.; Noetzel, N.; Brinkkotter, P.; Pilotto, A.; Benzing, T.; Polidori, M.C. Role of a multidimensional prognosis in-hospital monitoring for older patients with prolonged stay. Int. J. Clin. Pract. 2021, 75, e13989. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health 2019, 29, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Downs, T.D.; Cash, H.R.; Grotz, R.C. Progress in development of the index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Sancarlo, D.; D’Onofrio, G.; Franceschi, M.; Scarcelli, C.; Niro, V.; Addante, F.; Copetti, M.; Ferrucci, L.; Fontana, L.; Pilotto, A. Validation of a Modified-Multidimensional Prognostic Index (m-MPI) including the Mini Nutritional Assessment Short-Form (MNA-SF) for the prediction of one-year mortality in hospitalized elderly patients. J. Nutr. Health Aging 2011, 15, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Bliss, M.R.; McLaren, R.; Exton-Smith, A.N. Mattresses for preventing pressure sores in geriatric patients. Mon. Bull. Minist. Health Public Health Lab. Serv. 1966, 25, 238–268. [Google Scholar]
- Pilotto, A.; Cella, A.; Pilotto, A.; Daragjati, J.; Veronese, N.; Musacchio, C.; Mello, A.M.; Logroscino, G.; Padovani, A.; Prete, C.; et al. Three Decades of Comprehensive Geriatric Assessment: Evidence Coming From Different Healthcare Settings and Specific Clinical Conditions. J. Am. Med. Dir. Assoc. 2017, 18, 192.e1–192.e11. [Google Scholar] [CrossRef] [PubMed]
- Baake, C.P. Begutachtungsverfahren NBA—Pflegegrad bei Erwachsenen: So Funktionieren Feststellungsverfahren und Einstufung nach Dem Neuen Recht; Mit Praxisbeispielen zur Berechnung, 1st ed.; Walhalla: Regensburg, Germany, 2017. [Google Scholar]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilotto, A.; Sancarlo, D.; Aucella, F.; Fontana, A.; Addante, F.; Copetti, M.; Panza, F.; Strippoli, G.F.; Ferrucci, L. Addition of the multidimensional prognostic index to the estimated glomerular filtration rate improves prediction of long-term all-cause mortality in older patients with chronic kidney disease. Rejuvenation Res. 2012, 15, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, A.; Paroni, G.; Seripa, D.; Addante, F.; Dagostino, M.P.; Aucella, F. Frailty, disability and physical exercise in the aging process and in chronic kidney disease. Kidney Blood Press Res. 2014, 39, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Nixon, A.C.; Bampouras, T.M.; Pendleton, N.; Woywodt, A.; Mitra, S.; Dhaygude, A. Frailty and chronic kidney disease: Current evidence and continuing uncertainties. Clin. Kidney J. 2018, 11, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Schafer, M.; Korber, M.I.; Vimalathasan, R.; Mauri, V.; Iliadis, C.; Metze, C.; Ten Freyhaus, H.; Baldus, S.; Polidori, M.C.; Pfister, R. Risk Stratification of Patients Undergoing Percutaneous Repair of Mitral and Tricuspid Valves Using a Multidimensional Geriatric Assessment. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007624. [Google Scholar] [CrossRef]
- Chetrit, J.; Mendis, N.; Afilalo, J. Frailty: As Simple as Possible, but No Simpler. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e008227. [Google Scholar] [CrossRef]
- Tangri, N.; Kitsios, G.D.; Inker, L.A.; Griffith, J.; Naimark, D.M.; Walker, S.; Rigatto, C.; Uhlig, K.; Kent, D.M.; Levey, A.S. Risk prediction models for patients with chronic kidney disease: A systematic review. Ann. Intern. Med. 2013, 158, 596–603. [Google Scholar] [CrossRef]
- Gale, C.R.; Booth, T.; Starr, J.M.; Deary, I.J. Intelligence and socioeconomic position in childhood in relation to frailty and cumulative allostatic load in later life: The Lothian Birth Cohort 1936. J. Epidemiol. Community Health 2016, 70, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Veronese, N.; Siri, G.; Cella, A.; Daragjati, J.; Cruz-Jentoft, A.J.; Polidori, M.C.; Mattace-Raso, F.; Paccalin, M.; Topinkova, E.; Greco, A.; et al. Older women are frailer, but less often die then men: A prospective study of older hospitalized people. Maturitas 2019, 128, 81–86. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Jassal, S.V. The concept of frailty in geriatric chronic kidney disease (CKD) patients. Blood Purif. 2015, 39, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Inker, L.A.; Coresh, J. Chronic Kidney Disease in Older People. JAMA 2015, 314, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Gaillard, F.; van der Weijden, J.; Mjoen, G.; Ferhman-Ekholm, I.; Dubourg, L.; Ebert, N.; Schaeffner, E.; Akerfeldt, T.; Goffin, K.; et al. Age-adapted percentiles of measured glomerular filtration in healthy individuals: Extrapolation to living kidney donors over 65 years. Clin. Chem. Lab. Med. 2022, 60, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Jager, K.J.; Bokenkamp, A.; Christensson, A.; Dubourg, L.; Eriksen, B.O.; Gaillard, F.; Gambaro, G.; van der Giet, M.; Glassock, R.J.; et al. CKD: A Call for an Age-Adapted Definition. J. Am. Soc. Nephrol. 2019, 30, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Addante, F.; Copetti, M.; Paroni, G.; Fontana, A.; Sancarlo, D.; Pellegrini, F.; Ferrucci, L.; Pilotto, A. Identification of a metabolic signature for multidimensional impairment and mortality risk in hospitalized older patients. Aging Cell 2013, 12, 459–466. [Google Scholar] [CrossRef]
- Rohrig, G.; Becker, I.; Polidori, M.C.; Schulz, R.J.; Noreik, M. Association of anemia and hypoalbuminemia in German geriatric inpatients: Relationship to nutritional status and comprehensive geriatric assessment. Z. Gerontol. Geriatr. 2015, 48, 619–624. [Google Scholar] [CrossRef]
- Cabrerizo, S.; Cuadras, D.; Gomez-Busto, F.; Artaza-Artabe, I.; Marin-Ciancas, F.; Malafarina, V. Serum albumin and health in older people: Review and meta analysis. Maturitas 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Hong, X.; Yan, J.; Xu, L.; Shen, S.; Zeng, X.; Chen, L. Relationship between nutritional status and frailty in hospitalized older patients. Clin. Interv. Aging 2019, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Alp Ikizler, T.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.Y.; Chao, C.T.; Chan, D.C.; Huang, J.W.; Hung, K.Y. Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: A scoping review. Ther. Adv. Chronic Dis. 2019, 10, 2040622319880382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, S.R.; Wagner, M.; Tangri, N. Chronic kidney disease, frailty, and unsuccessful aging: A review. J. Ren. Nutr. 2014, 24, 364–370. [Google Scholar] [CrossRef]
- Lin, T.Y.; Hsu, Y.H. IL-20 in Acute Kidney Injury: Role in Pathogenesis and Potential as a Therapeutic Target. Int. J. Mol. Sci. 2020, 21, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Lai, S.; Molfino, A.; Coppola, B.; De Leo, S.; Tommasi, V.; Galani, A.; Migliaccio, S.; Greco, E.A.; Gnerre Musto, T.; Muscaritoli, M. Effect of personalized dietary intervention on nutritional, metabolic and vascular indices in patients with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3351–3359. [Google Scholar] [PubMed]
- Kim, J.S.; Wilson, J.M.; Lee, S.R. Dietary implications on mechanisms of sarcopenia: Roles of protein, amino acids and antioxidants. J. Nutr. Biochem. 2010, 21, 1–13. [Google Scholar] [CrossRef]
- Jay, C.L.; Washburn, K.; Dean, P.G.; Helmick, R.A.; Pugh, J.A.; Stegall, M.D. Survival Benefit in Older Patients Associated with Earlier Transplant with High KDPI Kidneys. Transplantation 2017, 101, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef]
- Rabbat, C.G.; Thorpe, K.E.; Russell, J.D.; Churchill, D.N. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J. Am. Soc. Nephrol. 2000, 11, 917–922. [Google Scholar] [CrossRef]
- Port, F.K.; Wolfe, R.A.; Mauger, E.A.; Berling, D.P.; Jiang, K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993, 270, 1339–1343. [Google Scholar] [CrossRef]
- McAdams-DeMarco, M.A.; Isaacs, K.; Darko, L.; Salter, M.L.; Gupta, N.; King, E.A.; Walston, J.; Segev, D.L. Changes in Frailty After Kidney Transplantation. J. Am. Geriatr. Soc. 2015, 63, 2152–2157. [Google Scholar] [CrossRef] [Green Version]
- Novais, T.; Pongan, E.; Gervais, F.; Coste, M.H.; Morelon, E.; Krolak-Salmon, P.; Vernaudon, J. Pretransplant Comprehensive Geriatric Assessment in Older Patients with Advanced Chronic Kidney Disease. Nephron 2021, 145, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Kurschat, C. Kidney transplantation in old age. Z. Gerontol. Geriatr. 2016, 49, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Von Gersdorff, G.; Von Korn, P.; Duvinage, A.; Ihorst, G.; Josef, A.; Kaufmann, M.; Baer, T.; Fellerhoff, T.; Fuhrmann, I.; Koesel, E.; et al. Cluster Randomized Controlled Trial on the Effects of 12 Months of Combined Exercise Training during Hemodialysis in Patients with Chronic Kidney Disease—Study Protocol of the Dialysis Training Therapy (DiaTT) Trial. Methods Protoc. 2021, 4, 60. [Google Scholar] [CrossRef] [PubMed]




| Total n = 375 | MPI-1 n = 86 (23%) | MPI-2 n = 210 (56%) | MPI-3 n = 79 (21%) | p-Value ° | ||
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age (years), mean (SD) | 77.5 (6.1) | 75.4 (5.5) | 77.7 (5.7) | 79.6 (7.0) | <0.001 | |
| Female, n (%) | 133 (36) | 21 (24) | 81 (39) | 31 (39) | 0.091 | |
| LHS, median (Q1-3) | 11 (6–19) | 8 (5–15) | 10 (6–18) | 17 (10–26) | 0.002 | |
| Education (years), median (Q1-3) | 11 (10–14) | 12 (11–15) | 11 (10–14) | 11 (9–12) | 0.006 | |
| Admission status, n (%) | New admission to hospital | 174 (47) | 58 (69) | 67 (32) | 42 (53) | <0.001 |
| Transferred from internal ward | 122 (33) | 13 (16) | 67 (32) | 42 (53) | ||
| Transferred from external ward | 74 (20) | 13 (16) | 44 (21) | 17 (22) | ||
| Hospitalisation last 12 months, n (%) | 267 (71) | 55 (64) | 152 (72) | 60 (76) | 0.153 | |
| Falls last 12 months, n (%) | 155 (41) | 21 (24) | 96 (46) | 38 (48) | 0.009 | |
| Comorbidities, n (%) | ||||||
| Hypertension | 263 (70) | 57 (66) | 145 (69) | 61 (77) | 0.188 | |
| Heart disease | 270 (72) | 48 (56) | 165 (79) | 57 (72) | <0.001 | |
| Cardiac arrhythmia | 229 (61) | 41 (48) | 131 (62) | 57 (72) | 0.036 | |
| Diabetes mellitus | 182 (49) | 37 (43) | 108 (51) | 37 (47) | 0.269 | |
| Chronic obstructive pulmonal disease | 61 (16) | 10 (12) | 36 (17) | 15 (19) | 0.341 | |
| Dementia | 14 (4) | 1 (1) | 5 (2) | 8 (10) | 0.033 | |
| Depression | 29 (8) | 5 (6) | 12 (6) | 12 (15) | 0.048 | |
| Peripheral artery disease | 81 (22) | 10 (12) | 51 (24) | 20 (25) | 0.012 | |
| Eye disease | 65 (17) | 7 (8) | 42 (20) | 16 (20) | 0.031 | |
| Geriatric profile | ||||||
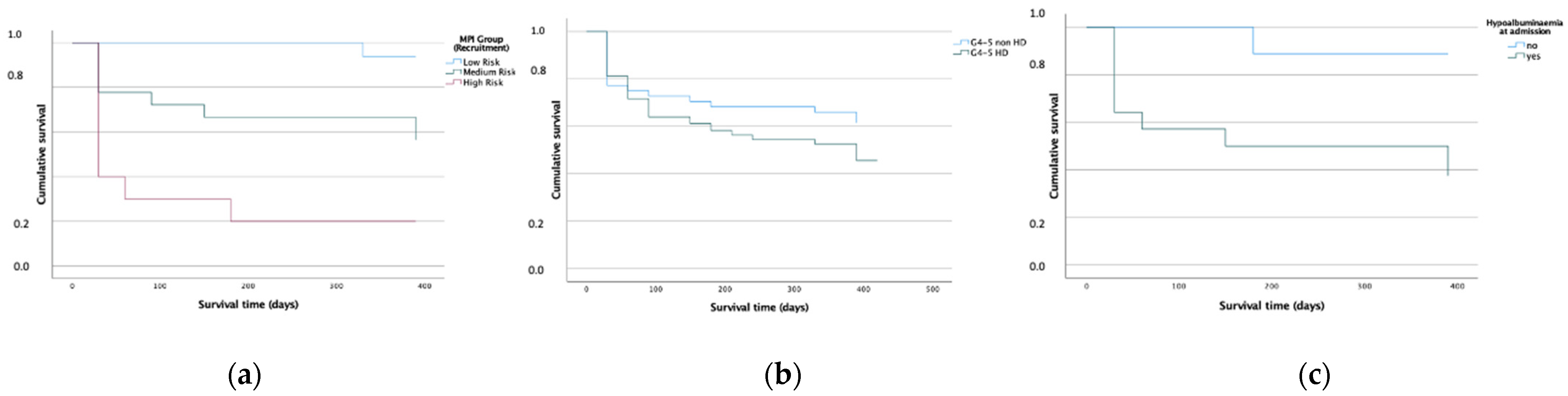
| MPI-value admission, mean (SD) | 0.48 (0.17) | 0.27 (0.05) | 0.49 (0.09) | 0.74 (0.06) | - | |
| MPI-value discharge, mean (SD) | 0.47 (0.15) | 0.30 (0.08) | 0.47 (0.1) | 0.68 (0.1) | - | |
| Number of geriatric resources, median (Q1-3) | 6 (4–7) | 7 (6–8) | 6 (5–7) | 5 (3–6) | <0.001 | |
| Number of geriatric syndromes, median (Q1-3) | 6 (4–8) | 4 (3–6) | 6 (4–7) | 8 (7–9) | <0.001 | |
| Number of medications, mean (SD) | 10.1 (3.6) | 8.8 (3.8) | 10.5 (3.4) | 10.6 (3.3) | <0.001 | |
| Living condition, n (%) | with relatives | 250 (67) | 81 (94) | 129 (61) | 40 (51) | <0.001 |
| institutionalised/private attendant | 34 (9) | 1 (1) | 15 (7) | 18 (23) | ||
| alone | 91 (24) | 4 (5) | 66 (31) | 21 (27) | ||
| Weight loss in the last 3 months, n (%) | 218 (59) | 42 (49) | 123 (59) | 53 (67) | 0.116 | |
| BMI, mean (SD) | 26.7 (5.6) | 26.9 (4.9) | 27.2 (5.8) | 25.0 (5.5) | 0.285 | |
| Use of home services, n (%) | 106 (28) | 1 (1) | 59 (28) | 46 (58) | <0.001 | |
| Grade of care (n = 495), n (%) | none | 207 (55) | 73 (85) | 116 (56) | 18 (23) | <0.001 |
| 1–2 | 103 (28) | 8 (9) | 68 (33) | 27 (34) | ||
| 3 | 48 (13) | 5 (6) | 22 (11) | 21 (27) | ||
| 4–5 | 16 (4) | 0 | 3 (1) | 13 (17) | ||
| Medical findings | ||||||
| Renal Replacement Therapy (RRT), n (%) | 138 (37) | 24 (28) | 75 (36) | 39 (49) | 0.181 | |
| Kidney-transplant recipients, n (%) | 45 (12) | 15 (18) | 23 (11) | 7 (9) | 0.864 | |
| Haemoglobin admission (g/dL), mean (SD) | 10.0 (2.2) | 10.3 (2.2) | 9.9 (2.1) | 10.0 (2.2) | 0.227 | |
| Haemoglobin discharge (g/dL), mean (SD) | 10.2 (1.9) | 10.7 (1.5) | 10.0 (2.1) | 9.7 (1.7) | 0.316 | |
| Total protein admission (g/L), mean (SD) | 63.3 (9.6) | 65.1 (7.0) | 63.3 (9.3) | 60.9 (12.4) | 0.007 | |
| Total protein discharge (g/L), mean (SD) | 63.3 (11.0) | 66.8 (10.4) | 62.5 (11.2) | 59.9 (9.9) | 0.235 | |
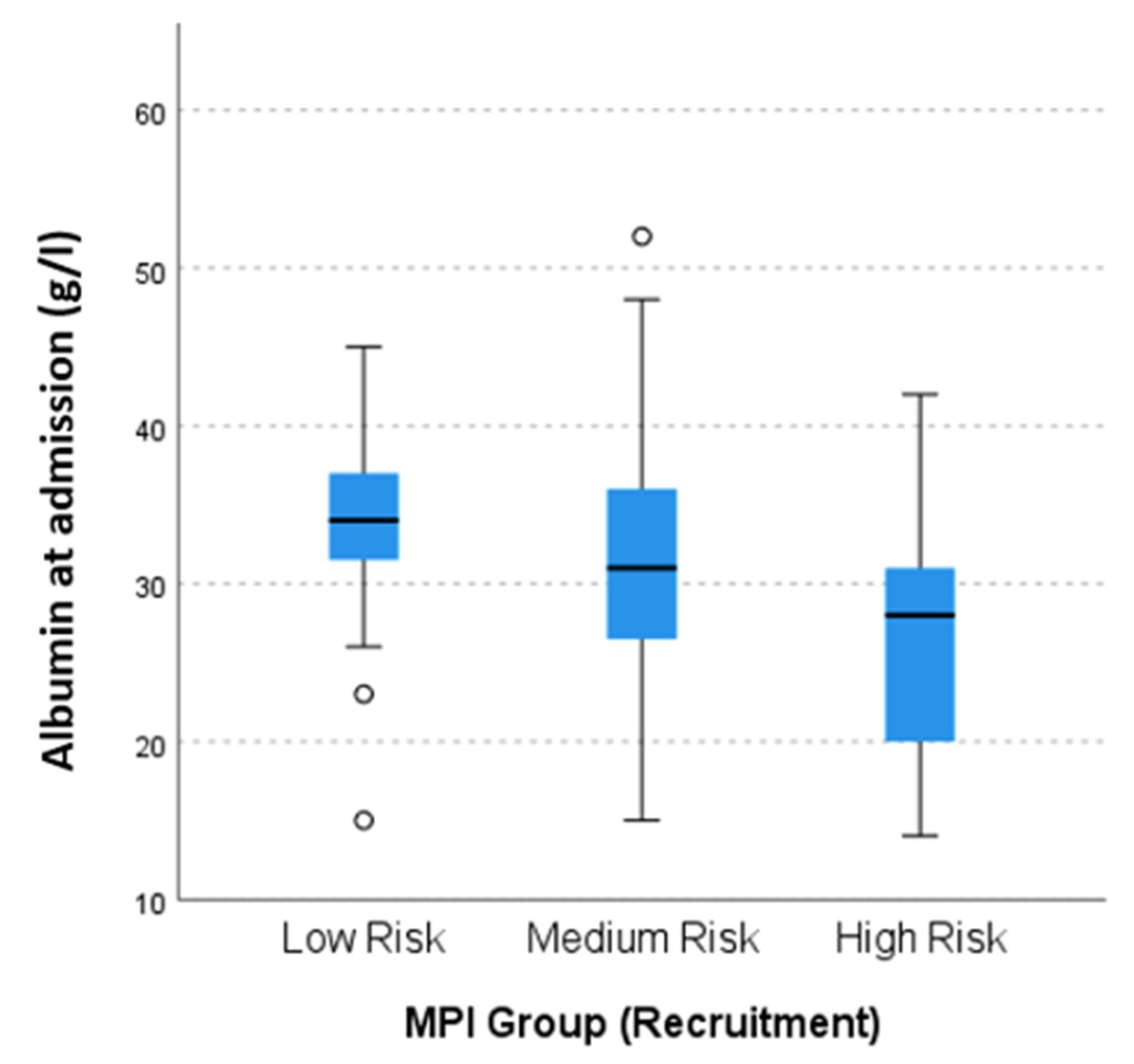
| Albumin admission (g/L), mean (SD) | 30.9 (6.9) | 33.6 (5.7) | 31.1 (6.6) | 26.9 (7.6) | <0.001 | |
| Albumin discharge (g/L), mean (SD) | 30.9 (6.8) | 34.1 (5.3) | 30.7 (6.5) | 26.3 (7.8) | <0.001 | |
| C-reactive protein admission (mg/dL), median (Q1-3) | 32 (12–83) | 22 (6–57) | 33 (10–75) | 50 (22–106) | 0.016 | |
| C-reactive protein discharge (mg/dL), median (Q1-3) | 21 (8–49) | 8 (5–39) | 22 (9–46) | 35 (12–83) | 0.020 | |
| Proteinuria (g/g Creatinine), median (Q1-3) [n = 205] | 0.5 (0.2–1.4) | 0.6 (0.1–2.4) | 0.4 (0.2–0.9) | 0.9 (0.4–1.9) | 0.521 | |
| Albuminuria (g/g Creatinine), median (Q1-3) [n = 186] | 0.1 (0.0–0.4) | 0.1 (0.0–0.4) | 0.1 (0.0–0.4) | 0.2 (0.1–0.7) | 0.667 | |
| Alpha-1-Microglobuline Urine (g/g Creatinine), median (Q1-3) [n = 189] | 0.1 (0.0–0.2) | 0.1 (0.0–0.2) | 0.1 (0.0–0.2) | 0.2 (0.1–0.4) | 0.122 | |
| Follow-Up 3 months (n = 346) | ||||||
| Alive, n (%) | 263 (76) | 79 (93) | 154 (80) | 30 (44) | <0.001 | |
| Rehospitalisation, (n = 271), n (%) | 160 (59) | 45 (56) | 90 (57) | 25 (78) | 0.140 | |
| Follow-Up 6 months (n = 341) | ||||||
| Alive, n (%) | 234 (69) | 76 (91) | 137 (71) | 21 (32) | <0.001 | |
| Rehospitalisation, (n = 269), n (%) | 192 (71) | 57 (70) | 105 (68) | 30 (91) | 0.027 | |
| Follow-Up 12 months (n = 306) | ||||||
| Alive, n (%) | 174 (57) | 58 (75) | 100 (61) | 16 (25) | 0.001 | |
| Rehospitalisation, (n = 247), n (%) | 210 (85) | 60 (80) | 118 (85) | 32 (91) | 0.109 | |
| Total n = 375 | G2 (GFR 60–89 mL/min/1.73 m2) n = 19 | G3a (GFR 45–59 mL/min/1.73 m2) n = 80 | G3b (GFR 30–44 mL/min/1.73 m2) n = 75 | G4 (GFR 15–29 mL/min/1.73 m2) n = 52 | G5 (GFR < 15 mL/min/1.73 m2) n = 149 | p-Value ° | ||
|---|---|---|---|---|---|---|---|---|
| KDIGO stage according to A (n = 178), n (%) | A1 | 41 (23.0) | 3 (37.5) | 21 (42.9) | 13 (25.0) | 0 | 4 (8.7) | <0.001 |
| A2 | 65 (36.5) | 3 (37.5) | 17 (34.7) | 26 (50.0) | 10 (43.5) | 9 (19.6) | ||
| A3 | 72 (40.4) | 2 (25.0) | 11 (22.4) | 13 (25.0) | 13 (56.5) | 33 (71.7) | ||
| Demographic | ||||||||
| Age (years), median (IQR) | 78.0 (9) | 73 (10) | 78 (9) | 77 (9) | 76 (9) | 78 (8) | 0.121 | |
| Female, n (%) | 133 (35.5) | 4 (21.1) | 27 (33.8) | 32 (42.7) | 16 (30.8) | 54 (36.2) | 0.565 | |
| Living conditions, n (%) | With relatives | 250 (66.7) | 15 (78.9) | 54 (67.5) | 46 (61.3) | 34 (65.4) | 101 (67.8) | 0.117 |
| Institutionalised/private attendant | 34 (9.1) | 0 | 5 (6.3) | 6 (8.0) | 2 (3.8) | 21 (14.1) | ||
| Alone | 91 (24.3) | 4 (21.1) | 21 (26.3) | 23 (30.7) | 16 (30.8) | 27 (18.1) | ||
| LHS, mean (SD) | 14.9 (16.1) | 17.1 (23.4) | 10.0 (9.6) | 11.7 (8.4) | 12.0 (7.2) | 20.0 (21.1) | <0.001 | |
| Body Mass Index (BMI), median (IQR) | 25.7 (7) | 27.7 (5) | 26.4 (8) | 26.6 (7) | 25.4 (9) | 24.8 (7) | 0.012 | |
| Care | ||||||||
| Grade of Care (n = 374), n (%) | none | 207 (55.3) | 13 (68.4) | 56 (70.0) | 45 (60.0) | 34 (65.4) | 59 (39.9) | 0.091 |
| 1–2 | 103 (27.5) | 5 (26.4) | 16 (20.1) | 19 (25.4) | 15 (28.9) | 48 (32.4) | ||
| 3 | 48 (12.8) | 1 (5.3) | 6 (7.5) | 9 (12.0) | 2 (3.8) | 30 (20.3) | ||
| 4–5 | 16 (4.3) | 0 | 2 (2.5) | 2 (2.7) | 1 (1.9) | 11 (7.5) | ||
| Home care, n (%) | 106 (28.3) | 3 (15.8) | 12 (15.0) | 19 (25.3) | 14 (26.9) | 58 (38.9) | 0.045 | |
| Medical findings | ||||||||
| Main Reason for CKD (n = 305), n (%) | Hypertensive Nephropathy | 72 (23.6) | 3 (23.1) | 17 (29.3) | 16 (25.8) | 7 (15.9) | 29 (22.7) | 0.632 |
| Diabetic Nephropathy | 67 (22.0) | 2 (15.4) | 10 (17.2) | 15 (24.2) | 12 (27.3) | 28 (21.9) | ||
| Glomerulonephritis | 46 (15.1) | 4 (30.8) | 9 (15.5) | 7 (11.3) | 6 (13.6) | 20 (15.6) | ||
| Ischemic Disease | 37 (12.1) | 0 | 6 (10.3) | 12 (19.4) | 3 (6.8) | 16 (12.5) | ||
| Other | 83 (39.3) | 4 (30.8) | 16 (27.6) | 12 (19.3) | 24 (27.3) | 35 (27.2) | ||
| Haemoglobin admission (g/dL), median (IQR) | 9.8 (2.9) | 10.7 (1.4) | 10.4 (2.9) | 9.6 (2.9) | 9.3 (3.2) | 9.7 (2.9) | 0.045 | |
| Haemoglobin discharge (g/dL), median (IQR) | 9.8 (2.3) | 8.9 (4.2) | 10.2 (2.0) | 9.8 (1.9) | 9.6 (3.5) | 10.0 (3.2) | 0.781 | |
| Total protein admission (g/L), median (IQR) | 66.0 (12) | 60.5 (7) | 68.0 (13 | 61.5 (11) | 62.5 (24) | 61.5 (14) | 0.728 | |
| Total protein discharge (g/L), median (IQR) | 64.0 (13) | 65.0 (10) | 68.0 (8) | 63.5 (12) | 61.0 (19) | 60.0 (12) | 0.254 | |
| Albumin admission (g/L), median (IQR) | 31 (9.75) | 31.5 (9) | 33.0 (9) | 32.0 (8) | 32.0 (11) | 28.0 (9) | 0.049 | |
| Albumin discharge (g/L), median (IQR) | 32.0 (9) | 34.5 (8) | 33.5 (10) | 31.0 (6) | 31.0 (10) | 29.0 (8) | 0.345 | |
| Parathyreoidal hormone admission, median (IQR) | 105.5 (153) | 81 (101) | 110.5 (81) | 125 (123) | 94 (184) | 109 (182) | 0.780 | |
| Geriatric profile | ||||||||
| MPI, median (IQR) | 0.44 (0.30) | 0.38 (0.31) | 0.44 (0.19) | 0.44 (0.19) | 0.47 (0.31) | 0.50 (0.31) | 0.003 | |
| MPI group, n (%) | MPI-1 | 86 (22.9) | 8 (42.1) | 23 (28.7) | 14 (18.7) | 17 (32.7) | 24 (16.1) | 0.018 |
| MPI-2 | 210 (56.0) | 9 (47.4) | 46 (57.5) | 50 (66.7) | 23 (44.2) | 82 (55.0) | ||
| MPI-3 | 79 (21.1) | 2 (10.5) | 11 (13.8) | 11 (14.7) | 12 (23.1) | 43 (28.9) | ||
| Geriatric resources (GR), mean (SD) | 5.8 (2.1) | 6.1 (1.9) | 6.6 (1.9) | 5.7 (2.0) | 5.8 (2.2) | 5.5 (2.1) | 0.006 | |
| Geriatric resources (extract) | ||||||||
| Physical, n (%) | 102 (27.2) | 10 (52.6) | 30 (37.5) | 17 (22.7) | 11 (21.2) | 34 (22.8) | 0.030 | |
| Good living conditions, n (%) | 259 (69.3) | 10 (52.6) | 63 (79.7) | 49 (65.3) | 29 (55.8) | 108 (72.5) | 0.007 | |
| Social resources, n (%) | 323 (86.4) | 13 (68.4) | 76 (96.2) | 60 (80.0) | 43 (82.7) | 131 (87.9) | 0.003 | |
| Geriatric Syndromes (GS), mean (SD) | 5.8 (2.4) | 5.4 (2.6) | 5.2 (2.4) | 6.0 (2.1) | 5.6 (2.5) | 6.3 (2.3) | 0.499 | |
| Geriatric syndromes (extract) | ||||||||
| Inanition, n (%) | 183 (48.8) | 10 (52.6) | 34 (42.5) | 30 (40.0) | 19 (36.5) | 90 (60.4) | 0.030 | |
| Polypharmacy, n (%) | 341 (90.9) | 15 (78.9) | 65 (81.3) | 66 (88.0) | 51 (98.1) | 144 (96.6) | <0.001 | |
| Follow-Up Discharge | ||||||||
| Alive, n (%) | 352 (93.9) | 19 (100) | 75 (93.8) | 73 (97.3) | 48 (92.3) | 137 (91.9) | 0.450 | |
| MPI, median (IQR) | 0.44 (0.19) | 0.38 (0.25) | 0.38 (0.19) | 0.44 (0.19) | 0.38 (0.30) | 0.50 (0.25) | <0.001 | |
| MPI (n = 349), n (%) | MPI-1 | 82 (23.5) | 9 (47.4) | 25 (33.3) | 14 (19.4) | 15 (31.3) | 19 (14.1) | <0.001 |
| MPI-2 | 222 (63.6) | 9 (47.4) | 43 (57.3) | 50 (69.4) | 25 (52.) | 95 (70.4) | ||
| MPI-3 | 45 (12.9) | 1 (5.3) | 7 (9.3) | 8 (11.1) | 8 (16.7) | 21 (15.6) | ||
| Follow-Up 3 months (n = 346) | ||||||||
| Alive, n (%) | 263 (76.0) | 17 (94.4) | 64 (83.1) | 54 (78.3) | 36 (73.5) | 92 (69.2) | 0.318 | |
| Grade of care, (n = 246), n (%) | 122 (49.6) | 6 (37.5) | 25 (44.6) | 26 (50.0) | 13 (36.1) | 52 (60.5) | 0.574 | |
| Rehospitalisation, (n = 271), n (%) | 160 (59.0) | 5 (29.4) | 33 (52.4) | 32 (59.3) | 26 (66.7) | 64 (65.3) | 0.081 | |
| Follow-Up 6 months (n = 341) | ||||||||
| Alive, n (%) | 234 (68.6) | 17 (94.4) | 58 (77.3) | 46 (67.6) | 33 (67.3) | 80 (61.6) | 0.124 | |
| Grade of care, (n = 221), n (%) | 120 (54.3) | 9 (52.9) | 19 (35.8) | 27 (60.0) | 9 (30.0) | 56 (73.7) | <0.001 | |
| Rehospitalisation, (n = 269), n (%) | 192 (71.4) | 8 (47.1) | 38 (61.3) | 41 (75.9) | 29 (76.3) | 76 (77.6) | 0.038 | |
| Follow-Up 12 months (n = 306) | ||||||||
| Alive, n (%) | 174 (56.9) | 13 (81.3) | 43 (63.2) | 34 (55.7) | 26 (57.8) | 58 (50.0) | <0.001 | |
| Grade of care, (n = 169), n (%) | 95 (56.2) | 9 (69.2) | 14 (35.0) | 21 (61.8) | 11 (42.3) | 40 (71.4) | 0.003 | |
| Rehospitalisation, (n = 247), n (%) | 210 (85.0) | 9 (64.3) | 40 (70.2) | 45 (90.0) | 31 (86.1) | 85 (94.4) | <0.001 | |
| Total n = 138 | HD n = 127 | PD n = 11 | p-Value ° | ||
|---|---|---|---|---|---|
| Age (years), median (IQR) | 78 (8) | 78.0 (9) | 78.0 (5) | 0.289 | |
| Female, n (%) | 49 (37.5) | 47 (37.0) | 2 (18.2) | 0.185 | |
| Hospitalisation last 12 months, n (%) | 99 (71.7) | 90 (70.9) | 9 (81.8) | 0.311 | |
| LHS (days), median (IQR) | 15 (19) | 15 (18) | 11.0 (23) | 0.818 | |
| BMI (kg/m2), median (IQR)) | 24.7 (6.3) | 24.8 (6.3) | 23.9 (5.3) | 0.716 | |
| Haemoglobin at admission (g/L) | 9.7 (3.0) | 9.7 (2.7) | 9.4 (2.7) | 0.402 | |
| Albumin at admission (g/L) | 38.5 (9) | 29.0 (12) | 27 (5.3) | 0.367 | |
| Living conditions, n (%) | With relatives | 95 (68.8) | 86 (67.7) | 9 (81.8) | 0.664 |
| Institutionalised/private attendant | 19 (13.8) | 18 (14.2) | 1 (9.1) | ||
| Alone | 24 (17.4) | 23 (18.1) | 1 (9.1) | ||
| Grade of Care, n (%) | none | 58 (42.0) | 50 (39.4) | 8 (72.7) | 0.478 |
| 1–2 | 43 (31.1) | 41 (32.2) | 2 (18.2) | ||
| 3 | 27 (19.6) | 26 (20.5) | 1 (9.1) | ||
| 4–5 | 10 (7.2) | 10 (7.9) | 0 | ||
| Home care, n (%) | 51 (37.0) | 49 (38.6) | 2 (18.2) | 0.218 | |
| MPI, median (IQR) | 0.50 (0.31) | 0.53 (0.31) | 0.49 (0.17) | 0.414 | |
| MPI group at admission, n (%) | MPI-1 | 24 (17.4) | 22 (17.3) | 2 (18.2) | 0.316 |
| MPI-2 | 75 (54.3) | 67 (52.8) | 8 (72.7) | ||
| MPI-3 | 39 (28.3) | 38 (29.9) | 1 (9.1) | ||
| CIRS, mean (SD) | 5.6 (1.6) | 5.6 (1.5) | 4.6 (1.4) | 0.026 | |
| ESS, mean (SD) | 14.2 (3.4) | 14.1 (3.5) | 15.3 (2.4) | 0.688 | |
| MNA-SF, mean (SD)) | 8.2 (3.3) | 8.2 (3.2) | 8.3 (4.1) | 0.503 | |
| ADL, mean (SD) | 3.6 (2.0) | 3.6 (2.1) | 4.2 (1.5) | 0.892 | |
| IADL, mean (SD) | 4.1 (2.5) | 4.0 (2.4) | 4.9 (2.7) | 0.498 | |
| SPMSQ, mean (SD) | 1.9 (1.9) | 1.9 (1.9) | 1.1 (1.6) | 0.199 | |
| Geriatric resources (GR), mean (SD) | 5.5 (2.0) | 5.5 (4.0) | 5.8 (1.8) | 0.848 | |
| Emotional, n (%) | 88 (63.8) | 78 (61.4) | 10 (90.9) | 0.045 | |
| Geriatric Syndromes (GS), mean (SD) | 6.4 (2.3) | 6.4 (2.3) | 5.6 (1.8) | 0.472 | |
| Sensorial Impairment, n (%) | 86 (62.3) | 83 (65.4) | 3 (27.3) | 0.019 | |
| Irritability/Depression, n (%) | 21 (15.2) | 21 (16.5) | 0 | 0.073 | |
| Insomnia, n (%) | 76 (55.1) | 69 (54.3) | 7 (63.6) | 0.487 | |
| Alive, n (%) | 129 (93.5) | 119 (93.7) | 10 (90.9) | 0.529 | |
| MPI, mean (SD) | 0.54 (0.17) | 0.54 (0.17) | 0.41 (0.15) | 0.021 | |
| Alive, n (%) | 85 (69.7) | 77 (68.8) | 8 (80.0) | 0.885 | |
| Grade of Care, n (%) | 47 (58.8) | 44 (61.1) | 3 (37.5) | 0.260 | |
| Rehospitalisation, n (%) | 60(65.9) | 55 (67.1) | 5 (55.6) | 0.596 | |
| Alive, n (%) | 74 (61.7) | 67 (60.9) | 7 (70.0) | 0.987 | |
| Grade of Care, n (%) | 51 (71.8) | 47 (74.6) | 4 (50.0) | 0.194 | |
| Rehospitalisation, n (%) | 71 (78.0) | 65 (79.3) | 6 (66.7) | 0.636 | |
| Alive, n (%) | 53 (50.0) | 49 (50.0) | 4 (50.0) | 0.691 | |
| Grade of Care, n (%) | 36 (69.2) | 35 (72.9) | 1 (25.0) | 0.083 | |
| Rehospitalisation, n (%) | 80 (95.2) | 73 (94.8) | 7 (100) | 0.332 | |
| Total n = 175 | Renal Replacement Therapy (RRT) n = 131 | Kidney Transplant Recipients n = 44 | p-Value ° | ||
|---|---|---|---|---|---|
| Age (years), median (IQR). | 77 (9) | 78.0 (8) | 73.0 (7) | <0.001 | |
| Female, n (%) | 61 (35) | 48 (37) | 13 (30) | 0.307 | |
| Period of education, mean (SD) | 12.4 (4.0) | 11.9 (3.5) | 13.7 (5.1) | 0.004 | |
| Hospitalisation last 12 months, n (%) | 130 (74) | 95 (73) | 35 (80) | 0.900 | |
| Falls last 12 months, n (%) | 76 (43) | 66 (50) | 10 (22) | 0.014 | |
| LHS (days), mean (SD) | 18.9 (20.8) | 19.9 (21.8) | 14.0 (17.1) | 0.077 | |
| BMI (kg/m2), median (IQR) | 25.7 (6.95) | 24.8 (6.3) | 25.4 (6.3) | <0.001 | |
| Living conditions, n (%) | With relatives | 121 (69) | 90 (69) | 31 (71) | 0.244 |
| Institutionalised/private attendant | 21 (12) | 19 (15) | 2 (5) | ||
| Alone | 33 (19) | 22 (17) | 11 (25) | ||
| Grade of Care, n (%) | none | 85 (48.6) | 54 (41) | 31 (71) | 0.031 |
| 1–2 | 50 (29) | 41 (31) | 9 (20) | ||
| 3 | 30 (17) | 26 (20) | 4 (9) | ||
| 4–5 | 10 (6) | 10 (8) | 0 | ||
| Home care, n (%) | 61 (35) | 51 (39) | 10 (23) | 0.751 | |
| MPI, mean (SD) | 0.51 (0.18) | 0.52 (0.17) | 0.43 (0.16) | 0.028 | |
| MPI group, n (%) | MPI-1 | 39 (22) | 23 (18) | 16 (36) | 0.038 |
| MPI-2 | 91 (52) | 70 (53) | 21 (48) | ||
| MPI-3 | 45 (26) | 38 (29) | 7 (16) | ||
| Geriatric resources, median (IQR) | 6.0 (3) | 6.0 (3) | 6.0 (3) | 0.664 | |
| Geriatric Syndromes, median (IQR) | 6.0 (4) | 6.0 (4) | 5.0 (4) | 0.106 | |
| Immobility, n (%) | 86 (49) | 70 (53) | 16 (36) | 0.101 | |
| Sensorial Impairment, n (%) | 103 (59) | 83 (63) | 20 (46) | 0.169 | |
| Inanition, n (%) | 97 (55) | 80 (61) | 17 (39) | 0.565 | |
| Instability, n (%) | 116 (66) | 95 (73) | 21 (48) | 0.439 | |
| Insomnia, n (%) | 90 (51) | 73 (56) | 17 (39) | 0.044 | |
| More GR than GS, n (%) | 120 (69) | 85 (65) | 35 (80) | 0.349 | |
| Follow-Up Discharge (n = 175) | |||||
| Alive, n (%) | 163 (93) | 122 (93) | 42 (93) | 0.518 | |
| MPI, mean (SD) | 0.51 (0.18) | 0.50 (0.15) | 0.42 (0.15) | 0.110 | |
| Falls, n (%) | 11 (7) | 8 (7) | 3 (7) | 0.787 | |
| Follow-Up 3 months (n = 157) | |||||
| Alive, n (%) | 113 (72) | 79 (69) | 34 (81) | 0.523 | |
| Grade of Care, n (%) | 56 (53) | 44 (60) | 12 (38) | 0.333 | |
| Rehospitalisation, n (%) | 77 (65) | 56 (66) | 21 (64) | 0.725 | |
| Falls, n (%) | 31 (28) | 25 (32) | 6 (18) | 0.253 | |
| Follow-Up 6 months (n = 155) | |||||
| Alive, n (%) | 102 (66) | 69 (61) | 33 (79) | 0.368 | |
| Grade of Care, n (%) | 57 (60) | 47 (72) | 10 (33) | 0.015 | |
| Rehospitalisation, n (%) | 94 (80) | 67 (79) | 27 (82) | 0.865 | |
| Falls, n (%) | 37 (37) | 29 (42) | 8 (26) | 0.207 | |
| Follow-Up 12 months (n = 141) | |||||
| Alive, n (%) | 78 (55) | 49 (49) | 29 (71) | 0.395 | |
| Grade of Care, n (%) | 43 (59) | 34 (71) | 9 (36) | 0.054 | |
| Rehospitalisation, n (%) | 103 (93) | 76 (96) | 27 (84) | 0.010 | |
| Falls, n (%) | 37 (42) | 29 (48) | 8 (29) | 0.999 | |
| Total n = 190 | CKD G4-5 Non-RRT n = 52 | Haemodialysis n = 138 | p-Value ° | ||
|---|---|---|---|---|---|
| Age (years), median (IQR) | 78.0 (9) | 76.0 (8) | 78.0 (9) | 0.027 | |
| Female, n (%) | 68 (36) | 19 (37) | 49 (36) | 0.732 | |
| LHS (days), mean (SD) | 18.1 (19.1) | 12.0 (7.0) | 20.4 (21.6) | 0.003 | |
| BMI (kg/m2), median (IQR) | 25.1 (7.4) | 25.9 (9.7) | 24.9 (6.9) | 0.046 | |
| Albumin at admission, median (IQR) | 32 (12) | 28.5 (11) | 0.011 | ||
| Living conditions, n (%) | With relatives | 126 (66) | 34 (65) | 92 (67) | 0.261 |
| Institutionalised/private attendant | 22 (12) | 3 (6) | 19 (14) | ||
| Alone | 42 (22) | 15 (29) | 27 (20) | ||
| Grade of Care, n (%) | none | 85 (45) | 32 (62) | 53 (39) | <0.001 |
| 1–2 | 61 (32) | 15 (29) | 46 (34) | ||
| 3 | 31 (16) | 4 (8) | 27 (20) | ||
| 4–5 | 12 (6) | 1 (2) | 11 (8) | ||
| Home care, n (%) | 70 (37) | 15 (29) | 55 (40) | <0.001 | |
| MPI, mean (SD) | 0.52 (0.17) | 0.47 (0.18) | 0.53 (0.16) | 0.052 | |
| MPI group, n (%) | MPI-1 | 39 (21) | 17 (33) | 22 (16) | 0.018 |
| MPI-2 | 97 (51) | 23 (44) | 74 (54) | ||
| MPI-3 | 54 (28) | 12 (23) | 42 (30) | ||
| Geriatric resources, mean (SD) | 5.5 (2.1) | 5.7 (2.3) | 5.5 (2.1) | 0.355 | |
| Geriatric Syndromes, median (IQR) | 6.0 (4) | 5.0 (3) | 6.0 (3) | 0.044 | |
| Follow-Up Discharge (n = 190) | |||||
| Alive, n (%) | 175 (92) | 47 (90) | 128 (93) | 0.460 | |
| MPI, mean (SD) | 0.50 (0.16) | 0.44 (0.17) | 0.52 (0.15) | 0.005 | |
| Follow-Up 3 months (n = 172) | |||||
| Alive, n (%) | 120 (70) | 36 (74) | 84 (68) | 0.186 | |
| Grade of Care, (n = 114) n (%) | 62 (54) | 13 (37) | 49 (62) | 0.010 | |
| Rehospitalisation, n (%) | 85 (66) | 25 (66) | 60 (67) | 0.839 | |
| Follow-Up 6 months (n = 170) | |||||
| Alive, n (%) | 106 (62) | 34 (69) | 72 (60) | 0.133 | |
| Grade of Care, (n = 98) n (%) | 61 (62) | 10 (32) | 51 (76) | 0.076 | |
| Rehospitalisation (n = 172), n (%) | 99 (78) | 28 (76) | 71 (79) | 0.862 | |
| Follow-Up 12 months (n = 153) | |||||
| Alive, n (%) | 80 (52) | 28 (62) | 52 (48) | 0.039 | |
| Grade of Care, (n = 78) n (%) | 50 (64) | 13 (46) | 37 (74) | 0.003 | |
| Rehospitalisation, (n = 119) n (%) | 109 (92) | 30 (86) | 79 (94) | 0.334 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, A.M.; Pickert, L.; Heeß, A.; Becker, I.; Kurschat, C.; Bartram, M.P.; Benzing, T.; Polidori, M.C. Prognostic Signature of Chronic Kidney Disease in Advanced Age: Secondary Analysis from the InGAH Study with One-Year Follow-Up. Biomolecules 2022, 12, 423. https://doi.org/10.3390/biom12030423
Meyer AM, Pickert L, Heeß A, Becker I, Kurschat C, Bartram MP, Benzing T, Polidori MC. Prognostic Signature of Chronic Kidney Disease in Advanced Age: Secondary Analysis from the InGAH Study with One-Year Follow-Up. Biomolecules. 2022; 12(3):423. https://doi.org/10.3390/biom12030423
Chicago/Turabian StyleMeyer, Anna Maria, Lena Pickert, Annika Heeß, Ingrid Becker, Christine Kurschat, Malte P. Bartram, Thomas Benzing, and Maria Cristina Polidori. 2022. "Prognostic Signature of Chronic Kidney Disease in Advanced Age: Secondary Analysis from the InGAH Study with One-Year Follow-Up" Biomolecules 12, no. 3: 423. https://doi.org/10.3390/biom12030423