SRF Rearrangements in Soft Tissue Tumors with Muscle Differentiation
Abstract
:1. Introduction
2. Materials and Methods
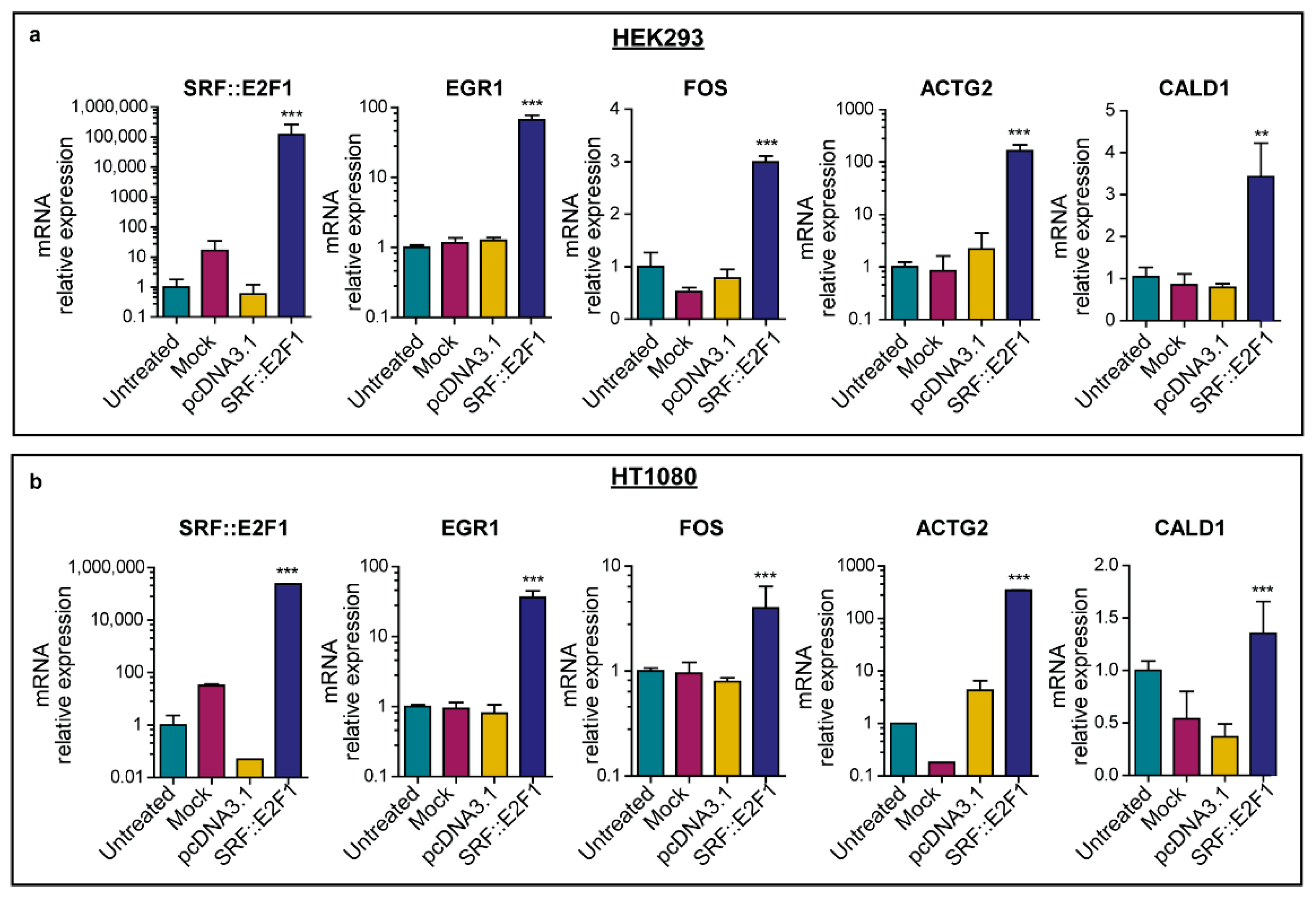
2.1. Cell Culture and Transfection of SRF::E2F1
2.2. RNA-Sequencing
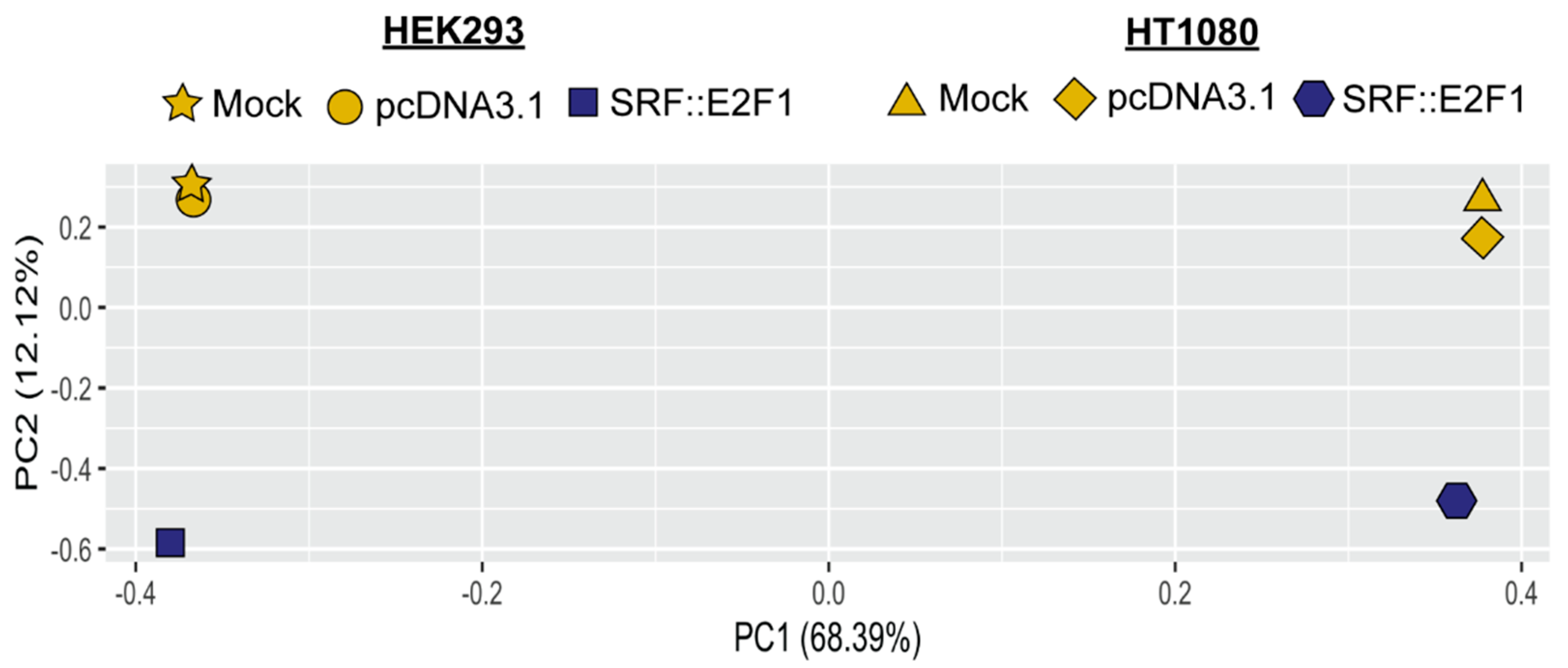
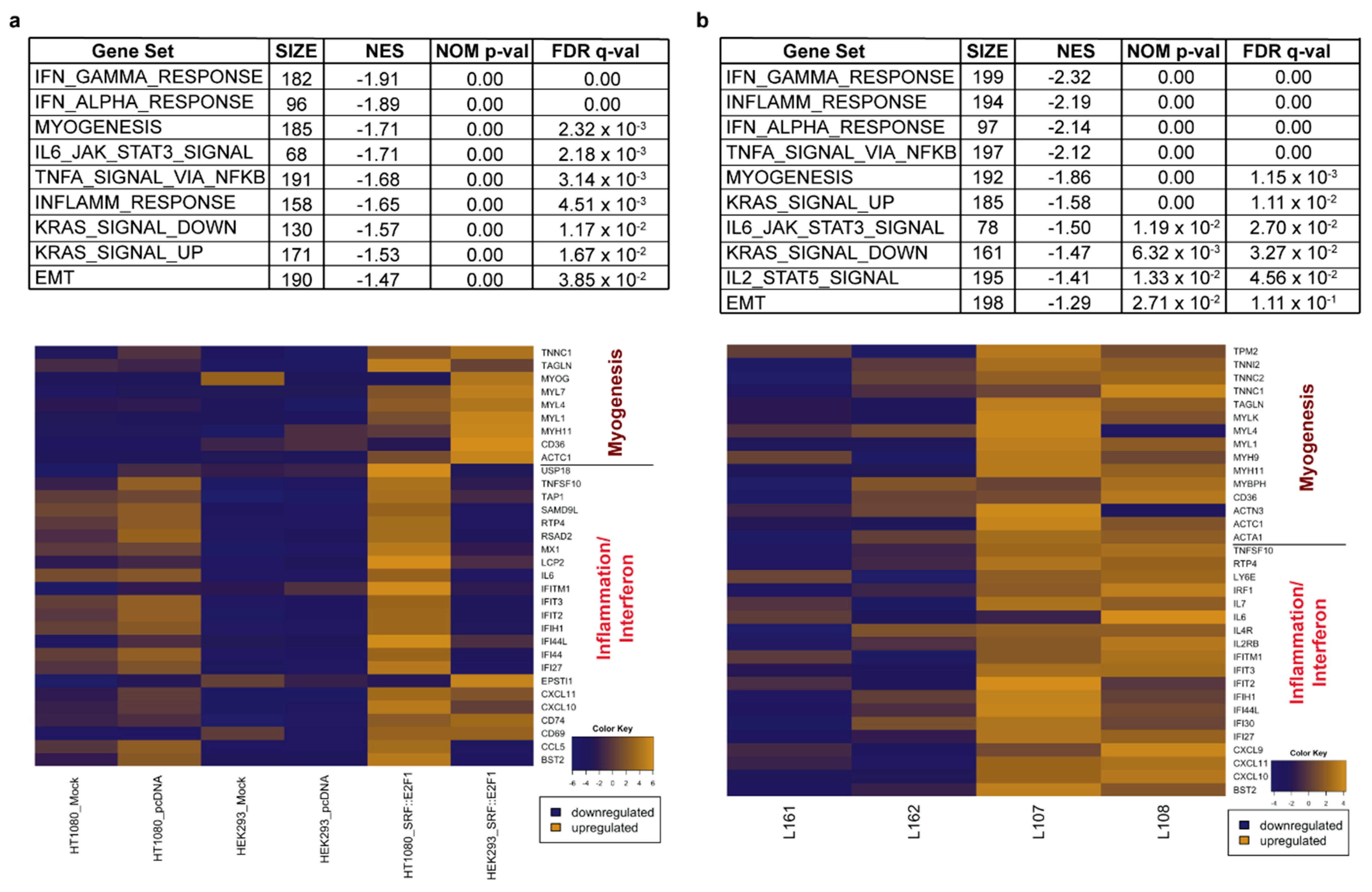
2.3. Bioinformatic Analysis
2.4. Real-Time PCR Analysis
3. Results
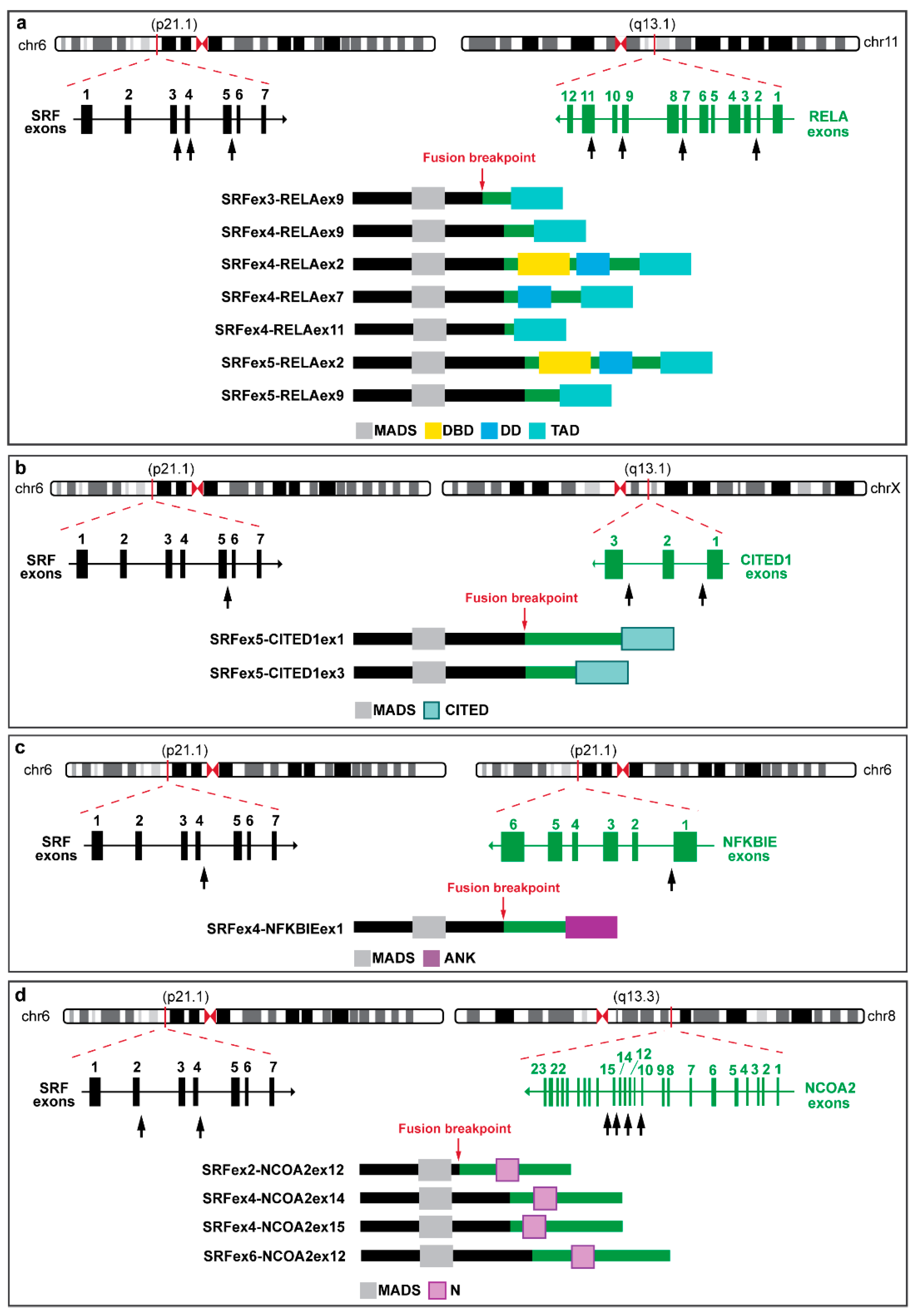
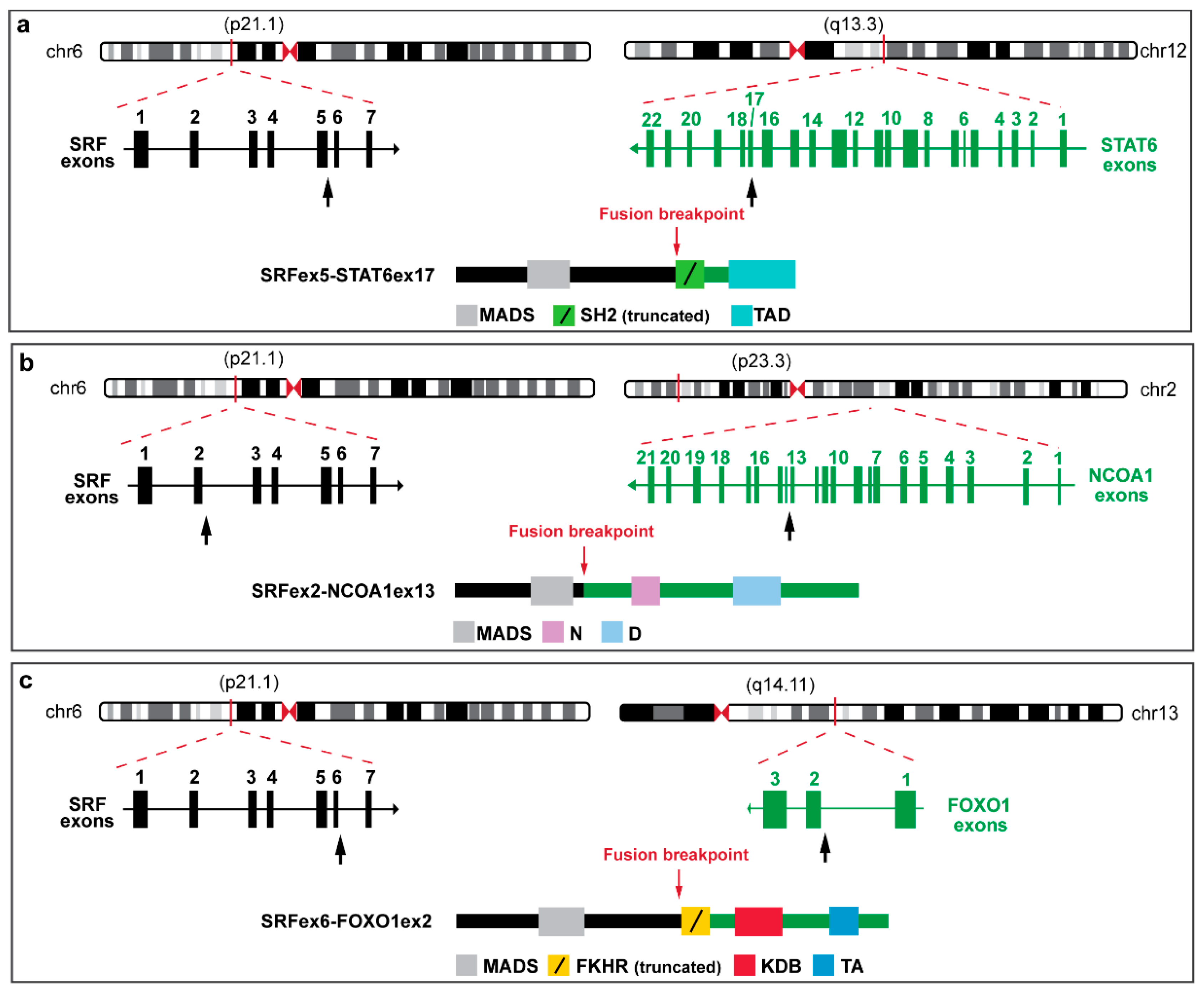
3.1. SRF Rearrangement in Myoepithelial Neoplasms
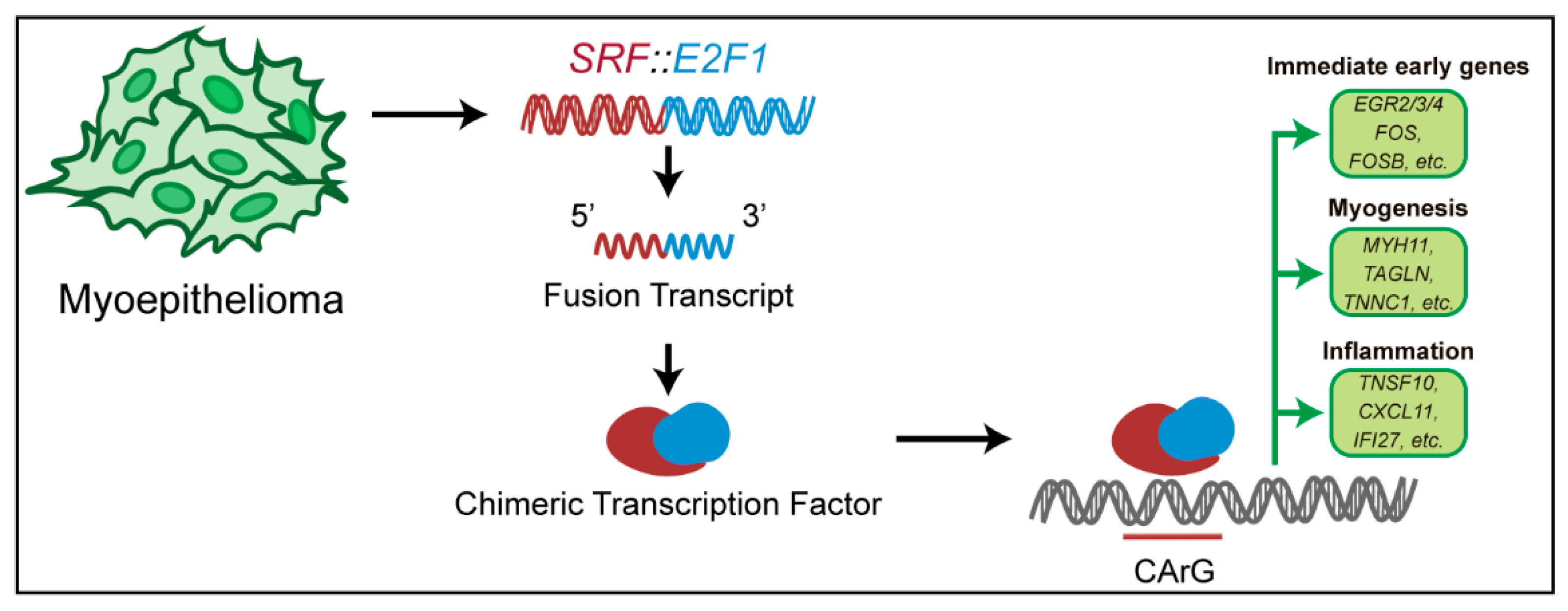
3.2. Gene Expression Analysis Driven by SRF Fusion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mertens, F.; Antonescu, C.R.; Mitelman, F. Gene Fusions in Soft Tissue Tumors: Recurrent and Overlapping Pathogenetic Themes. Genes Chromosom. Cancer 2016, 55, 291–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, F.; Johansson, B.; Fioretos, T.; Mitelman, F. The Emerging Complexity of Gene Fusions in Cancer. Nat. Rev. Cancer 2015, 15, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lanic, M.D.; Le Loarer, F.; Rainville, V.; Sater, V.; Viennot, M.; Beaussire, L.; Viailly, P.J.; Angot, E.; Hostein, I.; Jardin, F.; et al. Detection of Sarcoma Fusions by a Next-Generation Sequencing Based–Ligation-Dependent Multiplex RT-PCR Assay. Mod. Pathol. 2022, 35, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Sung, Y.; Zhang, L.; Agaram, N.P.; Fletcher, C.D. Recurrent SRF-RELA Fusions Define a Novel Subset of Cellular Variants in the Myofibroma/Myopericytoma Spectrum: A Potential Diagnostic Pitfall with Sarcomas with Myogenic Differentiation. Am J Surg Pathol. 2017, 41, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Karanian, M.; Kelsey, A.; Paindavoine, S.; Duc, A.; Vanacker, H.; Hook, L.; Weinbreck, N.; Delfour, C.; Minard, V.; Baillard, P.; et al. SRF Fusions Other Than with RELA Expand the Molecular Definition of SRF -Fused Perivascular Tumors. Am. J. Surg. Pathol. 2020, 44, 1725–1735. [Google Scholar] [CrossRef]
- Li, Y.; Huang, D.; Bi, R.; Zhou, X.; Yang, W. Uterine Tumours with Myogenic Differentiation Harbouring SRF :: RELA Fusions. Histopathology 2022, 81, 477–485. [Google Scholar] [CrossRef]
- Suurmeijer, A.J.; Dickson, B.C.; Swanson, D.; Sung, Y.S.; Zhang, L.; Antonescu, C.R. Novel SRF-ICA1L Fusions in Cellular Myoid Neoplasms with Potential for Malignant Behavior. Am. J. Surg. Pathol. 2020, 44, 55–60. [Google Scholar] [CrossRef]
- Karanian, M.; Pissaloux, D.; Gomez-Brouchet, A.; Chevenet, C.; Le Loarer, F.; Fernandez, C.; Minard, V.; Corradini, N.; Castex, M.P.; Duc-Gallet, A.; et al. SRF-FOXO1 and SRF-NCOA1 Fusion Genes Delineate a Distinctive Subset of Well-Differentiated Rhabdomyosarcoma. Am. J. Surg. Pathol. 2020, 44, 607–616. [Google Scholar] [CrossRef]
- Dachy, G.; Fraitag, S.; Boulouadnine, B.; Cordi, S.; Demoulin, J.B. Novel COL4A1-VEGFD Gene Fusion in Myofibroma. J. Cell. Mol. Med. 2021, 25, 4387–4394. [Google Scholar] [CrossRef]
- Urbini, M.; Astolfi, A.; Indio, V.; Tarantino, G.; Serravalle, S.; Saponara, M.; Nannini, M.; Gronchi, A.; Fiore, M.; Maestro, R.; et al. Identification of SRF-E2F1 Fusion Transcript in EWSR-Negative Myoepithelioma of the Soft Tissue. Oncotarget 2017, 8, 60036–60045. [Google Scholar] [CrossRef]
- Schratt, G.; Weinhold, B.; Lundberg, A.S.; Schuck, S.; Berger, J.; Schwarz, H.; Weinberg, R.A.; Rüther, U.; Nordheim, A. Serum Response Factor Is Required for Immediate-Early Gene Activation yet Is Dispensable for Proliferation of Embryonic Stem Cells. Mol. Cell. Biol. 2001, 21, 2933–2943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coletti, D.; Daou, N.; Hassani, M.; Li, Z.; Parlakian, A. Serum Response Factor in Muscle Tissues: From Development to Ageing. Eur. J. Transl. Myol. 2016, 26, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Colletti, M.; Galardi, A.; Miele, E.; Di Paolo, V.; Russo, I.; De Stefanis, C.; De Vito, R.; Rinelli, M.; Ciolfi, A.; De Angelis, B.; et al. Establishment and Characterization of a Cell Line (S-Rms1) Derived from an Infantile Spindle Cell Rhabdomyosarcoma with Srf-Ncoa2 Fusion Transcript. Int. J. Mol. Sci. 2021, 22, 5484. [Google Scholar] [CrossRef]
- Pipes, G.C.T.; Creemers, E.E.; Olson, E.N. The Myocardin Family of Transcriptional Coactivators: Versatile Regulators of Cell Growth, Migration, and Myogenesis. Genes Dev. 2006, 20, 1545–1556. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Liu, Z.; Yang, C.; Gu, L.; Deng, D. SRF Promotes Gastric Cancer Metastasis through Stromal Fibroblasts in an SDF1-CXCR4-Dependent Manner. Oncotarget 2016, 7, 46088–46099. [Google Scholar] [CrossRef] [Green Version]
- Park, M.Y.; Kim, K.R.; Park, H.S.; Park, B.H.; Choi, H.N.; Jang, K.Y.; Chung, M.J.; Kang, M.J.; Lee, D.G.; Moon, W.S. Expression of the Serum Response Factor in Hepatocellular Carcinoma: Implications for Epithelial-Mesenchymal Transition. Int. J. Oncol. 2007, 31, 1309–1315. [Google Scholar] [CrossRef] [Green Version]
- Liontos, M.; Niforou, K.; Velimezi, G.; Vougas, K.; Evangelou, K.; Apostolopoulou, K.; Vrtel, R.; Damalas, A.; Kontovazenitis, P.; Kotsinas, A.; et al. Modulation of the E2F1-Driven Cancer Cell Fate by the DNA Damage Response Machinery and Potential Novel E2F1 Targets in Osteosarcomas. Am. J. Pathol. 2009, 175, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Lin, M.; Li, C.; Liu, H.; Gong, C. A Comprehensive Review of the Roles of E2F1 in Colon Cancer. Am. J. Cancer Res. 2020, 10, 757–768. [Google Scholar]
- Alonso, M.M.; Alemany, R.; Fueyo, J.; Gomez-Manzano, C. E2F1 in Gliomas: A Paradigm of Oncogene Addiction. Cancer Lett. 2008, 263, 157–163. [Google Scholar] [CrossRef]
- Halaban, R.; Cheng, E.; Smicun, Y.; Germino, J. Deregulated E2F Transcriptional Activity in Autonomously Growing Melanoma Cells. J. Exp. Med. 2000, 191, 1005–1015. [Google Scholar] [CrossRef]
- Dray, M.S.; McCarthy, S.W.; Palmer, A.A.; Bonar, S.F.; Stalley, P.D.; Marjoniemi, V.; Millar, E.; Scolyer, R.A. Myopericytoma: A Unifying Term for a Spectrum of Tumours That Show Overlapping Features with Myofibroma. A Review of 14 Cases. J. Clin. Pathol. 2006, 59, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.M.; Sboner, A.; Zhang, L.; Kitabayashi, L.; Chen, C.L.; Sung, Y.S.; Wexler, L.H.; LaQuaglia, M.P.; Edelman, M.; Sreekantaiah, C.; et al. Recurrent NCOA2 Gene Rearrangements in Congenital/Infantile Spindle Cell Rhabdomyosarcoma Juan. Genes Chromosom. Cancer 2013, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaggio, R.; Zhang, L.; Sung, Y.S.; Huang, S.C.; Chen, C.L.; Bisogno, G.; Zin, A.; Agaram, N.P.; LaQuaglia, M.P.; Wexler, L.H.; et al. A Molecular Study of Pediatric Spindle and Sclerosing Rhabdomyosarcoma: Identification of Novel and Recurrent VGLL2-Related Fusions in Infantile Cases Rita. Am. J. Surg. Pathol. 2016, 40, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nihous, H.; Macagno, N.; Baud-Massière, J.; Haffner, A.; Jouve, J.L.; Gentet, J.C.; Touzery, C.; Le Loarer, F.; Bouvier, C. Genetic Variant of SRF-Rearranged Myofibroma with a Misleading Nuclear Expression of STAT6 and STAT6 Involvement as 3′ Fusion Partner. Virchows Arch. 2021, 478, 597–603. [Google Scholar] [CrossRef]
- Jo, V.Y. Soft Tissue Special Issue: Myoepithelial Neoplasms of Soft Tissue: An Updated Review with Emphasis on Diagnostic Considerations in the Head and Neck. Head Neck Pathol. 2020, 14, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Sullivan, A.L.; Collier, J.G.; Glass, C.K. Serum Response Factor Indirectly Regulates Type i Interferon-Signaling in Macrophages. J. Interf. Cytokine Res. 2013, 33, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Tang, W.; Bruscia, E.M.; Zhang, P.X.; Lin, A.; Gaines, P.; Wu, D.; Halene, S. SRF Is Required for Neutrophil Migration in Response to Inflammation. Blood 2014, 123, 3027–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Yu, C.; Xiang, K.; Gao, M.; Liu, Z.; Yang, B.; Yang, M.; Zhao, S. Inhibition of ANXA2 Regulated by SRF Attenuates the Development of Severe Acute Pancreatitis by Inhibiting the NF-ΚB Signaling Pathway. Inflamm. Res. 2022, 71, 1067–1078. [Google Scholar] [CrossRef]
- Sprenkeler, E.G.G.; Guenther, C.; Faisal, I.; Kuijpers, T.W.; Fagerholm, S.C. Molecular Mechanisms of Leukocyte Migration and Its Potential Targeting—Lessons Learned From MKL1/SRF-Related Primary Immunodeficiency Diseases. Front. Immunol. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Liu, L.; Bankell, E.; Rippe, C.; Morén, B.; Stenkula, K.G.; Nilsson, B.O.; Swärd, K. Cell Type Dependent Suppression of Inflammatory Mediators by Myocardin Related Transcription Factors. Front. Physiol. 2021, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Gozzellino, L.; Urbini, M.; Indio, V.; Nannini, M.; Pantaleo, M.A.; Stacchiotti, S.; Astolfi, A.; Pasquinelli, G. SRF Rearrangements in Soft Tissue Tumors with Muscle Differentiation. Biomolecules 2022, 12, 1678. https://doi.org/10.3390/biom12111678
Costa A, Gozzellino L, Urbini M, Indio V, Nannini M, Pantaleo MA, Stacchiotti S, Astolfi A, Pasquinelli G. SRF Rearrangements in Soft Tissue Tumors with Muscle Differentiation. Biomolecules. 2022; 12(11):1678. https://doi.org/10.3390/biom12111678
Chicago/Turabian StyleCosta, Alice, Livia Gozzellino, Milena Urbini, Valentina Indio, Margherita Nannini, Maria Abbondanza Pantaleo, Silvia Stacchiotti, Annalisa Astolfi, and Gianandrea Pasquinelli. 2022. "SRF Rearrangements in Soft Tissue Tumors with Muscle Differentiation" Biomolecules 12, no. 11: 1678. https://doi.org/10.3390/biom12111678





