Heterogeneous Clinical Phenotypes of dHMN Caused by Mutation in HSPB1 Gene: A Case Series
Abstract
:1. Introduction
2. Methods and Materials
2.1. Clinical Data Collection
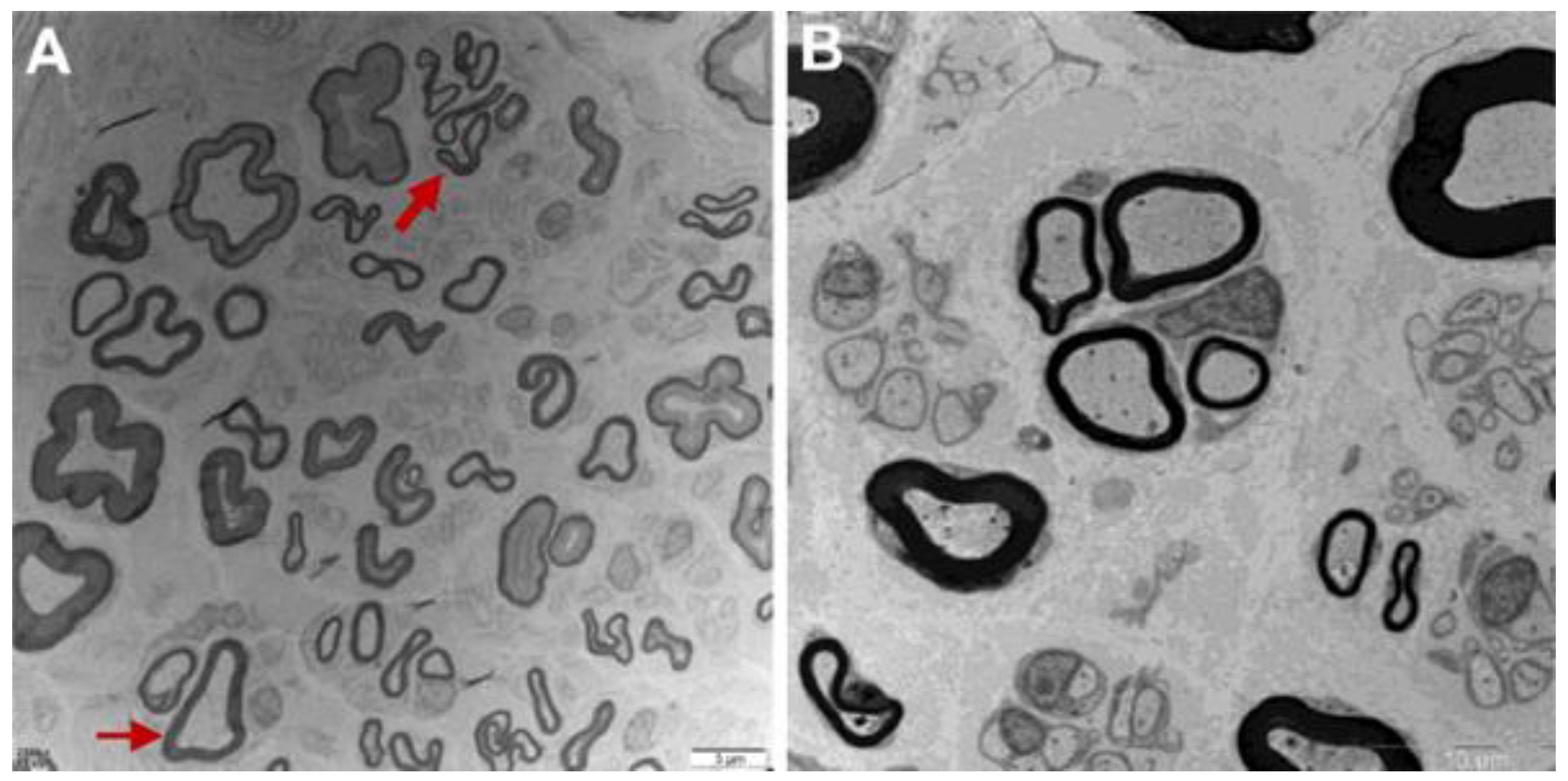
2.2. Sural Nerve Biopsy and Ultrastructural Observation
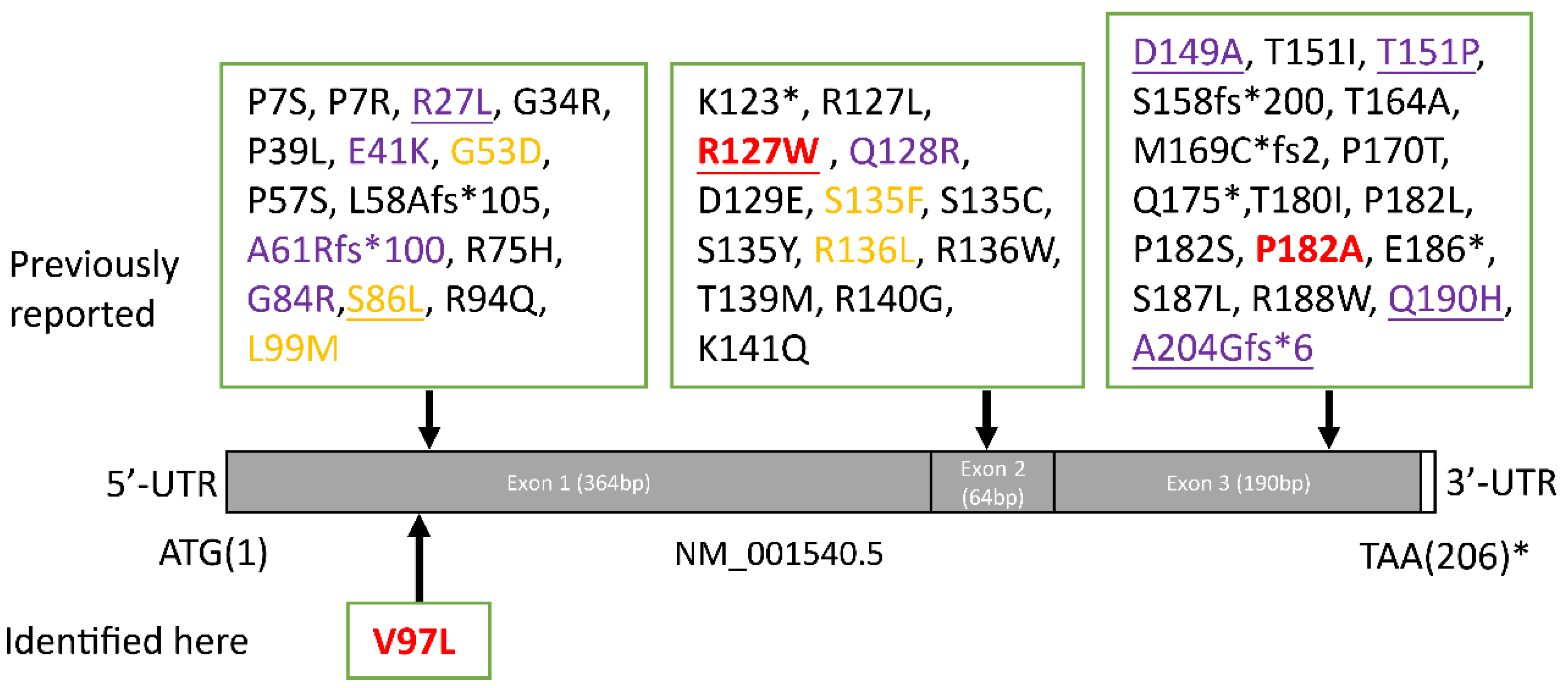
2.3. Genetic Analysis
2.4. Cell Culture
2.5. Transfection
2.6. Cell Viability Assay and Morphological Analysis
2.7. TUNEL Assay
2.8. Western Blot Analysis
3. Results
3.1. Clinical Data
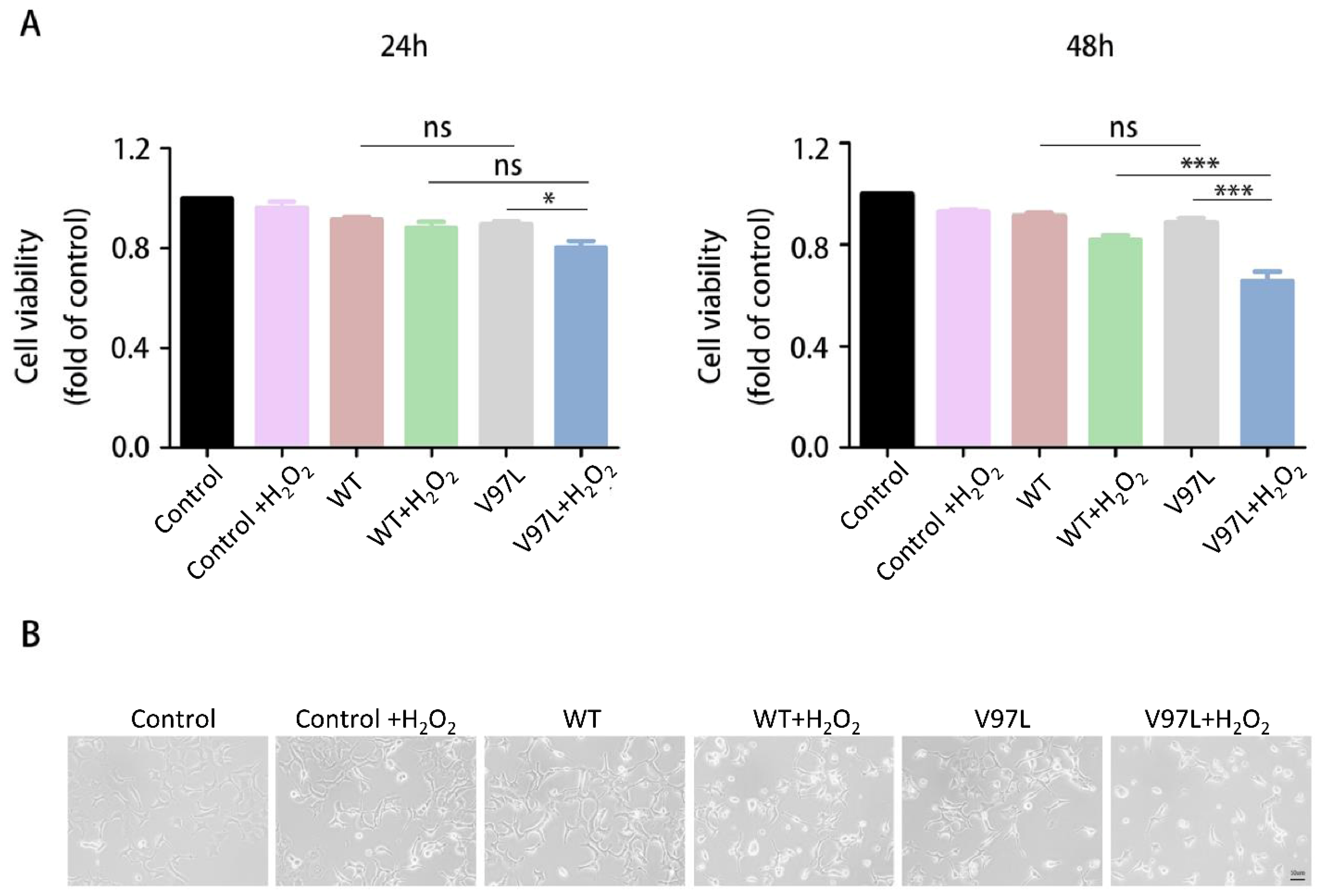
3.2. Effect of HSPB1-p.V97L Mutation on H2O2-Treated SH-SY5Y Cells
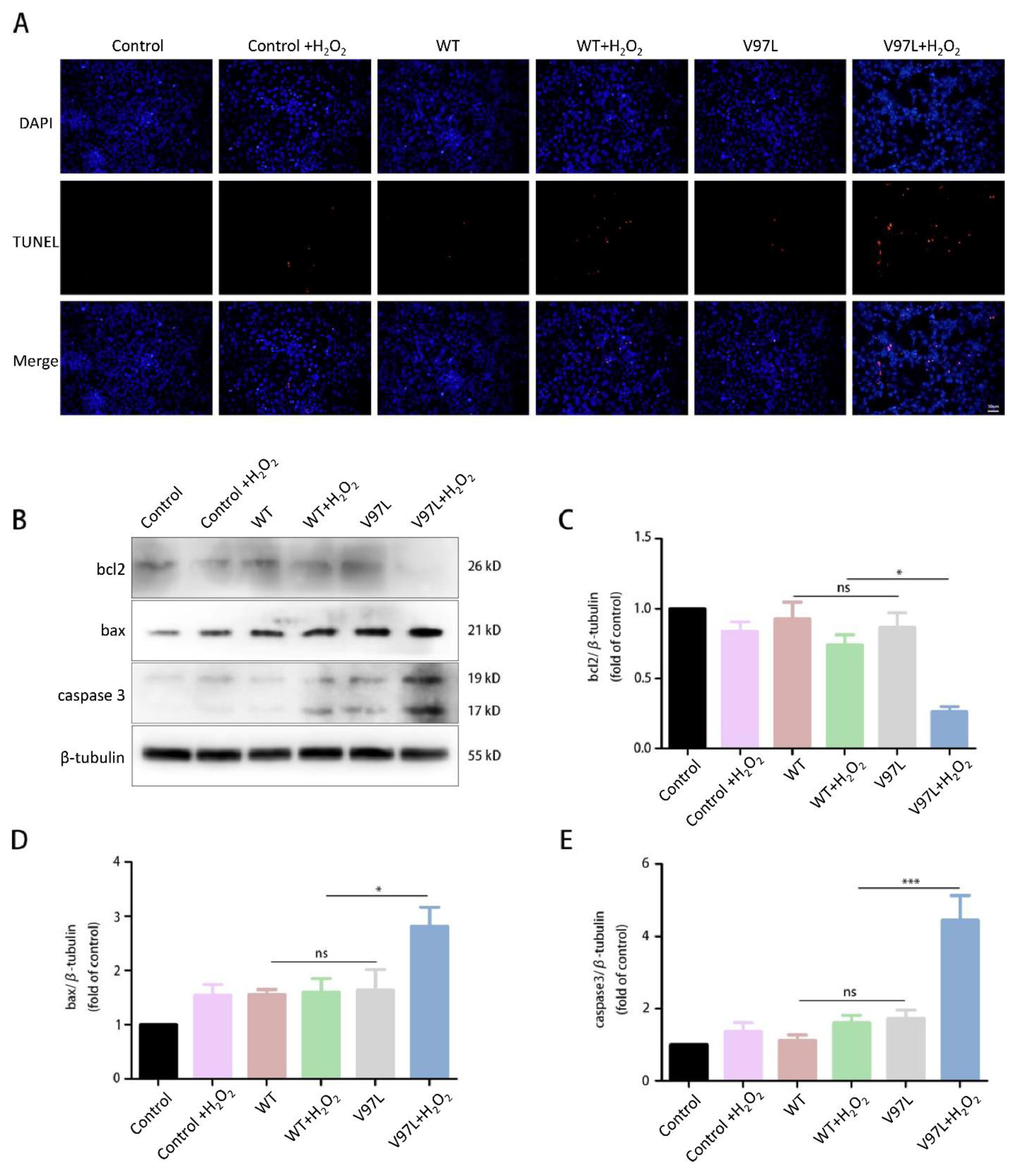
3.3. Effects of HSPB1-p.V97L Mutation on H2O2-Induced Apoptosis in SH-SY5Y Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismailov, S.M.; Fedotov, V.P.; Dadali, E.L.; Polyakov, A.V.; Van Broeckhoven, C.; Ivanov, V.I.; De Jonghe, P.; Timmerman, V.; Evgrafov, O.V. A new locus for autosomal dominant Charcot-Marie-Tooth disease type 2 (CMT2F) maps to chromosome 7q11-q21. Eur. J. Hum. Genet. 2001, 9, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Evgrafov, O.V.; Mersiyanova, I.; Irobi, J.; Van Den Bosch, L.; Dierick, I.; Leung, C.L.; Schagina, O.; Verpoorten, N.; Van Impe, K.; Fedotov, V.; et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 2004, 36, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Davis, M.; Garton, F.C.; Henderson, R.; Bharti, V.; Wray, N.; McCombe, P. Mutations in heat shock protein beta-1 (HSPB1) are associated with a range of clinical phenotypes related to different patterns of motor neuron dysfunction: A case series. J. Neurol. Sci. 2020, 413, 116809. [Google Scholar] [CrossRef] [PubMed]
- Houlden, H.; Laura, M.; Wavrant-De, V.F.; Blake, J.; Wood, N.; Reilly, M.M. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology 2008, 71, 1660–1668. [Google Scholar] [CrossRef]
- Abati, E.; Magri, S.; Meneri, M.; Manenti, G.; Velardo, D.; Balistreri, F.; Pisciotta, C.; Saveri, P.; Bresolin, N.; Comi, G.P.; et al. Charcot-Marie-Tooth disease type 2F associated with biallelic HSPB1 mutations. Ann. Clin. Transl. Neurol. 2021, 8, 1158–1164. [Google Scholar] [CrossRef]
- Greenbaum, L.; Ben-David, M.; Nikitin, V.; Gera, O.; Barel, O.; Hersalis-Eldar, A.; Shamash, J.; Shimshoviz, N.; Reznik-Wolf, H.; Shohat, M.; et al. Early and late manifestations of neuropathy due to HSPB1 mutation in the Jewish Iranian population. Ann. Clin. Transl. Neurol. 2021, 8, 1260–1268. [Google Scholar] [CrossRef]
- Amornvit, J.; Yalvac, M.E.; Chen, L.; Sahenk, Z. A novel p.T139M mutation in HSPB1 highlighting the phenotypic spectrum in a family. Brain Behav. 2017, 7, e774. [Google Scholar] [CrossRef]
- Solla, P.; Vannelli, A.; Bolino, A.; Marrosu, G.; Coviello, S.; Murru, M.R.; Tranquilli, S.; Corongiu, D.; Benedetti, S.; Marrosu, M.G. Heat shock protein 27 R127W mutation: Evidence of a continuum between axonal Charcot-Marie-Tooth and distal hereditary motor neuropathy. J. Neurol. Neurosurg. Psychiatry 2010, 81, 958–962. [Google Scholar] [CrossRef]
- Capponi, S.; Geuens, T.; Geroldi, A.; Origone, P.; Verdiani, S.; Cichero, E.; Adriaenssens, E.; De Winter, V.; Bandettini di Poggio, M.; Barberis, M.; et al. Molecular Chaperones in the Pathogenesis of Amyotrophic Lateral Sclerosis: The Role of HSPB1. Hum. Mutat. 2016, 37, 1202–1208. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Xu, Y.; Fan, D. Rare variants of HSPB1 are probably associated with amyotrophic lateral sclerosis. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 75–78. [Google Scholar]
- Scarlato, M.; Viganò, F.; Carrera, P.; Previtali, S.C.; Bolino, A. A novel heat shock protein 27 homozygous mutation: Widening of the continuum between MND/dHMN/CMT2. J. Peripher. Nerv. Syst. 2015, 20, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Frasquet, M.; Rojas-García, R.; Argente-Escrig, H.; Vázquez-Costa, J.F.; Muelas, N.; Vílchez, J.J.; Sivera, R.; Millet, E.; Barreiro, M.; Díaz-Manera, J.; et al. Distal hereditary motor neuropathies: Mutation spectrum and genotype-phenotype correlation. Eur. J. Neurol. 2021, 28, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Capponi, S.; Geroldi, A.; Fossa, P.; Grandis, M.; Ciotti, P.; Gulli, R.; Schenone, A.; Mandich, P.; Bellone, E. HSPB1 and HSPB8 in inherited neuropathies: Study of an Italian cohort of dHMN and CMT2 patients. J. Peripher. Nerv. Syst. 2011, 16, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, X.; Zhang, Y.; Sun, A.; Fan, D. Molecular analysis and clinical diversity of distal hereditary motor neuropathy. Eur. J. Neurol. 2020, 27, 1319–1326. [Google Scholar] [CrossRef]
- Wu, X.; Qin, K.; Iroegbu, C.D.; Xiang, K.; Peng, J.; Guo, J.; Yang, J.; Fan, C. Genetic analysis of potential biomarkers and therapeutic targets in ferroptosis from coronary artery disease. J. Cell Mol. Med. 2022, 26, 2177–2190. [Google Scholar] [CrossRef]
- Oh, A.; Jeon, S.; Jeong, M.G.; Kim, H.K.; Kang, J.; Lee, Y.-S.; Hwang, E.S. HSPB1 inhibitor J2 attenuates lung inflammation through direct modulation of Ym1 production and paracrine signaling. Biomed. Pharmacother. 2021, 143, 112225. [Google Scholar] [CrossRef]
- Mule, S.N.; Gomes, V.D.M.; Wailemann, R.A.; Macedo-Da-Silva, J.; Rosa-Fernandes, L.; Larsen, M.R.; Labriola, L.; Palmisano, G. HSPB1 influences mitochondrial respiration in ER-stressed beta cells. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140680. [Google Scholar] [CrossRef]
- van Marion, D.; Ramos, K.S.; Lanters, E.; Bulte, L.B.; Bogers, A.; de Groot, N.; Brundel, B.J. Atrial heat shock protein levels are associated with early postoperative and persistence of atrial fibrillation. Heart Rhythm 2021, 18, 1790–1798. [Google Scholar] [CrossRef]
- Hung, C.S.; Huang, C.Y.; Hsu, Y.W.; Makondi, P.T.; Chang, W.C.; Chang, Y.J.; Wang, J.; Wei, P. HSPB1 rs2070804 polymorphism is associated with the depth of primary tumor. J. Cell Biochem. 2020, 121, 63–69. [Google Scholar] [CrossRef]
- Sooraj, K.; Shukla, S.; Kaur, R.; Titiyal, J.S.; Kaur, J. The protective role of HSP27 in ocular diseases. Mol. Biol. Rep. 2022, 49, 5107–5115. [Google Scholar] [CrossRef]
- Sun, Y.; Macrae, T.H. Small heat shock proteins: Molecular structure and chaperone function. Cell Mol. Life Sci. 2005, 62, 2460–2476. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, B.; Cristofani, R.; Ferrari, V.; Cozzi, M.; Rusmini, P.; Casarotto, E.; Chierichetti, M.; Mina, F.; Galbiati, M.; Piccolella, M.; et al. Insights on Human Small Heat Shock Proteins and Their Alterations in Diseases. Front. Mol. Biosci. 2022, 9, 842149. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Shinohara, H.; Goto, S.; Inaguma, Y.; Morishita, R.; Asano, T. Copurification of small heat shock protein with alpha B crystallin from human skeletal muscle. J. Biol. Chem. 1992, 267, 7718–7725. [Google Scholar] [CrossRef]
- Holmgren, A.; Bouhy, D.; De Winter, V.; Asselbergh, B.; Timmermans, J.P.; Irobi, J.; Timmerman, V. Charcot-Marie-Tooth causing HSPB1 mutations increase Cdk5-mediated phosphorylation of neurofilaments. Acta Neuropathol. 2013, 126, 93–108. [Google Scholar] [CrossRef]
- Benn, S.C.; Perrelet, D.; Kato, A.C.; Scholz, J.; Decosterd, I.; Mannion, R.J.; Bakowska, J.C.; Woolf, C.J. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron 2002, 36, 45–56. [Google Scholar] [CrossRef]
- Kalmar, B.; Innes, A.; Wanisch, K.; Kolaszynska, A.K.; Pandraud, A.; Kelly, G.; Abramov, A.Y.; Reilly, M.M.; Schiavo, G.; Greensmith, L. Mitochondrial deficits and abnormal mitochondrial retrograde axonal transport play a role in the pathogenesis of mutant Hsp27-induced Charcot Marie Tooth Disease. Hum. Mol. Genet. 2017, 26, 3313–3326. [Google Scholar] [CrossRef]
- Wyttenbach, A.; Sauvageot, O.; Carmichael, J.; Diaz-Latoud, C.; Arrigo, A.P.; Rubinsztein, D.C. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 2002, 11, 1137–1151. [Google Scholar] [CrossRef]
- Haidar, M.; Asselbergh, B.; Adriaenssens, E.; De Winter, V.; Timmermans, J.; Auer-Grumbach, M.; Juneja, M.; Timmerman, V. Neuropathy-causing mutations in HSPB1 impair autophagy by disturbing the formation of SQSTM1/p62 bodies. Autophagy 2019, 15, 1051–1068. [Google Scholar] [CrossRef]
- Bruey, J.M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.P.; Kroemer, G.; Solary, E.; et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000, 2, 645–652. [Google Scholar] [CrossRef]
- Rossor, A.M.; Morrow, J.M.; Polke, J.M.; Murphy, S.M.; Houlden, H.; Laura, M.; Manji, H.; Blake, J.; Reilly, M.M. Pilot phenotype and natural history study of hereditary neuropathies caused by mutations in the HSPB1 gene. Neuromuscul. Disord. 2017, 27, 50–56. [Google Scholar] [CrossRef]
- Kijima, K.; Numakura, C.; Goto, T.; Takahashi, T.; Otagiri, T.; Umetsu, K.; Hayasaka, K. Small heat shock protein 27 mutation in a Japanese patient with distal hereditary motor neuropathy. J. Hum. Genet. 2005, 50, 473–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acunzo, J.; Katsogiannou, M.; Rocchi, P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol. 2012, 44, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Higuchi, Y.; Yuan, J.; Hashiguchi, A.; Yoshimura, A.; Ishihara, S.; Nozuma, S.; Okamoto, Y.; Matsuura, E.; Ishiura, H.; et al. Clinical and genetic features of Charcot-Marie-Tooth disease 2F and hereditary motor neuropathy 2B in Japan. J. Peripher. Nerv. Syst. 2018, 23, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Alderson, T.R.; Adriaenssens, E.; Asselbergh, B.; Pritišanac, I.; Van Lent, J.; Gastall, H.Y.; A Wälti, M.; Louis, J.M.; Timmerman, V.; Baldwin, A.J.; et al. A weakened interface in the P182L variant of HSP27 associated with severe Charcot-Marie-Tooth neuropathy causes aberrant binding to interacting proteins. EMBO J. 2021, 40, e103811. [Google Scholar] [CrossRef]
- Chalova, A.S.; Sudnitsyna, M.V.; Strelkov, S.V.; Gusev, N.B. Characterization of human small heat shock protein HspB1 that carries C-terminal domain mutations associated with hereditary motor neuron diseases. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014, 1844, 2116–2126. [Google Scholar] [CrossRef]
- Liu, X.M.; Tang, B.S.; Zhao, G.H.; Xia, K.; Zhang, F.F.; Pan, Q.; Cai, F.; Hu, Z.M.; Zhang, C.; Chen, B.; et al. Mutation analysis of small heat shock protein 27 gene in Chinese patients with Charcot-Marie-Tooth disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2005, 22, 510–513. [Google Scholar]
- Ylikallio, E.; Konovalova, S.; Dhungana, Y.; Hilander, T.; Junna, N.; Partanen, J.V.; Toppila, J.P.; Auranen, M.; Tyynismaa, H. Truncated HSPB1 causes axonal neuropathy and impairs tolerance to unfolded protein stress. BBA Clin. 2015, 3, 233–242. [Google Scholar] [CrossRef]
- Oberstadt, M.; Mitter, D.; Classen, J.; Baum, P. Late onset dHMN II caused by c.404C>G mutation in HSPB1 gene. J. Peripher. Nerv. Syst. 2016, 21, 111–113. [Google Scholar] [CrossRef]
- Tang, B.; Liu, X.; Zhao, G.; Luo, W.; Xia, K.; Pan, Q.; Cai, F.; Hu, Z.; Zhang, C.; Chen, B.; et al. Mutation analysis of the small heat shock protein 27 gene in chinese patients with Charcot-Marie-Tooth disease. Arch. Neurol. 2005, 62, 1201–1207. [Google Scholar] [CrossRef]
- Nefedova, V.V.; Sudnitsyna, M.V.; Strelkov, S.V.; Gusev, N.B. Structure and properties of G84R and L99M mutants of human small heat shock protein HspB1 correlating with motor neuropathy. Arch. Biochem. Biophys. 2013, 538, 16–24. [Google Scholar] [CrossRef]
- Raju, I.; Abraham, E.C. Mutants of human αB-crystallin cause enhanced protein aggregation and apoptosis in mammalian cells: Influence of co-expression of HspB1. Biochem. Biophys. Res. Commun. 2013, 430, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zourlidou, A.; Payne, S.M.; Latchman, D.S. HSP27 but not HSP70 has a potent protective effect against alpha-synuclein-induced cell death in mammalian neuronal cells. J. Neurochem. 2004, 88, 1439–1448. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Zhang, J.; Zhan, F.; Tian, W.; Jiang, Q.; Luan, X.; Zhang, X.; Cao, L. Heterogeneous Clinical Phenotypes of dHMN Caused by Mutation in HSPB1 Gene: A Case Series. Biomolecules 2022, 12, 1382. https://doi.org/10.3390/biom12101382
Shen X, Zhang J, Zhan F, Tian W, Jiang Q, Luan X, Zhang X, Cao L. Heterogeneous Clinical Phenotypes of dHMN Caused by Mutation in HSPB1 Gene: A Case Series. Biomolecules. 2022; 12(10):1382. https://doi.org/10.3390/biom12101382
Chicago/Turabian StyleShen, Xiya, Jiawei Zhang, Feixia Zhan, Wotu Tian, Qingqing Jiang, Xinghua Luan, Xiaojie Zhang, and Li Cao. 2022. "Heterogeneous Clinical Phenotypes of dHMN Caused by Mutation in HSPB1 Gene: A Case Series" Biomolecules 12, no. 10: 1382. https://doi.org/10.3390/biom12101382





