Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects
Abstract
:1. Introduction
2. Extracellular Hemoglobin in Mammals
2.1. Endogeous Extracellular Hemoglobin Removal and Degradation
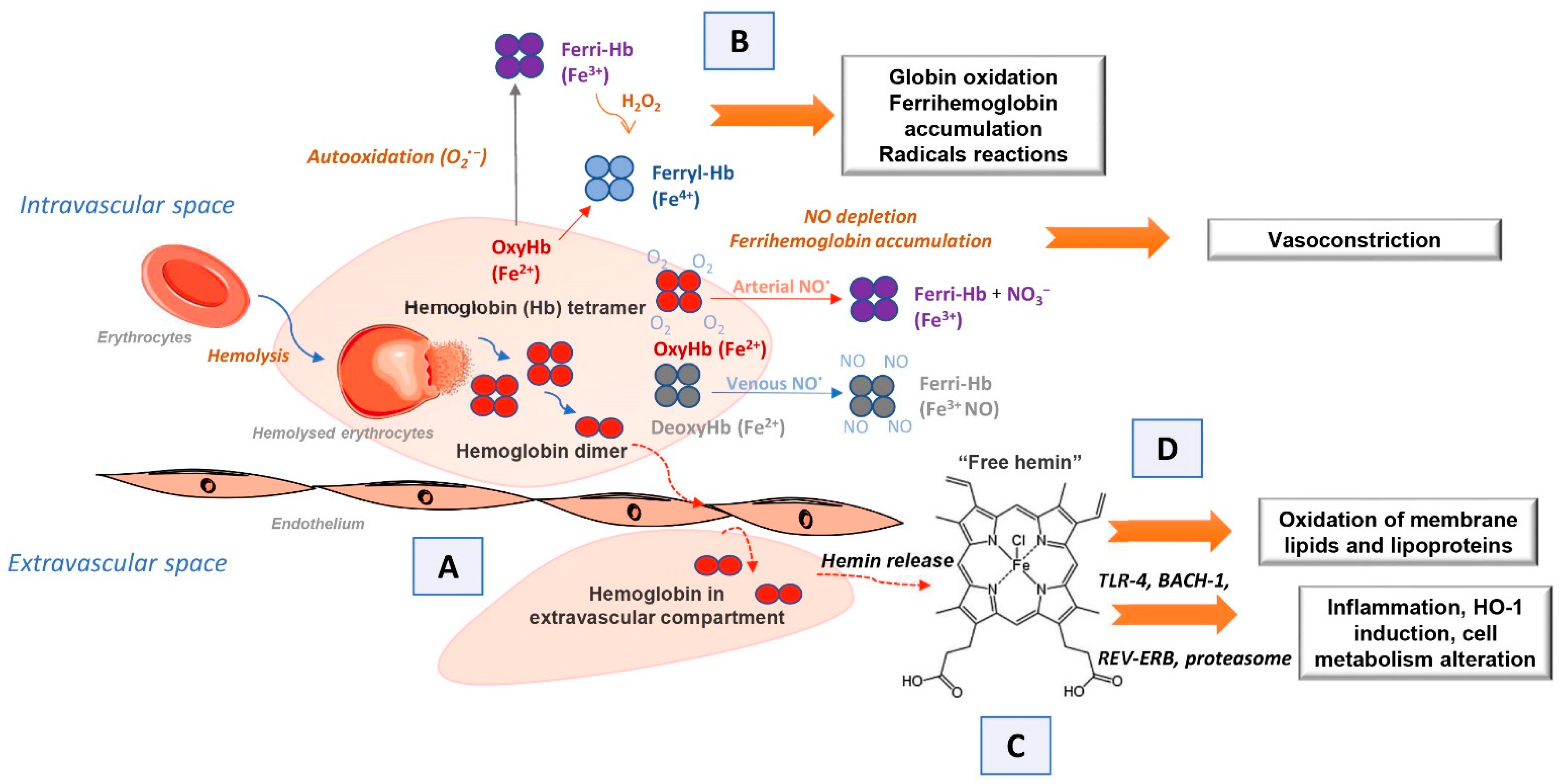
2.2. Endogenous Extracellular Hemoglobin Mode of Action
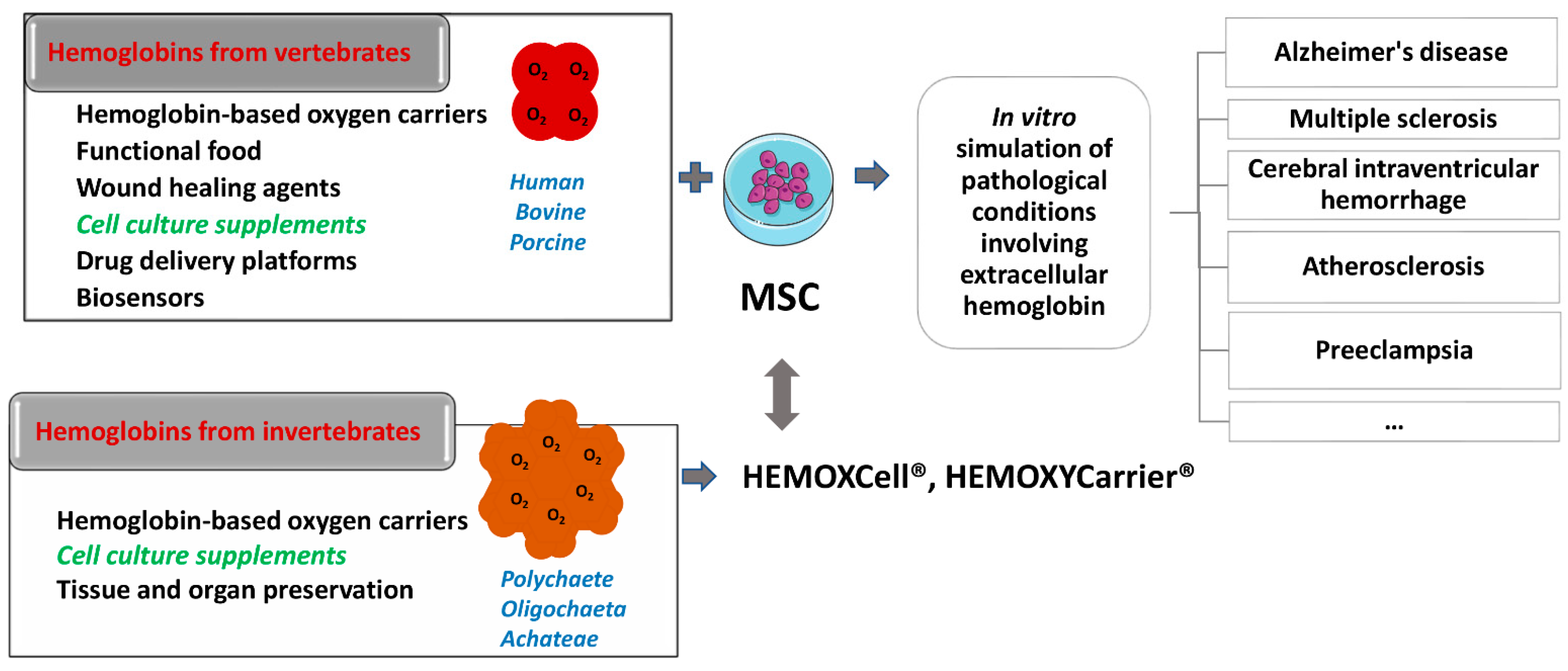
3. Exogenous Administration of Extracellular Hemoglobin
4. Evidence on Capacity of Mammalian Endogenous and Exogenous Extracellular Hemoglobin to Regulate Cellular Functions and Molecular Signaling in Health and Disease
5. Evidence of Capacity of Invertebrate Hemoglobin to Regulate Cellular Functions and Molecular Signaling
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quaye, I.K. Extracellular Hemoglobin: The Case of a Friend Turned Foe. Front. Physiol. 2015, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Decker, H. Immunological Properties of Oxygen-Transport Proteins: Hemoglobin, Hemocyanin and Hemerythrin. Cell. Mol. Life Sci. 2016, 74, 293–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, J.S. Lessons Learned from 50 Years of Hemoglobin Research: Unstirred and Cell-Free Layers, Electrostatics, Baseball Gloves, and Molten Globules. Antioxid. Redox Signal. 2020, 32, 228–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The Pathophysiology of Extracellular Hemoglobin Associated with Enhanced Oxidative Reactions. Front. Physiol. 2015, 5, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, F.A.; Kahn, S.A.; Da Fonseca, A.C.C.; Bahia, C.P.; Pinho, J.P.C.; Graca-Souza, A.V.; Houzel, J.C.; De Oliveira, P.L.; Moura-Neto, V.; Oliveira, M.F. On the fate of extracellular hemoglobin and heme in brain. J. Cereb. Blood Flow Metab. 2009, 29, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Rother, R.P.; Bell, L.; Hillmen, P. The clinical sequelae of Intravascular Hemolysis A Novel Mechanism of Human Disease. JAMA 2015, 293, 1653–1662. [Google Scholar] [CrossRef]
- Ogasawara, N.; Oguro, T.; Sakabe, T.; Matsushima, M.; Takikawa, O.; Isobe, K.I.; Nagase, F. Hemoglobin Induces the Expression of Indoleamine 2,3-Dioxygenase in Dendritic Cells through the Activation of PI3K, PKC, and NF-ΚB and the Generation of Reactive Oxygen Species. J. Cell. Biochem. 2009, 108, 716–725. [Google Scholar] [CrossRef]
- Jeney, V.; Eaton, J.W.; Balla, G.; Balla, J. Natural History of the Bruise: Formation, Elimination, and Biological Effects of Oxidized Hemoglobin. Oxid. Med. Cell. Longev. 2013, 2013, 703571. [Google Scholar] [CrossRef] [Green Version]
- Panja, C.; Setty, R.K.S.; Vaidyanathan, G.; Ghosh, S. Label-Free Proteomic Analysis of Flavohemoglobin Deleted Strain of Saccharomyces Cerevisiae. Int. J. Proteomics 2016, 2016, 8302423. [Google Scholar] [CrossRef] [Green Version]
- Bahl, N.; Winarsih, I.; Tucker-Kellogg, L.; Ding, J.L. Extracellular Haemoglobin Upregulates and Binds to Tissue Factor on Macrophages: Implications for Coagulation and Oxidative Stress. Thromb. Haemost. 2014, 111, 67–78. [Google Scholar] [CrossRef]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and Free Hemoglobin Revisited: Exploring Hemoglobin and Hemin Scavengers as a Novel Class of Therapeutic Proteins. Blood 2013, 121, 1276–1285. [Google Scholar] [CrossRef] [Green Version]
- Jeney, V. Pro-Inflammatory Actions of Red Blood Cell-Derived DAMPs. In Inflammasomes: Clinical and Therapeutic Implications, Experientia Supplementum; Cordero, M., Alcocer-Gómez, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 108, pp. 211–233. [Google Scholar] [CrossRef]
- Garton, T.; Keep, R.F.; Hua, Y.; Xi, G. CD163, a Hemoglobin/Haptoglobin Scavenger Receptor, After Intracerebral Hemorrhage: Functions in Microglia/Macrophages Versus Neurons. Transl. Stroke Res. 2017, 8, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; McCulloh, R.J. Hemopexin and Haptoglobin: Allies against Heme Toxicity from Hemoglobin Not Contenders. Front. Physiol. 2015, 6, 187. [Google Scholar] [CrossRef]
- McCormick, D.J.; Atassi, M.Z. Hemoglobin Binding with Haptoglobin: Delineation of the Haptoglobin Binding Site on the α-Chain of Human Hemoglobin. J. Protein Chem. 1990, 9, 735–742. [Google Scholar] [CrossRef]
- Kaempfer, T.; Duerst, E.; Gehrig, P.; Roschitzki, B.; Rutishauser, D.; Grossmann, J.; Schoedon, G.; Vallelian, F.; Schaer, D.J. Extracellular Hemoglobin Polarizes the Macrophage Proteome toward Hb-Clearance, Enhanced Antioxidant Capacity and Suppressed HLA Class 2 Expression. J. Proteome Res. 2011, 10, 2397–2408. [Google Scholar] [CrossRef]
- Smeds, E.; Romantsik, O.; Jungner, Å.; Erlandsson, L.; Gram, M. Pathophysiology of Extracellular Haemoglobin: Use of Animal Models to Translate Molecular Mechanisms into Clinical Significance. ISBT Sci. Ser. 2017, 12, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Abdulla, F.; Zhang, P.; Nguyen, H.; Nguyen, P.; Killeen, T.; Miescher, S.M.; Brinkman, N.; et al. Haptoglobin and Hemopexin Inhibit Vaso-Occlusion and Inflammation in Murine Sickle Cell Disease: Role of Heme Oxygenase-1 Induction. PLoS ONE 2018, 13, e0196455. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Ding, J.L. A Perspective on the Role of Extracellular Hemoglobin on the Innate Immune System. DNA Cell Biol. 2013, 32, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Ponka, P.; Sheftel, A.D.; English, A.M.; Scott Bohle, D.; Garcia-Santos, D. Do Mammalian Cells Really Need to Export and Import Heme? Trends Biochem. Sci. 2017, 42, 395–406. [Google Scholar] [CrossRef]
- Sverrisson, K.; Axelsson, J.; Rippe, A.; Gram, M.; Åkerström, B.; Hansson, S.R.; Rippe, B. Extracellular Fetal Hemoglobin Induces Increases in Glomerular Permeability: Inhibition with A1 -Microglobulin and Tempol. Am. J. Physiol. Ren. Physiol. 2014, 306, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Penders, J.; Delanghe, J.R. Alpha 1-Microglobulin: Clinical Laboratory Aspects and Applications. Clin. Chim. Acta 2004, 346, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Olofsson, T.; Tapper, H.; Åkerström, B. The Lipocalin Aa1-Microglobulin Protects Erythroid K562 Cells against Oxidative Damage Induced by Heme and Reactive Oxygen Species. Free Radic. Res. 2008, 42, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.C.; Pires, S.I.; Baek, H.J.; Buehler, W.P.; Palmer, F.A.; Cabrales, P. Apohemoglobin-Haptoglobin Complex Attenuates the Pathobiology of Circulating Acellular 2 Hemoglobin and Heme. Am. J. Physiol. Circ. Physiol. 2020, 318, H1296–H1307. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Du, R.; Tan, N.S.; Ho, B.; Ding, J.L. CD163 and IgG Codefend against Cytotoxic Hemoglobin via Autocrine and Paracrine Mechanisms. J. Immunol. 2013, 190, 5267–5278. [Google Scholar] [CrossRef] [Green Version]
- Nakai, K.; Sakuma, I.; Ohta, T.; Ando, J.; Kitabatake, A.; Nakazato, Y.; Takahashi, T.A. Permeability Characteristics of Hemoglobin Derivatives across Cultured Endothelial Cell Monolayers. J. Lab. Clin. Med. 1998, 132, 313–319. [Google Scholar] [CrossRef]
- Matheson, B.; Razynska, A.; Kwansa, H.; Bucci, E. Appearance of Dissociable and Cross-Linked Hemoglobins in the Renal Hilar Lymph. J. Lab. Clin. Med. 2000, 135, 459–464. [Google Scholar] [CrossRef]
- Schechter, A.N. Hemoglobin Research and the Origins of Molecular Medicine. Blood 2008, 112, 3927–3938. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Hemmi, H. Recognition of Pathogen-Associated Molecular Patterns by TLR Family. Immunol. Lett. 2003, 85, 85–95. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-inflammatory Actions of Heme and Other Hemoglobin-Derived DAMPs. Front. Immunol. 2020, 30, 1323. [Google Scholar] [CrossRef]
- Helms, C.; Kim-Shapiro, D.B. Hemoglobin-Mediated Nitric Oxide Signaling. Free Radic. Biol. Med. 2013, 61, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Alayash, A.I. Oxygen therapeutics: Can we tame haemoglobin? Nat. Rev. Drug Discov. 2004, 3, 152–159. [Google Scholar] [CrossRef]
- Doherty, D.H.; Doyle, M.P.; Curry, S.R.; Vali, R.J.; Fattor, T.J.; Olson, J.S.; Lemon, D.D. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 1998, 16, 672–676. [Google Scholar] [CrossRef]
- Reeder, B.J. The Redox Activity of Hemoglobins: From Physiologic Functions to Pathologic Mechanisms. Antioxidants Redox Signal. 2010, 13, 1087–1123. [Google Scholar] [CrossRef]
- Bamm, V.V.; Henein, M.E.L.; Sproul, S.L.J.; Lanthier, D.K.; Harauz, G. Potential Role of Ferric Hemoglobin in MS Pathogenesis: Effects of Oxidative Stress and Extracellular Methemoglobin or Its Degradation Products on Myelin Components. Free Radic. Biol. Med. 2017, 112, 494–503. [Google Scholar] [CrossRef]
- Chintagari, N.R.; Jana, S.; Alayash, A.I. Oxidized Ferric and Ferryl Forms of Hemoglobin Trigger Mitochondrial Dysfunction and Injury in Alveolar Type i Cells. Am. J. Respir. Cell Mol. Biol. 2016, 55, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Buehler, P.W.; D’Agnillo, F.; Hoffman, V.; Alayash, A.I. Effects of Endogenous Ascorbate on Oxidation, Oxygenation, and Toxicokinetics of Cell-Free Modified Hemoglobin after Exchange Transfusion in Rat and Guinea Pig. J. Pharmacol. Exp. Ther. 2007, 323, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A Possible Physiological Mediator of Low Density Lipoprotein Oxidation and Endothelial Injury. Arterioscler. Thromb. Vasc. Biol. 1991, 11, 1700–1711. [Google Scholar] [CrossRef] [Green Version]
- Nagy, E.; Eaton, J.W.; Jeney, V.; Soares, M.P.; Varga, Z.; Galajda, Z.; Szentmiklósi, J.; Méhes, G.; Csonka, T.; Smith, A.; et al. Red Cells, Hemoglobin, Heme, Iron, and Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1347–1353. [Google Scholar] [CrossRef]
- Ogawa, K.; Sun, J.; Taketani, S.; Nakajima, O.; Nishitani, C.; Sassa, S.; Hayashi, N.; Yamamoto, M.; Shibahara, S.; Fujita, H.; et al. Heme Mediates Derepression of Maf Recognition Element through Direct Binding to Transcription Repressor Bach1. EMBO J. 2001, 20, 2835–2843. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of Heme as the Ligand for the Orphan Nuclear Receptors REV-ERBα and REV-ERBβ. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Alayash, A.I. Hemoglobin-Based Blood Substitutes: Oxygen Carriers, Pressor Agents, or Oxidants? Nat. Biotechnol. 1999, 17, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Bialas, C.; Moser, C.; Sims, C.A. Artificial Oxygen Carriers and Red Blood Cell Substitutes: A Historic Overview and Recent Developments toward Military and Clinical Relevance. J. Trauma Acute Care Surg. 2019, 87 (Suppl. S1), S48–S58. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.F. Blood substitutes. Haemoglobin therapeutics in clinical practice. Crit. Care 1999, 3, R99–R102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amberson, W.R. Clinical experience with hemoglobin-saline solutions. Science 1947, 106, 117. [Google Scholar] [CrossRef]
- Savitsky, J.P.; Doczi, J.; Black, J.; Arnold, J.D. A clinical safety trial of stroma-free hemoglobin. Clin. Pharmacol. Ther. 1978, 23, 73–80. [Google Scholar] [CrossRef]
- Bunn, H.F.; Esham, W.T.; Bull, R.W. The renal handling of hemoglobin. I. Glomerular filtration. J. Exp. Med. 1969, 129, 909–923. [Google Scholar] [CrossRef]
- Sen Gupta, A. Hemoglobin-based Oxygen Carriers: Current State-of-the-art and Novel Molecules. Shock 2019, 52 (Suppl. S1), 70–83. [Google Scholar] [CrossRef]
- Sakai, H.; Sou, K.; Horinouchi, H.; Kobayashi, K.; Tsuchida, E. Review of hemoglobin-vesicles as artificial oxygen carriers. Artif. Organs 2009, 33, 139–145. [Google Scholar] [CrossRef]
- Hoffman, S.J.; Looker, D.L.; Roehrich, J.M.; Cozart, P.E.; Durfee, S.L.; Tedesco, J.L.; Stetler, G.L. Expression of fully functional tetrameric human hemoglobin in Escherichia coli. Proc. Natl. Acad. Sci. USA 1990, 87, 8521–8525. [Google Scholar] [CrossRef]
- Resta, T.C.; Walker, B.R.; Eichinger, M.R.; Doyle, M.P. Rate of NO scavenging alters effects of recombinant hemoglobin solutions on pulmonary vasoreactivity. J. Appl. Physiol. 2002, 93, 1327–1336. [Google Scholar] [CrossRef] [Green Version]
- Varnado, C.L.; Mollan, T.L.; Birukou, I.; Smith, B.J.; Henderson, D.P.; Olson, J.S. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013, 18, 2314–2328. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Scerbo, M.; Kramer, G. A Review of Blood Substitutes: Examining the History, Clinical Trial Results, and Ethics of Hemoglobin-Based Oxygen Carriers. Clinics 2009, 64, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Lamy, M.L.; Daily, E.K.; Brichant, J.F.; Larbuisson, R.P.; Demeyere, R.H.; Vandermeersch, E.A.; Lehot, J.J.; Parsloe, M.R.; Berridge, J.C.; Sinclair, C.J.; et al. Randomized Trial of Diaspirin Cross-Linked Hemoglobin Solution as an Alternative to Blood Transfusion after Cardiac Surgery. Anesthesiology 2000, 92, 646–656. [Google Scholar] [CrossRef]
- Winslow, R.M. New transfusion strategies: Red Cell Substitutes. Annu. Rev. Med. 1999, 50, 337–353. [Google Scholar] [CrossRef]
- Jahr, S.J.; Moallempour, M.; Lim, C.J. HBOC-201, Hemoglobin Glutamer-250 (Bovine), Hemopure (Biopure Corporation). Expert Opin. Biol. Ther. 2008, 8, 1425–1433. [Google Scholar] [CrossRef]
- Gould, S.A.; Moore, E.E.; Hoyt, D.B.; Burch, J.M.; Haenel, J.B.; Garcia, J.; DeWoskin, R.; Moss, G.S. The First Randomized Trial of Human Polymerized Hemoglobin as a Blood Substitute in Acute Trauma and Emergent Surgery. J. Am. Coll. Surg. 1998, 187, 113–120. [Google Scholar] [CrossRef]
- Cheng, D.C.H.; Mazer, C.D.; Martineau, R.; Ralph-Edwards, A.; Karski, J.; Robblee, J.; Finegan, B.; Hall, R.I.; Latimer, R.; Vuylsteke, A. A Phase II Dose-Response Study of Hemoglobin Raffimer (Hemolink) in Elective Coronary Artery Bypass Surgery. J. Thorac. Cardiovasc. Surg. 2004, 127, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, C.; Ahl, T.; Johansson, T.; Larsson, S.; Nellgård, P.; Ponzer, S.; Fagrell, B.; Przybelski, R.; Keipert, P.; Winslow, N.; et al. A Multicenter Clinical Study of the Safety and Activity of Maleimide-Polyethylene Glycol-Modified Hemoglobin (Hemospan®) in Patients Undergoing Major Orthopedic Surgery. Anesthesiology 2006, 105, 1153–1163. [Google Scholar] [CrossRef]
- Abuchowski, A. SANGUINATE (PEGylated Carboxyhemoglobin Bovine): Mechanism of Action and Clinical Update. Artif. Organs 2017, 41, 346–350. [Google Scholar] [CrossRef]
- Tomita, D.; Kimura, T.; Hosaka, H.; Daijima, Y.; Haruki, R.; Ludwig, K.; Böttcher, C.; Komatsu, T. Covalent Core-Shell Architecture of Hemoglobin and Human Serum Albumin as an Artificial O2 Carrier. Biomacromolecules 2013, 14, 1816–1825. [Google Scholar] [CrossRef]
- Iwasaki, H.; Yokomaku, K.; Kureishi, M.; Igarashi, K.; Hashimoto, R.; Kohno, M.; Iwazaki, M.; Haruki, R.; Akiyama, M.; Asai, K.; et al. Hemoglobin–Albumin Cluster: Physiological Responses after Exchange Transfusion into Rats and Blood Circulation Persistence in Dogs. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S621–S629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.M. Semipermeable microcapsules. Science 1964, 146, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Bugarski, B.; Dovezenski, N.; Stojanovic, N.; Bugarski, D.; Koncern, H. Emulsion Containing Hydrophobic Nanodrops with Bound Hemoglobin Molecules in a Hydrophilic Phase as a Blood Substitute. Deutsches Patentamt DE 2002-10209860 WO 2003074022, 9 December 2003. [Google Scholar]
- Banerjee, U.; Wolfe, S.; O’Boyle, Q.; Cuddington, C.; Palmer, A.F. Scalable production and complete biophysical characterization of poly(ethylene glycol) surface conjugated liposome encapsulated hemoglobin (PEG-LEH). PLoS ONE 2022, 17, e0269939. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Amano, T.; Kamiyama, N.; Takehara, N.; Jingu, M.; Takagi, H.; Sugita, O.; Kobayashi, N.; Kure, T.; Shimizu, T.; et al. First-in-human phase 1 trial of hemoglobin vesicles as artificial red blood cells developed for use as a transfusion alternative. Blood Adv. 2022, 6, 5711–5715. [Google Scholar] [CrossRef] [PubMed]
- Arenberger, P.; Engels, P.; Arenbergerova, M.; Gkalpakiotis, S.; García Luna Martínez, F.J.; Villarreal Anaya, A.; Jimenez Fernandez, L. Clinical Results of the Application of a Hemoglobin Spray to Promote Healing of Chronic Wounds. GMS Krankenhhyg. Interdiszip. 2011, 6, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Elg, F.; Hunt, S. Hemoglobin Spray as Adjunct Therapy in Complex Wounds: Meta-Analysis versus Standard Care Alone in Pooled Data by Wound Type across Three Retrospective Cohort Controlled Evaluations. SAGE Open Med. 2018, 6, 205031211878431. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, Y.; Li, Z.; Li, X.; Jin, Q.; Ji, J. Hemoglobin as a Smart PH-Sensitive Nanocarrier to Achieve Aggregation Enhanced Tumor Retention. Biomacromolecules 2018, 19, 2007–2013. [Google Scholar] [CrossRef]
- Qi, W.; Yan, X.; Duan, L.; Cui, Y.; Yang, Y.; Li, J. Glucose-Sensitive Microcapsules from Glutaraldehyde Cross-Linked Hemoglobin and Glucose Oxidase. Biomacromolecules 2009, 10, 1212–1216. [Google Scholar] [CrossRef]
- Stančić, A.Z.; Drvenica, I.T.; Obradović, H.N.; Bugarski, B.M.; Lj Ilić, V.; Bugarski, D.S. Native Bovine Hemoglobin Reduces Differentiation Capacity of Mesenchymal Stromal Cells in Vitro. Int. J. Biol. Macromol. 2020, 144, 909–920. [Google Scholar] [CrossRef]
- Dutheil, D.; Rousselot, M.; Hauet, T.; Zal, F. Organ-Preserving Composition and Uses. U.S. Patent US 2014/0113274 A1, 24 April 2014. Available online: http://www.freepatentsonline.com/y2014/0113274.html (accessed on 8 November 2022).
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Goodwin, M.B.; Boatman, E.; Theil, E.; Marcus, M.A.; Fakra, S.C. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proc. Biol. Sci. B 2013, 281, 20132741. [Google Scholar] [CrossRef] [Green Version]
- Le Pape, F.; Cosnuau-Kemmat, L.; Gaëlle, R.; Dubrana, F.; Férec, C.; Zal, F.; Leize, E.; Delépine, P. HEMOXCell, a New Oxygen Carrier Usable as an Additive for Mesenchymal Stem Cell Culture in Platelet Lysate-Supplemented Media. Artif. Organs 2017, 41, 359–371. [Google Scholar] [CrossRef]
- Magaldi, A.G.; Ghiretti, F.; Tognon, G.; Zanotti, G. The Structure of the Extracellular Hemoglobin of Annelids. In Invertebrate Oxygen Carriers; Linzen, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar] [CrossRef]
- Zal, F.; Rousselot, M. Extracellular Hemoglobins from Annelids, and Their Potential Use in Biotechnology. In Outstanding Marine Molecules: Chemistry, Biology, Analysis; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 361–376. [Google Scholar]
- Batool, F.; Delpy, E.; Zal, F.; Leize-Zal, E.; Huck, O. Therapeutic Potential of Hemoglobin Derived from the Marine Worm Arenicola Marina (M101): A Literature Review of a Breakthrough Innovation. Mar. Drugs 2021, 19, 376. [Google Scholar] [CrossRef]
- Zal, F.; Green, B.N.; Lallier, F.H.; Vinogradov, S.N.; Toulmond, A. Quaternary Structure of the Extracellular Haemoglobin of the Lugworm Arenicola Marina. A Multi-Angle-Laser-Light-Scattering and Electrospray-Ionisation-Mass-Spectrometry Analysis. Eur. J. Biochem. 1997, 243, 85–92. [Google Scholar] [CrossRef]
- Rousselot, M.; Delpy, E.; La Rochelle, C.D.; Lagente, V.; Pirow, R.; Rees, J.F.; Hagege, A.; Le Guen, D.; Hourdez, S.; Zal, F. Arenicola Marina Extracellular Hemoglobin: A New Promising Blood Substitute. Biotechnol. J. 2006, 1, 333–345. [Google Scholar] [CrossRef]
- Elmer, J.; Palmer, A.F. Biophysical Properties of Lumbricus terrestris Erythrocruorin and Its Potential Use as a Red Blood Cell Substitute. J. Funct. Biomater. 2012, 3, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.A.; Moestrup, S.K. Identification of the Haemoglobin Scavenger Receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Liebman, H.A.; Feinstein, D.I. Thrombosis in Patients with Paroxysmal Noctural Hemoglobinuria Is Associated with Markedly Elevated Plasma Levels of Leukocyte-Derived Tissue Factor. Thromb. Res. 2003, 111, 235–238. [Google Scholar] [CrossRef]
- Setty, B.N.Y.; Betal, S.G.; Zhang, J.; Stuart, M.J. Heme Induces Endothelial Tissue Factor Expression: Potential Role in Hemostatic Activation in Patients with Hemolytic Anemia. J. Thromb. Haemost. 2008, 6, 2202–2209. [Google Scholar] [CrossRef]
- Gonzales, J.; Holbert, K.; Czysz, K.; George, J.; Fernandes, C.; Fraidenburg, D.R. Hemin-Induced Endothelial Dysfunction and Endothelial to Mesenchymal Transition in the Pathogenesis of Pulmonary Hypertension Due to Chronic Hemolysis. Int. J. Mol. Sci. 2022, 23, 4763. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, M.; Lu, Z.; Babu, V.S.; Li, Y.; Shi, F.; Zhan, F.; Liu, C.; Li, J.; Lin, L. The Oxidative Injury of Extracellular Hemoglobin Is Associated with Reactive Oxygen Species Generation of Grass Carp (Ctenopharyngodon idella). Front. Immunol. 2022, 13, 843662. [Google Scholar] [CrossRef]
- Schonberger, S.J.; Edgar, P.F.; Kydd, R.; Faull, R.L.M.; Cooper, G.J.S. Proteomic Analysis of the Brain in Alzheimer’s Disease: Molecular Phenotype of a Complex Disease Process. Proteomics 2001, 1, 1519–1528. [Google Scholar] [CrossRef]
- Pandav, R.S.; Chandra, V.; Dodge, H.H.; DeKosky, S.T.; Ganguli, M. Hemoglobin Levels and Alzheimer Disease: An Epidemiologic Study in India. Am. J. Geriatr. Psychiatry 2004, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Gómez, A.; Carmona, M.; Huesa, G.; Porta, S.; Riera-Codina, M.; Biagioli, M.; Gustincich, S.; Aso, E. Neuronal Hemoglobin Is Reduced in Alzheimer’s Disease, Argyrophilic Grain Disease, Parkinson’s Disease, and Dementia with Lewy Bodies. J. Alzheimer’s Dis. 2011, 23, 537–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altinoz, M.A.; Guloksuz, S.; Schmidt-Kastner, R.; Kenis, G.; Ince, B.; Rutten, B.P.F. Involvement of Hemoglobins in the Pathophysiology of Alzheimer’s Disease. Exp. Gerontol. 2019, 126, 110680. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Alkhayer, K.; Clements, R.; Singhal, N.; Gregory, R.; Azzam, S.; Li, S.; Freeman, E.; McDonough, J. Neuronal Hemoglobin Expression and Its Relevance to Multiple Sclerosis Neuropathology. J. Mol. Neurosci. 2016, 59, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Bamm, V.V.; Lanthier, D.K.; Stephenson, E.L.; Smith, G.S.T.; Harauz, G. In Vitro Study of the Direct Effect of Extracellular Hemoglobin on Myelin Components. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Ley, D.; Romantsik, O.; Vallius, S.; Sveinsdóttir, K.; Sveinsdóttir, S.; Agyemang, A.A.; Baumgarten, M.; Mörgelin, M.; Lutay, N.; Bruschettini, M.; et al. High Presence of Extracellular Hemoglobin in the Periventricular White Matter Following Preterm Intraventricular Hemorrhage. Front. Physiol. 2016, 7, 330. [Google Scholar] [CrossRef] [Green Version]
- Bellos, I.; Pergialiotis, V.; Loutradis, D.; Papapanagiotou, A.; Daskalakis, G. The Role of Hemoglobin Degradation Pathway in Preeclampsia: A Systematic Review and Meta-Analysis. Placenta 2020, 92, 9–16. [Google Scholar] [CrossRef]
- Anderson, U.D.; Jälmby, M.; Faas, M.M.; Hansson, S.R. The Hemoglobin Degradation Pathway in Patients with Preeclampsia—Fetal Hemoglobin, Heme, Heme Oxygenase-1 and Hemopexin—Potential Diagnostic Biomarkers? Pregnancy Hypertens. 2018, 14, 273–278. [Google Scholar] [CrossRef]
- Janz, D.R.; Ware, L.B. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J. Intensive Care. 2015, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Tellone, E.; Barreca, D.; Ficarra, S.; Laganà, G. Implication of COVID-19 on Erythrocytes Functionality: Red Blood Cell Biochemical Implications and Morpho-Functional Aspects. Int. J. Mol. Sci. 2022, 23, 2171. [Google Scholar] [CrossRef]
- Roth, R. Hemoglobin Enhances the Production of Tissue Factor by Endothelial Cells in Response to Bacterial Endotoxin. Blood 1994, 83, 2860–2865. [Google Scholar] [CrossRef]
- McFaul, S.J.; Bowman, P.D.; Villa, V.M.; Gutierrez-Ibanez, M.J.; Johnson, M.; Smith, D. Hemoglobin stimulates mononuclear leukocytes to release interleukin-8 and tumor necrosis factor α. Blood 1994, 84, 3175–3181. [Google Scholar] [CrossRef] [Green Version]
- Wegiel, B.; Hauser, C.J.; Otterbein, L.E. Heme as a Danger Molecule in Pathogen Recognition. Free Radic. Biol. Med. 2015, 89, 651–661. [Google Scholar] [CrossRef]
- Lee, S.K.; Goh, S.Y.; Wong, Y.Q.; Ding, J.L. Response of Neutrophils to Extracellular Haemoglobin and LTA in Human Blood System. EBioMedicine 2015, 2, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; Boyd, T.; Alvarado, C.; Gunn, E.; Adams, J.; Ness, T.; Dunwoody, R.; Lamb, J.; House, B.; Knapp, J.; et al. Reporter Cell Assessment of TLR4-Induced NF-ΚB Responses to Cell-Free Hemoglobin and the Influence of Biliverdin. Biomedicines 2019, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Shaver, C.M.; Upchurch, C.P.; Janz, D.R.; Grove, B.S.; Putz, N.D.; Wickersham, N.E.; Dikalov, S.I.; Ware, L.B.; Bastarache, J.A. Cell-Free Hemoglobin: A Novel Mediator of Acute Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L532–L541. [Google Scholar] [CrossRef] [Green Version]
- Amri, F.; Ghouili, I.; Tonon, M.C.; Amri, M.; Masmoudi-Kouki, O. Hemoglobin-Improved Protection in Cultured Cerebral Cortical Astroglial Cells: Inhibition of Oxidative Stress and Caspase Activation. Front. Endocrinol. 2017, 8, 67. [Google Scholar] [CrossRef]
- Agyemang, A.A.; Kvist, S.V.; Brinkman, N.; Gentinetta, T.; Illa, M.; Ortenlöf, N.; Holmqvist, B.; Ley, D.; Gram, M. Cell-Free Oxidized Hemoglobin Drives Reactive Oxygen Species Production and pro-Inflammation in an Immature Primary Rat Mixed Glial Cell Culture. J. Neuroinflammation 2021, 18, 42. [Google Scholar] [CrossRef]
- Pandya, C.D.; Vekaria, H.; Joseph, B.; Slone, S.A.; Gensel, J.C.; Sullivan, P.G.; Miller, B.A. Hemoglobin Induces Oxidative Stress and Mitochondrial Dysfunction in Oligodendrocyte Progenitor Cells. Transl. Res. 2021, 231, 13–23. [Google Scholar] [CrossRef]
- Biagioli, M.; Pinto, M.; Cesselli, D.; Zaninello, M.; Lazarevic, D.; Roncaglia, P.; Simone, R.; Vlachouli, C.; Plessy, C.; Bertin, N.; et al. Unexpected Expression of α- and β-Globin in Mesencephalic Dopaminergic Neurons and Glial Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15454–15459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, F.; Meurers, B.H.; Zhu, C.; Medvedeva, V.P.; Chesselet, M.F. Neurons Express Hemoglobin α- and β-Chains in Rat and Human Brains. J. Comp. Neurol. 2009, 515, 538–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regan, R.F.; Guo, Y.; Kumar, N. Heme Oxygenase-1 Induction Protects Murine Cortical Astrocytes from Hemoglobin Toxicity. Neurosci. Lett. 2000, 282, 1–4. [Google Scholar] [CrossRef]
- Yang, Y.; Xi, Z.; Xue, Y.; Ren, J.; Sun, Y.; Wang, B.; Zhong, Z.; Yang, G.; Sun, Q.; Bian, L. Hemoglobin Pretreatment Endows Rat Cortical Astrocytes Resistance to Hemin-Induced Toxicity via Nrf2/HO-1 Pathway. Exp. Cell Res. 2017, 361, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Blalock, E.M.; Chen, K.C.; Sharrow, K.; Herman, J.P.; Porter, N.M.; Foster, T.C.; Landfield, P.W. Gene Microarrays in Hippocampal Aging: Statistical Profiling Identifies Novel Processes Correlated with Cognitive Impairment. J. Neurosci. 2003, 23, 3807–3819. [Google Scholar] [CrossRef]
- Schweser, F.; Raffaini Duarte Martins, A.L.; Hagemeier, J.; Lin, F.; Hanspach, J.; Weinstock-Guttman, B.; Hametner, S.; Bergsland, N.; Dwyer, M.G.; Zivadinov, R. Mapping of Thalamic Magnetic Susceptibility in Multiple Sclerosis Indicates Decreasing Iron with Disease Duration: A Proposed Mechanistic Relationship between Inflammation and Oligodendrocyte Vitality. Neuroimage 2018, 167, 438–452. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Petrone, L.; Pietraforte, D.; Siracusano, A.; Margutti, P.; Delunardo, F.; Ortona, E.; Minetti, M.; Salvati, B.; et al. Free Hemoglobin: A Dangerous Signal for the Immune System in Patients with Carotid Atherosclerosis? Ann. N. Y. Acad. Sci. 2007, 1107, 42–50. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Di Cristofano, C.; Pietraforte, D.; Lionetti, V.; Capoano, R.; Salvati, B.; Businaro, R.; Di Giammarco, G.; Riganò, R. Haemoglobin Triggers Chemotaxis of Human Monocyte-Derived Dendritic Cells: Possible Role in Atherosclerotic Lesion Instability. Atherosclerosis 2011, 215, 316–322. [Google Scholar] [CrossRef]
- Posta, N.; Csősz, É.; Oros, M.; Pethő, D.; Potor, L.; Kalló, G.; Hendrik, Z.; Sikura, K.É.; Méhes, G.; Tóth, C.; et al. Hemoglobin Oxidation Generates Globin-Derived Peptides in Atherosclerotic Lesions and Intraventricular Hemorrhage of the Brain, Provoking Endothelial Dysfunction. Lab. Investig. 2020, 100, 986–1002. [Google Scholar] [CrossRef] [Green Version]
- Centlow, M.; Carninci, P.; Nemeth, K.; Mezey, E.; Brownstein, M.; Hansson, S.R. Placental expression profiling in preeclampsia: Local overproduction of hemoglobin may drive pathological changes. Fertil. Steril. 2008, 90, 1834–1843. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.G.; Centlow, M.; Rutardottir, S.; Stenfors, I.; Larsson, J.; Hosseini-Maaf, B.; Olsson, M.L.; Hansson, S.R.; Akerström, B. Increased levels of cell-free hemoglobin, oxidation markers, and the antioxidative heme scavenger α1-microglobulin in preeclampsia. Free Rad. Biol. Med. 2010, 48, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; He, S.; Liu, X.; Jiang, W.; Ye, T.; Lin, Z.; Sang, Y.; Su, C.; Wan, Y.; Shen, G.; et al. Extravascular Red Blood Cells and Hemoglobin Promote Tumor Growth and Therapeutic Resistance as Endogenous Danger Signals. J. Immunol. 2015, 194, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A.; Belcher, D.A.; Munoz, C.; Williams, A.T.; Palmer, A.F.; Cabrales, P. Polymerized human hemoglobin increases the effectiveness of cisplatin-based chemotherapy in non-small cell lung cancer. Oncotarget 2020, 11, 3770–3781. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory Properties of Mesenchymal Stem Cells: Cytokines and Factors. Am. J. Reprod. Immunol. 2012, 67, 1–8. [Google Scholar] [CrossRef] [Green Version]
- de Vasconcellos Machado, C.; da Silva Telles, P.D.; Nascimento, I.L.O. Immunological Characteristics of Mesenchymal Stem Cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged No More: Measuring the Immunogenicity of Allogeneic Adult Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, P.M.; Fuentes-Julian, S.; Blazquez-Martinez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive Properties of Mesenchymal Stem Cells: Advances and Applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef]
- Kukolj, T.; Trivanović, D.; Djordjević, I.O.; Mojsilović, S.; Krstić, J.; Obradović, H.; Janković, S.; Santibanez, J.F.; Jauković, A.; Bugarski, D. Lipopolysaccharide Can Modify Differentiation and Immunomodulatory Potential of Periodontal Ligament Stem Cells via ERK1,2 Signaling. J. Cell Physiol. 2018, 233, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, H.; Krstic, J.; Trivanovic, D.; Mojsilovic, S.; Okic, I.; Kukolj, T.; Ilic, V.; Jaukovic, A.; Terzic, M.; Bugarski, D. Improving Stemness and Functional Features of Mesenchymal Stem Cells from Wharton’s Jelly of a Human Umbilical Cord by Mimicking the Native, Low Oxygen Stem Cell Niche. Placenta 2019, 82, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilopoulou-Sellin, R.; Oyedeji, C.O.; Foster, P.L.; Thompson, M.M.; Saman, N.A. Hemoglobin as a Direct Inhibitor of Cartilage Growth in Vitro. Horm. Metab. Res. 1989, 21, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Yildizgoren, M.T.; Helvaci, M.R.; Ustun, N.; Osmanoglu, K.; Turhanoglu, A.D. Ultrasonographic Assessment of the Distal Femoral Cartilage Thickness in Patients with Homozygous Sickle Cell Disease. Cartilage 2016, 7, 217–221. [Google Scholar] [CrossRef]
- Tajima, T.; Yoshida, E.; Yamashita, A.; Ohmura, S.; Tomitaka, Y.; Sugiki, M.; Asada, Y.; Maruyama, M. Hemoglobin Stimulates the Expression of Matrix Metalloproteinases, MMP-2 and MMP-9 by Synovial Cells: A Possible Cause of Joint Damage after Intra-Articular Hemorrhage. J. Orthop. Res. 2005, 23, 891–898. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yu, W.; Gao, D.; You, G.; Li, P.; Zhang, S.; Zhang, J.; Hu, T.; Zhao, L.; et al. A PEGylated Bovine Hemoglobin as a Potent Hemoglobin-Based Oxygen Carrier. Biotechnol. Prog. 2017, 33, 252–260. [Google Scholar] [CrossRef]
- Kostić, I.T.; Ilić, V.L.; Đorđević, V.B.; Bukara, K.M.; Mojsilović, S.B.; Nedović, V.A.; Bugarski, D.S.; Veljović, D.N.; Mišić, D.M.; Bugarski, B.M. Erythrocyte Membranes from Slaughterhouse Blood as Potential Drug Vehicles: Isolation by Gradual Hypotonic Hemolysis and Biochemical and Morphological Characterization. Colloids Surf. B Biointerfaces 2014, 122, 250–259. [Google Scholar] [CrossRef]
- Stančić, A.Z.; Drvenica, I.T.; Ilić, V.L.; Bugarski, B.M.; Bugarski, D.S. Modulation of Functional Characteristics of Mesenchymal Stromal Cells by Acellular Preparation of Porcine Hemoglobin. Processes 2022, 10, 32. [Google Scholar] [CrossRef]
- Borriello, A.; Caldarelli, I.; Speranza, M.C.; Scianguetta, S.; Tramontano, A.; Bencivenga, D.; Stampone, E.; Negri, A.; Nobili, B.; Locatelli, F.; et al. Iron Overload Enhances Human Mesenchymal Stromal Cell Growth and Hampers Matrix Calcification. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1211–1223. [Google Scholar] [CrossRef]
- Tang, A.; Strat, A.N.; Rahman, M.; Zhang, H.; Bao, W.; Liu, Y.; Shi, D.; An, X.; Manwani, D.; Shi, P.; et al. Murine Bone Marrow Mesenchymal Stromal Cells Have Reduced Hematopoietic Maintenance Ability in Sickle Cell Disease. Blood 2021, 138, 2570–2582. [Google Scholar] [CrossRef]
- Crippa, S.; Rossella, V.; Aprile, A.; Silvestri, L.; Rivis, S.; Scaramuzza, S.; Pirroni, S.; Avanzini, M.A.; Basso-Ricci, L.; Hernandez, R.J.; et al. Bone Marrow Stromal Cells from β-Thalassemia Patients Have Impaired Hematopoietic Supportive Capacity. J. Clin. Investig. 2019, 129, 1566–1580. [Google Scholar] [CrossRef] [Green Version]
- Stančić, A.Z.; Drvenica, I.T.; Bugarski, B.M.; Ilić, V.L.; Bugarski, D.S. Extracellular Xenogeneic Hemoglobin Suppresses the Capacity for C2C12 Myoblast Myogenic Differentiation. Arch. Biol. Sci. 2020, 72, 379–391. [Google Scholar] [CrossRef]
- Hirsch, R.E.; Jelicks, L.A.; Wittenberg, B.A.; Kaul, D.K.; Shear, H.L.; Harrington, J.P. A first evaluation of the natural high molecular weight polymeric Lumbricus terrestris hemoglobin as an oxygen carrier. Artif. Cells Blood Substit. Immobil. Biotechnol. 1997, 25, 429–444. [Google Scholar] [CrossRef]
- Harrington, J.P.; Kobayashi, S.; Dorman, S.C.; Zito, S.L.; Hirsch, R.E. Acellular invertebrate hemoglobins as model therapeutic oxygen carriers: Unique redox potentials. Artif. Cells Blood Substit. Immobil. Biotechnol. 2007, 35, 53–67. [Google Scholar] [CrossRef]
- Mot, A.C.; Roman, A.; Lupan, I.; Kurtz, D.M., Jr.; Silaghi-Dumitrescu, R. Towards the development of hemerythrin-based blood substitutes. Protein J. 2010, 29, 387–393. [Google Scholar] [CrossRef]
- Muzzelo, C.; Neely, C.; Shah, P.; Abdulmalik, O.; Elmer, J. Prolonging the shelf life of Lumbricus terrestris erythrocruorin for use as a novel blood substitute. Artif. Cells Nanomed. Biotechnol. 2018, 46, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Elmer, J.; Zorc, K.; Rameez, S.; Zhou, Y.; Cabrales, P.; Palmer, A.F. Hypervolemic infusion of Lumbricus terrestris erythrocruorin purified by tangential-flow filtration. Transfusion 2012, 52, 1729–1740. [Google Scholar] [CrossRef]
- Elmer, J.; Palmer, A.F.; Cabrales, P. Oxygen delivery during extreme anemia with ultra-pure earthworm hemoglobin. Life Sci. 2012, 91, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Mallet, V.; Dutheil, D.; Polard, V.; Rousselot, M.; Leize, E.; Hauet, T.; Goujon, J.M.; Zal, F. Dose-Ranging Study of the Performance of the Natural Oxygen Transporter HEMO2life in Organ Preservation. Artif. Organs 2014, 38, 691–701. [Google Scholar] [CrossRef]
- Asong-Fontem, N.; Panisello-Rosello, A.; Lopez, A.; Imai, K.; Zal, F.; Delpy, E.; Rosello-Catafau, J.; Adam, R. A Novel Oxygen Carrier (M101) Attenuates Ischemia-Reperfusion Injuries during Static Cold Storage in Steatotic Livers. Int. J. Mol. Sci. 2021, 22, 8542. [Google Scholar] [CrossRef] [PubMed]
- Alix, P.; Val-Laillet, D.; Turlin, B.; Ben Mosbah, I.; Burel, A.; Bobillier, E.; Bendavid, C.; Delpy, E.; Zal, F.; Corlu, A.; et al. Adding the Oxygen Carrier M101 to a Cold-Storage Solution Could Be an Alternative to HOPE for Liver Graft Preservation. JHEP Rep. 2020, 2, 100119. [Google Scholar] [CrossRef] [PubMed]
- Lupon, E.; Lellouch, A.G.; Zal, F.; Cetrulo, C.L.; Lantieri, L.A. Combating Hypoxemia in COVID-19 Patients with a Natural Oxygen Carrier, HEMO2Life® (M101). Med. Hypotheses 2021, 146, 110421. [Google Scholar] [CrossRef]
- Batool, F.; Stutz, C.; Petit, C.; Benkirane-Jessel, N.; Delpy, E.; Zal, F.; Leize-Zal, E.; Huck, O. A Therapeutic Oxygen Carrier Isolated from Arenicola Marina Decreased P. Gingivalis Induced Inflammation and Tissue Destruction. Sci. Rep. 2020, 10, 14745. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, H.; Batool, F.; Corre, M.; Garlaschelli, A.; Conzatti, G.; Stutz, C.; Petit, C.; Delpy, E.; Zal, F.; Leize-Zal, E.; et al. Characterization of a Hyaluronic Acid-Based Hydrogel Containing an Extracellular Oxygen Carrier (M101) for Periodontitis Treatment: An in Vitro Study. Int. J. Pharm. 2021, 605, 120810. [Google Scholar] [CrossRef]
- Le Daré, B.; Ferron, P.J.; Bellamri, N.; Ribault, C.; Delpy, E.; Zal, F.; Lagente, V.; Gicquel, T. A Therapeutic Oxygen Carrier Isolated from Arenicola Marina Decreases Amanitin-Induced Hepatotoxicity. Toxicon 2021, 200, 87–91. [Google Scholar] [CrossRef]
- Le Pape, F.; Bossard, M.; Dutheil, D.; Rousselot, M.; Polard, V.; Férec, C.; Leize, E.; Delépine, P.; Zal, F. Advancement in Recombinant Protein Production Using a Marine Oxygen Carrier to Enhance Oxygen Transfer in a CHO-S Cell Line. Artif. Cells Nanomed. Biotechnol. 2015, 43, 186–195. [Google Scholar] [CrossRef]
- Le Pape, F.; Gaëlle, R.; Porchet, E.; Sourice, S.; Dubrana, F.; Férec, C.; Polard, V.; Pace, R.; Weiss, P.; Zal, F.; et al. Adhesion, Proliferation and Osteogenic Differentiation of Human MSCs Cultured under Perfusion with a Marine Oxygen Carrier on an Allogenic Bone Substitute. Artif. Cells Nanomed. Biotechnol. 2017, 46, 95–107. [Google Scholar] [CrossRef]
- Rodriguez-Brotons, A.; Bietiger, W.; Peronet, C.; Langlois, A.; Magisson, J.; Mura, C.; Sookhareea, C.; Polard, V.; Jeandidier, N.; Zal, F.; et al. Comparison of Perfluorodecalin and HEMOXCell as Oxygen Carriers for Islet Oxygenation in an in Vitro Model of Encapsulation. Tissue Eng. Part A 2016, 22, 1327–1336. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drvenica, I.T.; Stančić, A.Z.; Maslovarić, I.S.; Trivanović, D.I.; Ilić, V.L. Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects. Biomolecules 2022, 12, 1708. https://doi.org/10.3390/biom12111708
Drvenica IT, Stančić AZ, Maslovarić IS, Trivanović DI, Ilić VL. Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects. Biomolecules. 2022; 12(11):1708. https://doi.org/10.3390/biom12111708
Chicago/Turabian StyleDrvenica, Ivana T., Ana Z. Stančić, Irina S. Maslovarić, Drenka I. Trivanović, and Vesna Lj. Ilić. 2022. "Extracellular Hemoglobin: Modulation of Cellular Functions and Pathophysiological Effects" Biomolecules 12, no. 11: 1708. https://doi.org/10.3390/biom12111708







