Isolation and Biological Characterization of Homoisoflavanoids and the Alkylamide N-p-Coumaroyltyramine from Crinum biflorum Rottb., an Amaryllidaceae Species Collected in Senegal
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Purification of Compounds 1–5
2.4. Spectroscopic Data of Compounds 1–5
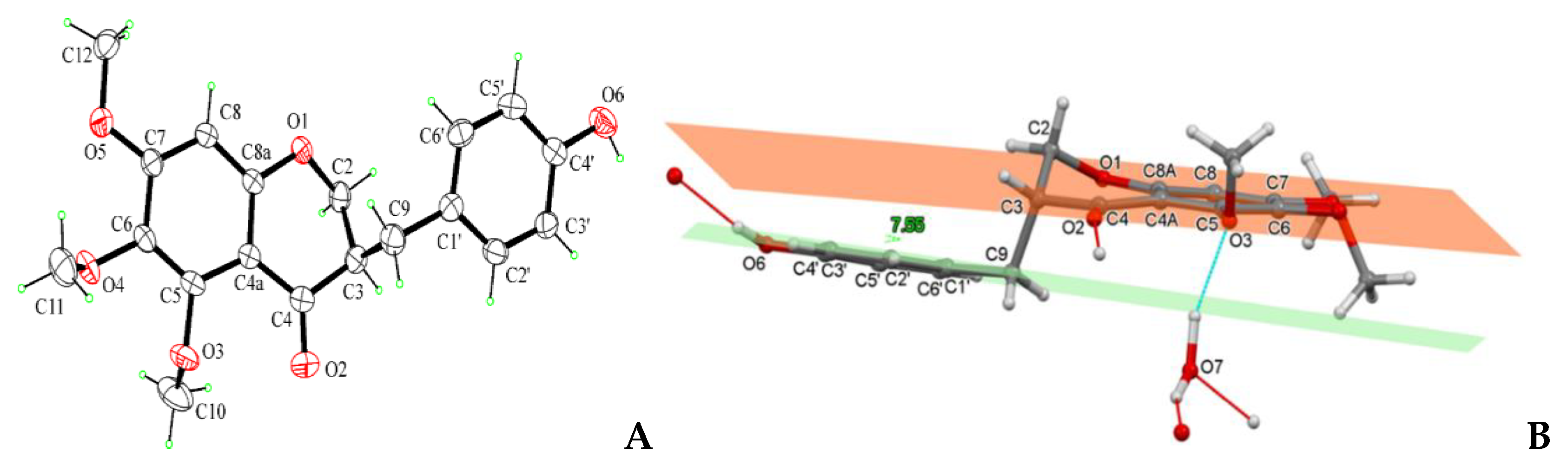
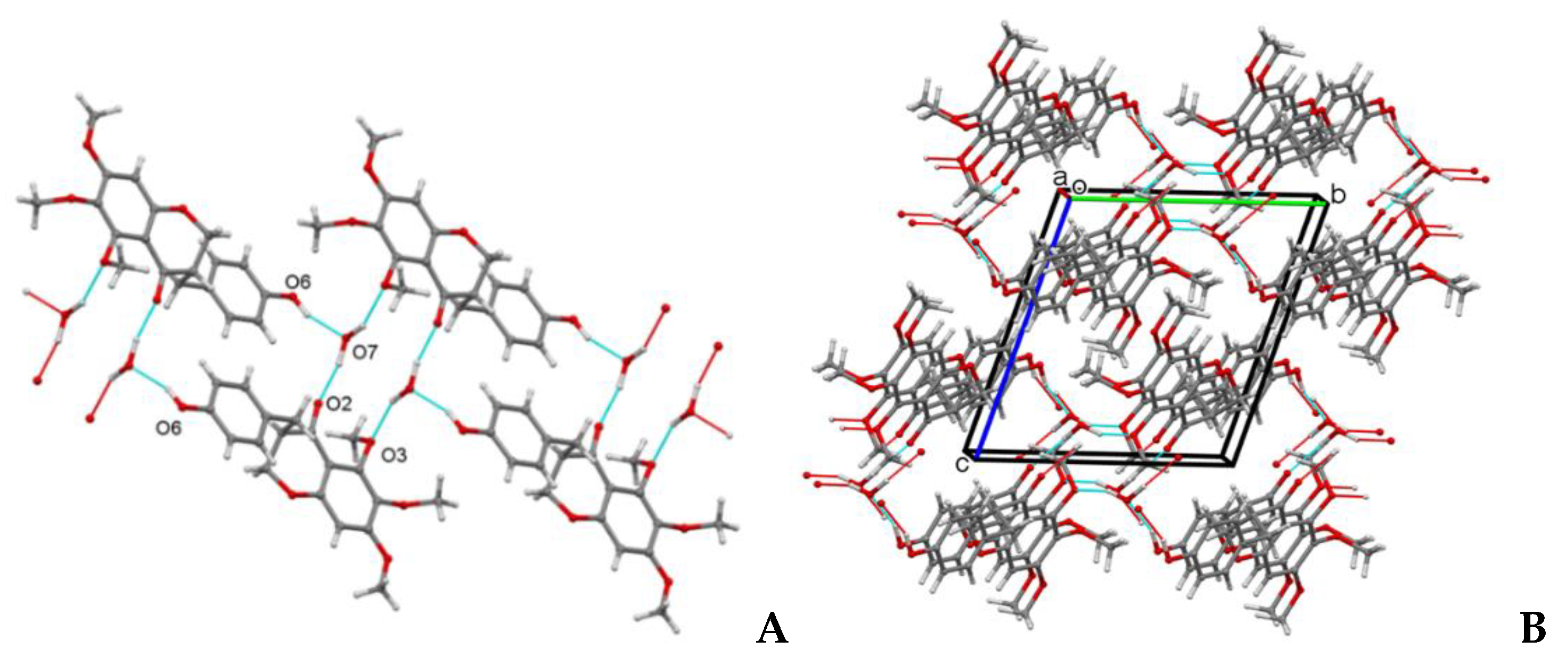
2.5. Crystal Structure Determination of Compound 1
2.6. Cell Culture and Reagents
2.7. MTT Assay
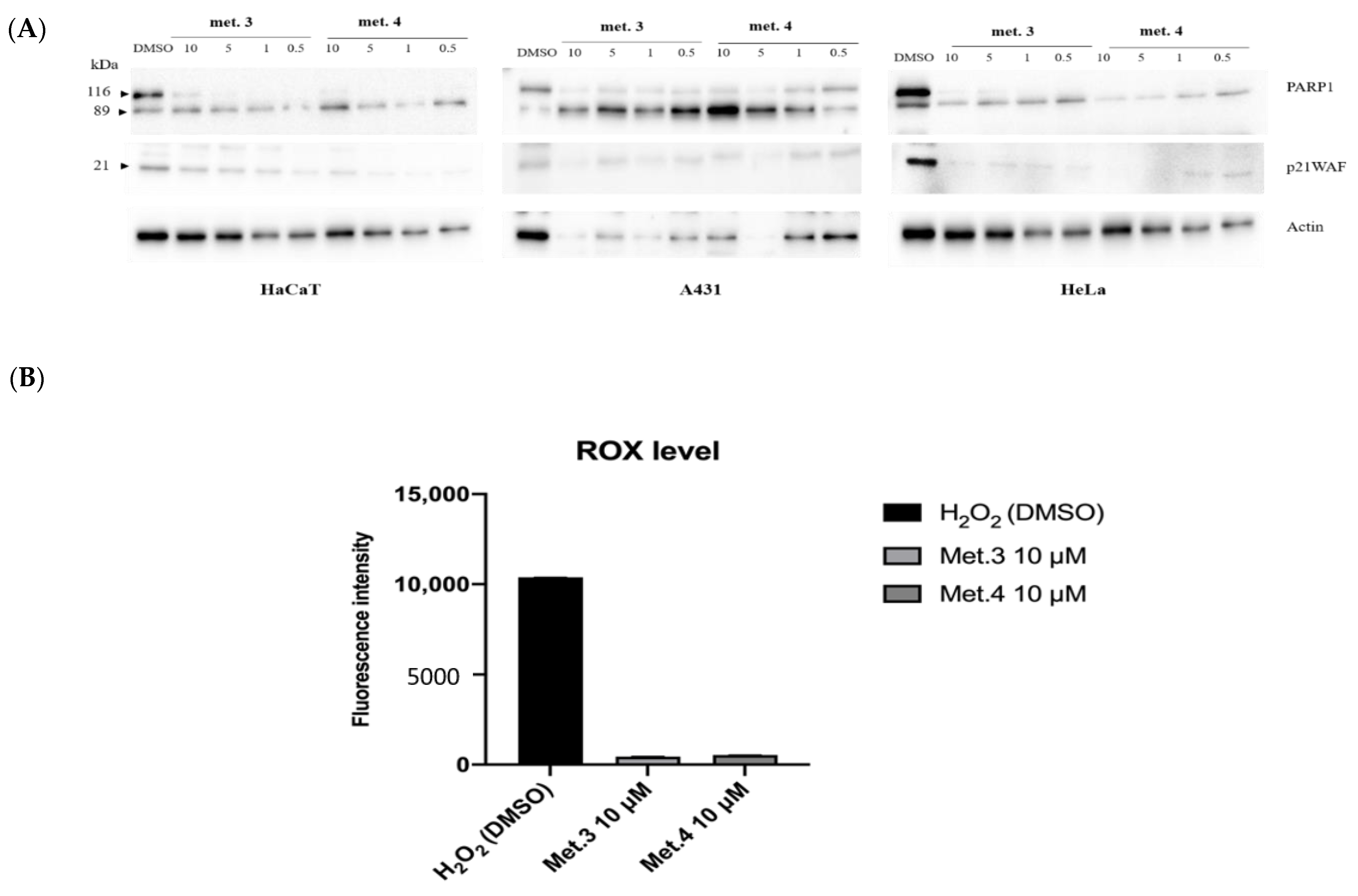
2.8. Detection of DNA Damage
2.9. Western Blot Analysis
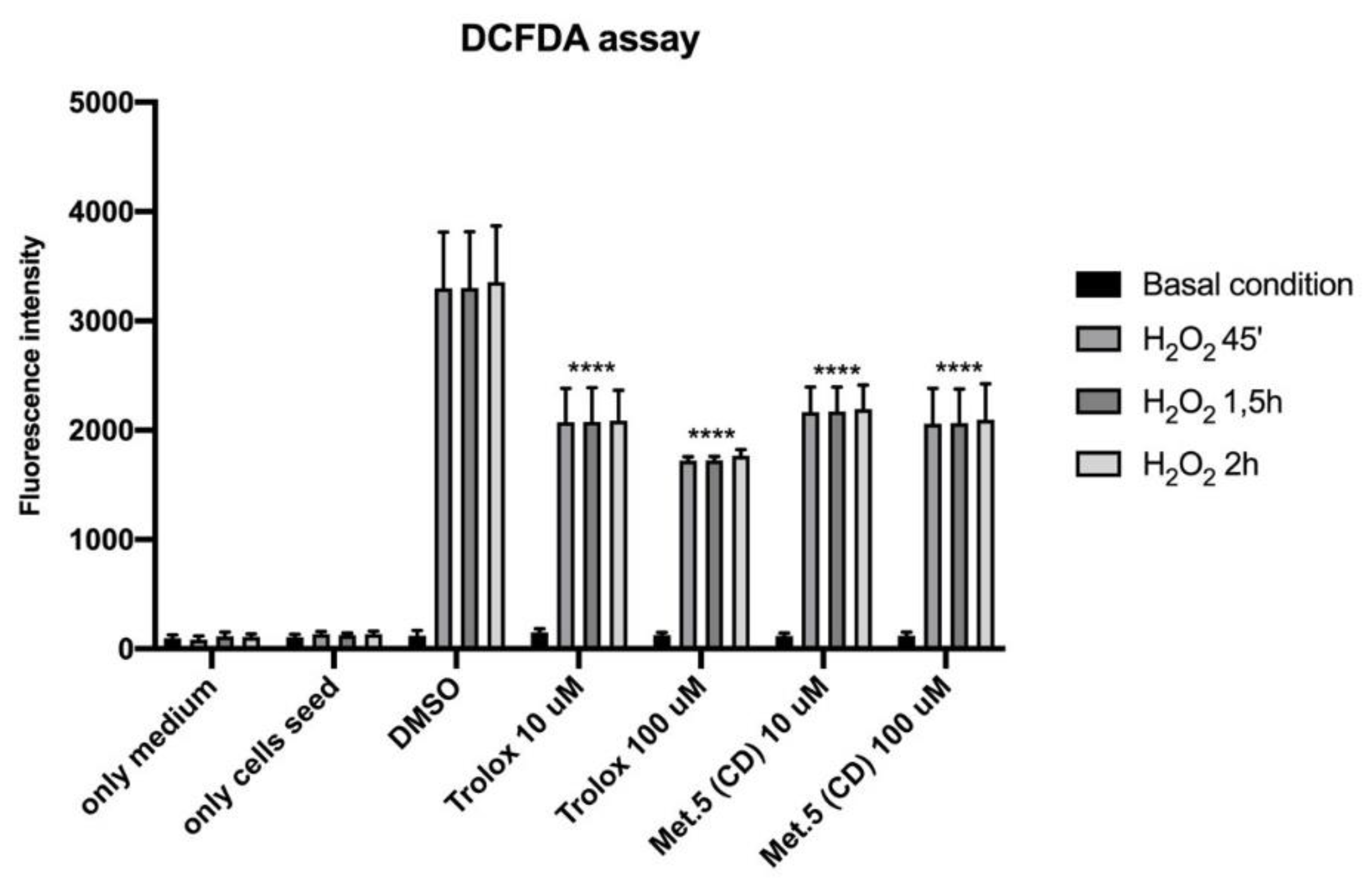
2.10. DCFDA Assay
2.11. 2Pseudotyped HIV-1GFP Infectivity Assay
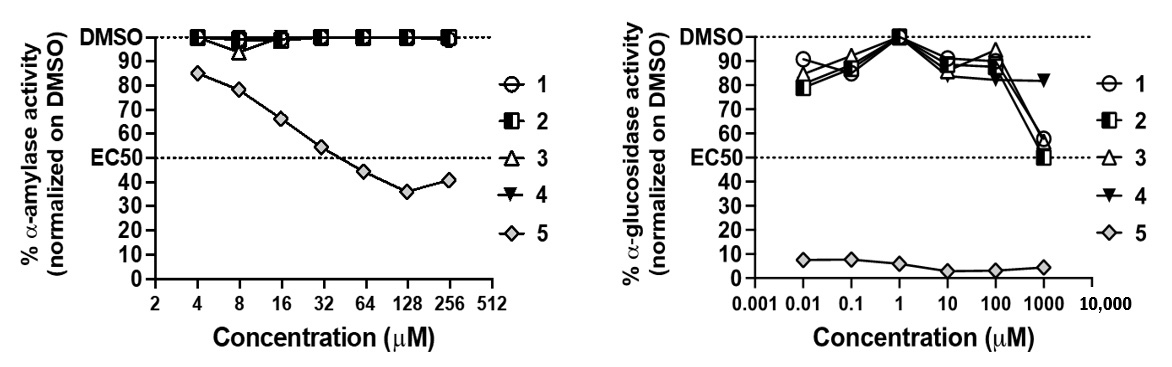
2.12. α-Glucosidase and α-Amylase Inhibitor Assay
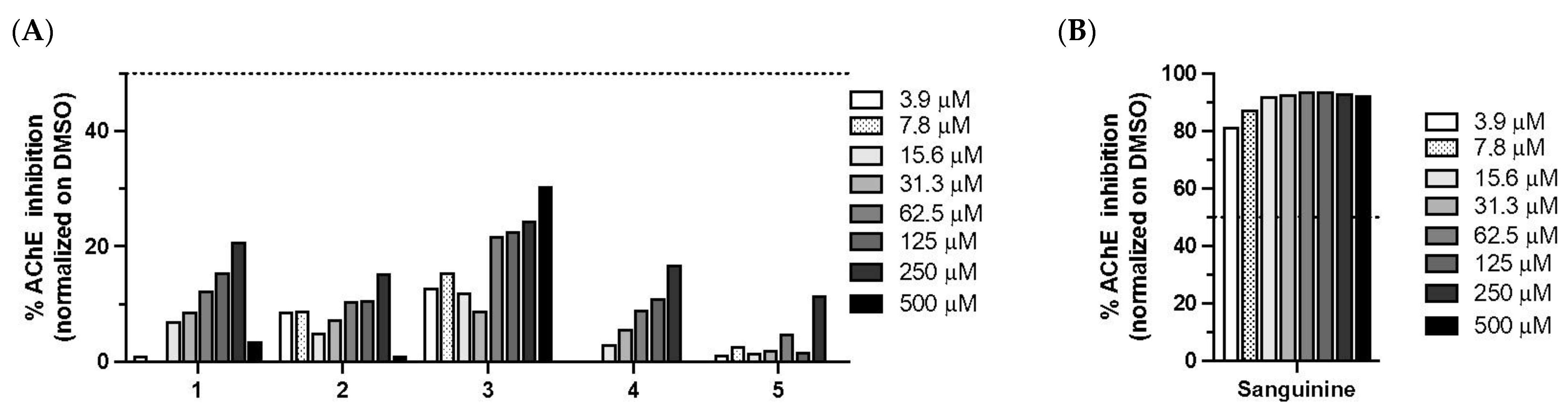
2.13. Anti-Acetylcholinesterase Assays
2.14. Statistical Analysis
3. Results
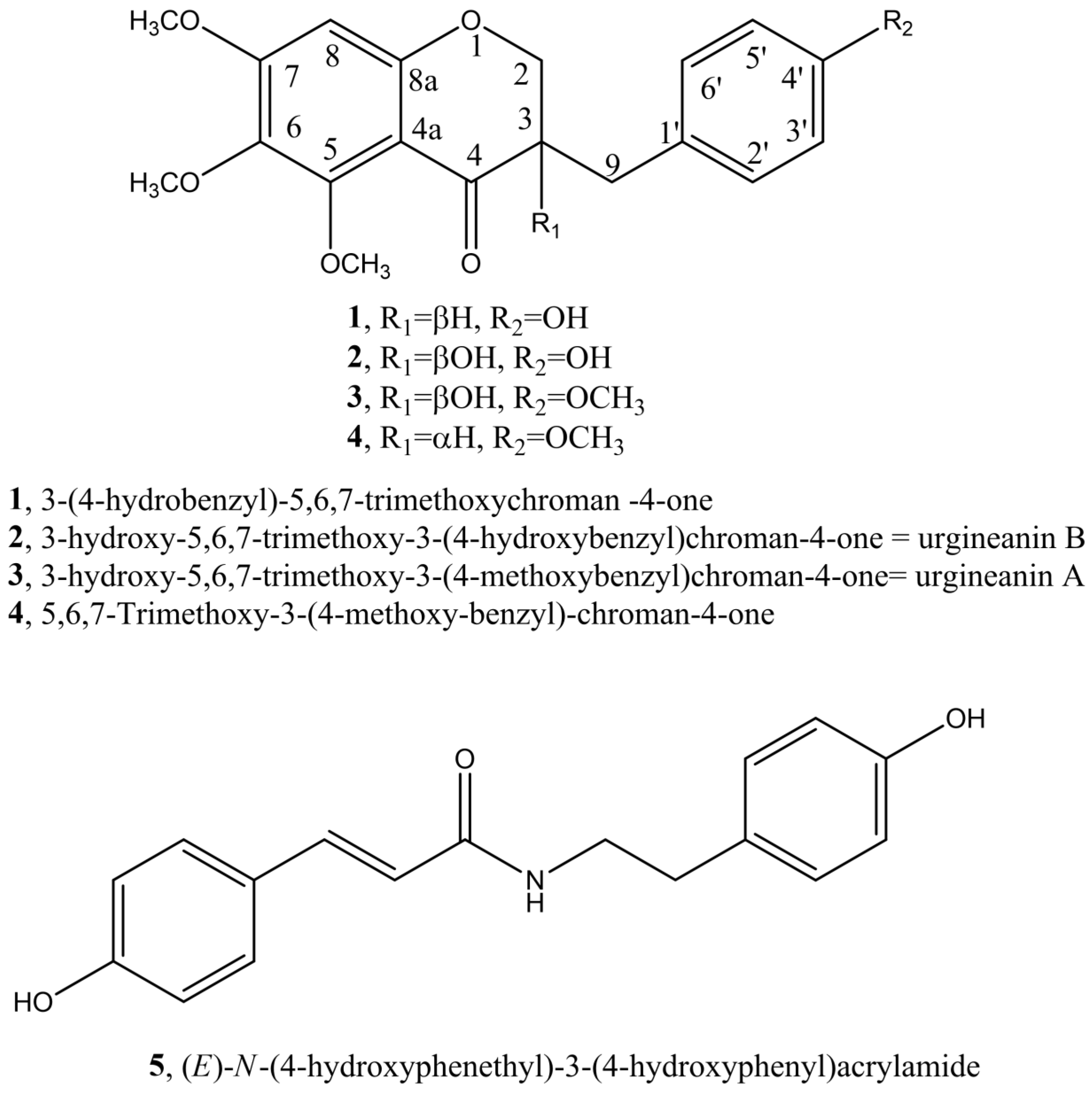
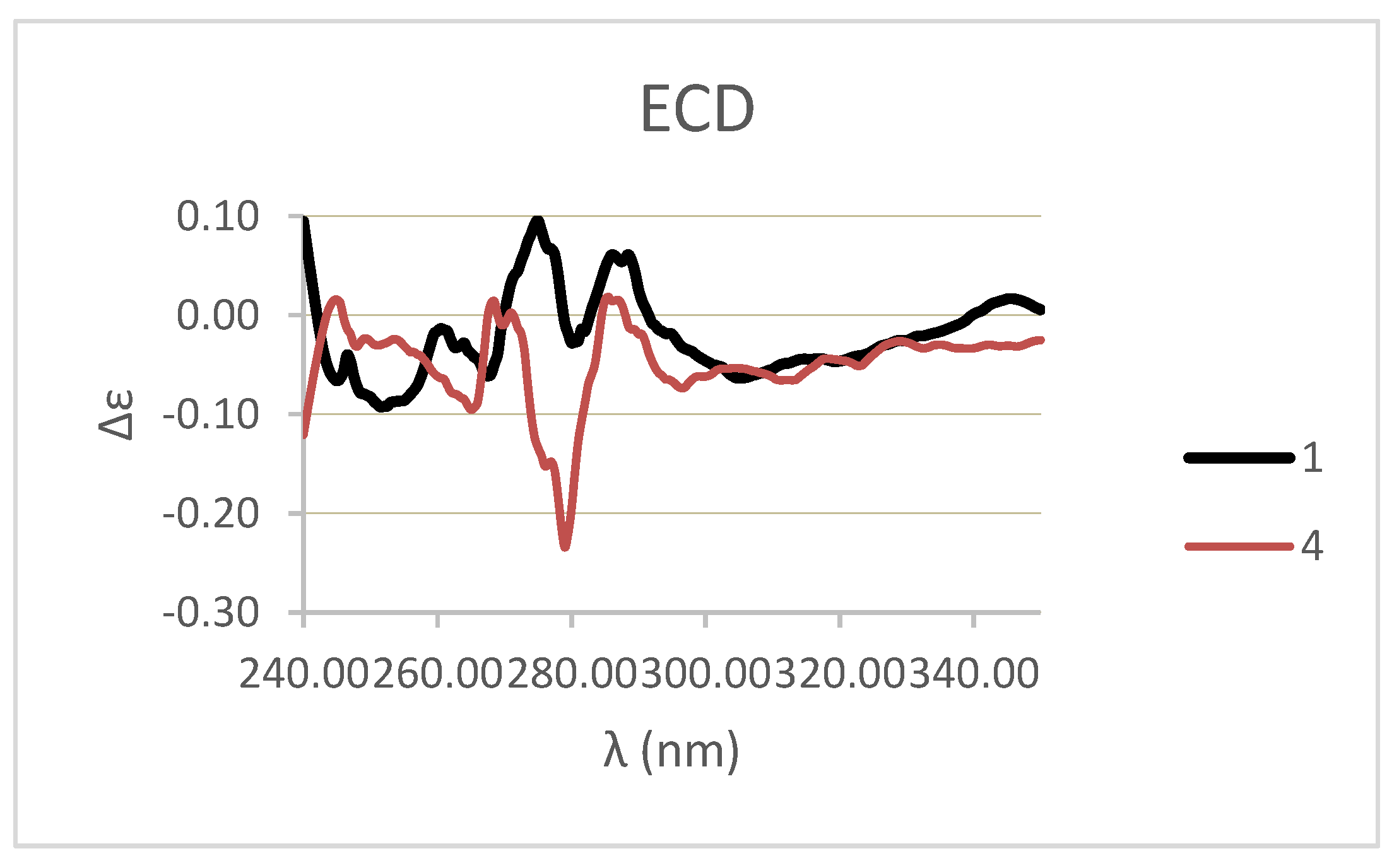
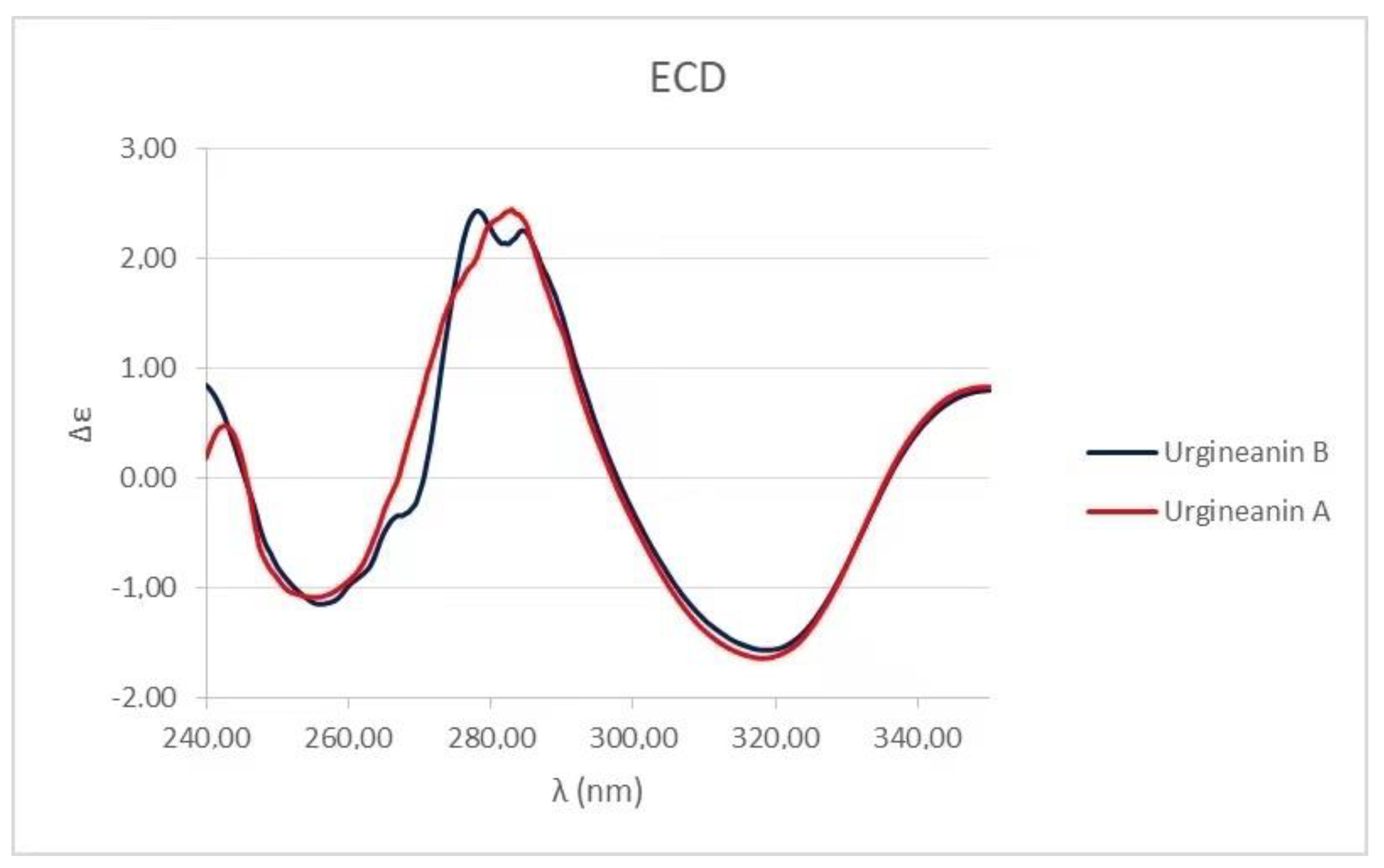
3.1. Identification of Metabolites Isolated from C. biflorum
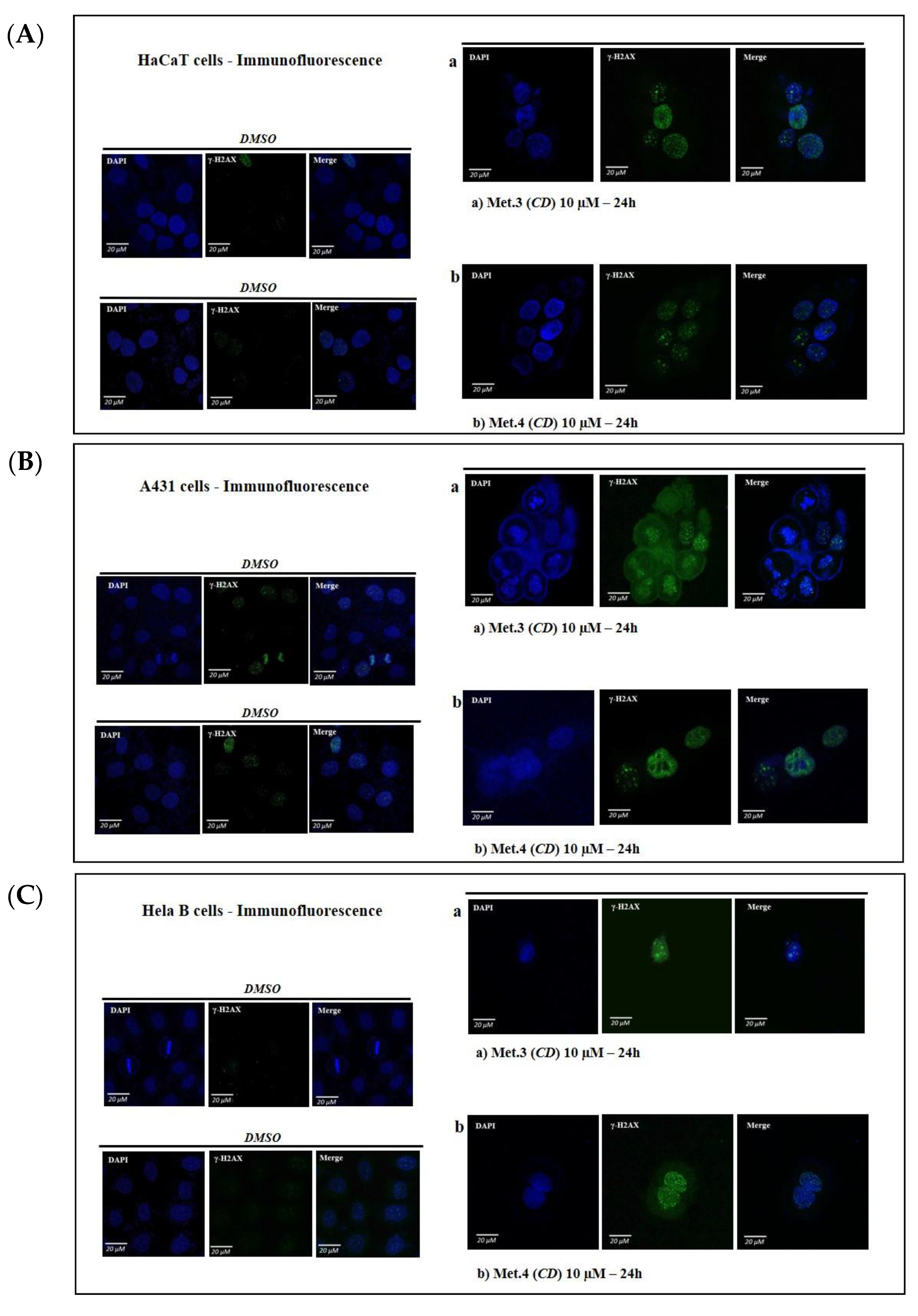
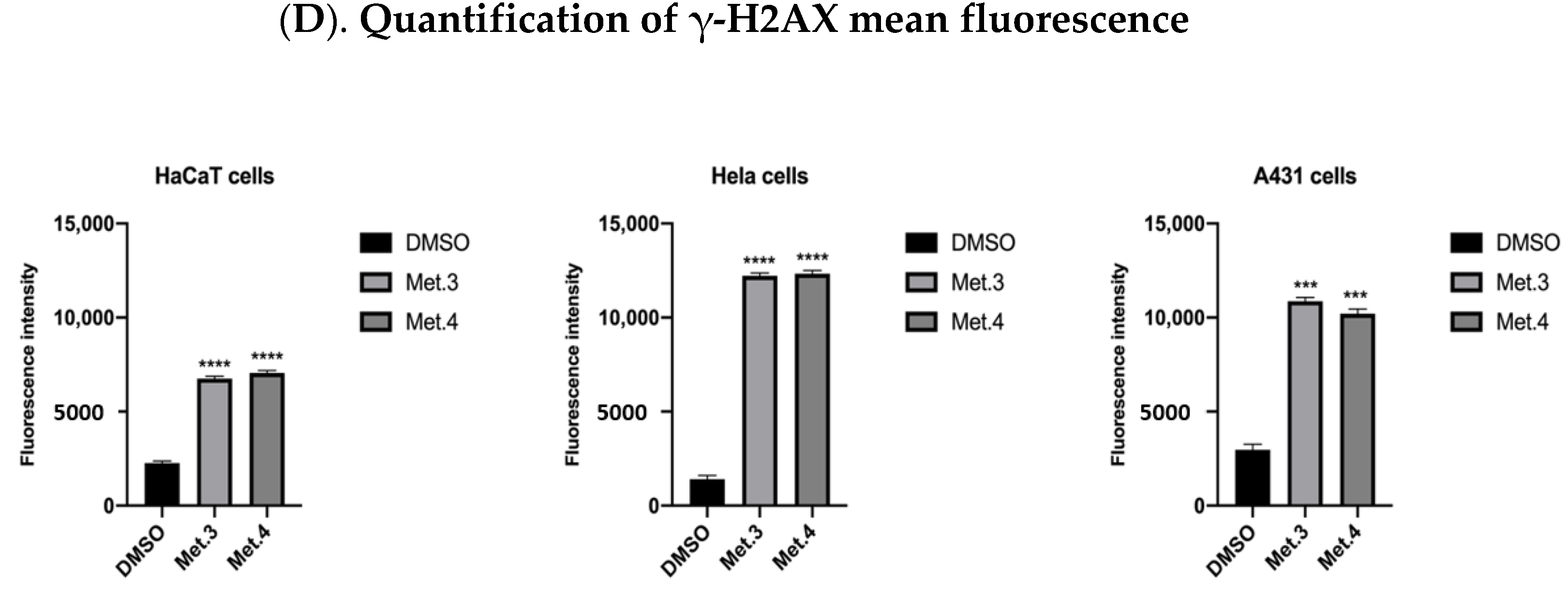
3.2. In Vitro Cytotoxicity
3.3. Antidiabetic and Anti-Acetylcholinesterase Properties
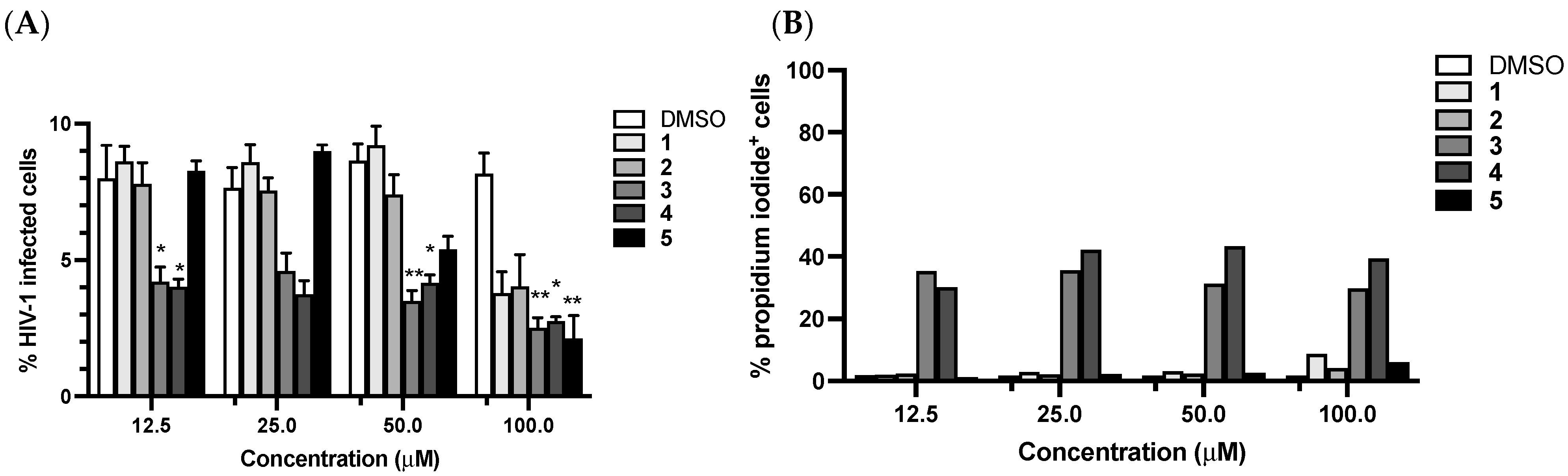
3.4. Antiviral Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hoshino, O. The amaryllidaceae alkaloids. Alkaloids 1998, 51, 323–376. [Google Scholar]
- Evidente, A.; Kornienko, A. Anticancer evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives. Phytochem. Rev. 2009, 8, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insight to the South Africa Amaryllidaceae. Food Chem. Toxicol. 2013, 62, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yao, G. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2019, 36, 1462–1488. [Google Scholar] [CrossRef]
- Nair, J.J.; Bastida, J.; Codina, C.; Viladomat, F.; van Staden, J. Alkaloids of the South African Amaryllidaceae: A review. Nat. Prod. Commun. 2013, 8, 1335–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 26, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Bastida, J.; Berkov, S. Amaryllidaceae Alkaloids. Molecules 2020. [Google Scholar]
- Masi, M.; Di Lecce, R.; Cimmino, A.; Evidente, A. Advances in the chemical and biological characterization of Amaryllidaceae alkaloids and natural analogues isolated in the last decade. Molecules 2020, 25, 5621. [Google Scholar] [CrossRef]
- Ghosal, S.; Saini, K.S.; Razdan, S. Crinum alkaloids: Their chemistry and biology. Phytochemistry 1985, 24, 2141–2156. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ka, S.; Masi, M.; Merindol, N.; Di Lecce, R.; Plourde, M.B.; Seck, M.; Marcin, G.; Pescitelli, G.; Desgagne-Penix, I.; Evidente, A. Gigantelline, gigantellinine and gigancrinine, cherylline-and crinine-type alkaloids isolated from Crinum jagus with anti-acetylcholinesterase activity. Phytochemistry 2020, 175, 112390. [Google Scholar] [CrossRef]
- Ka, S.; Merindol, N.; Sow, A.A.; Singh, A.; Landelouci, K.; Plourde, M.B.; Pepin, G.; Masi, M.; Di Lecce, R.; Evidente, A.; et al. Amaryllidaceae alkaloid cherylline inhibits the replication of dengue and Zika viruses. Antimicrob. Agents Chemother. 2021, 65, 9. [Google Scholar] [CrossRef] [PubMed]
- Ibrakav, A.S.; Akinfewa, A.O.; Hussein, A.A. A comprehensive review on non alkaloids chemical constituents from Amaryllidaceae. South Afr. J. Bot. 2020. In press. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Osbourn, A.E.; Lanzotti, V. Plant-Derived Natural Products Dordrecht; Springer: Berlin, Germany, 2009; pp. 361–384. [Google Scholar]
- Abd El-Hafiz, M.A. Aliphatic hydroxyketones from Crinum augustum. Phytochemistry 1991, 30, 3127–3129. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. The medicinal and poisonous plants of Southern and Eastern Africa. Kew Bull. 1964, 17, 463. [Google Scholar]
- Likhitwitayawuid, K.; Ruangrungsi, N.; Cordell, G.A. Amabiloside, a new glycoside from Crinum amabile. Nat. Prod. Lett. 1993, 3, 1–4. [Google Scholar] [CrossRef]
- Nam, N.H.; Jae, Y.Y. NF-κB Inhibitory activities of the methanol extracts and some constituents therein of some Vietnamese medicinal plants. Sci. Pharm. 2009, 77, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Jin, A.; Li, X.; Zhu, Y.Y.; Yu, H.Y.; Pi, H.F.; Zhang, P.; Ruan, H.L. Four new compounds from the bulbs of Lycoris aurea with neuroprotective effects against CoCl2 and H2O2-induced SH-SY5Y cell injuries. Arch. Pharm. Res. 2014, 37, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, W.D.; Shen, Y.H.; Zhang, C.; Li, H.L. A new phenolic compound from Crinum asiaticum L. Chin. Chem. Lett. 2008, 19, 447–449. [Google Scholar] [CrossRef]
- Nkanwen, E.R.S.; Gatsing, D.; Ngamga, D.; Fodouop, S.P.C.; Tane, P. Antibacterial agents from the leaves of Crinum purpurascens herb (Amaryllidaceae). Afric. Health Sci. 2009, 9, 264–269. [Google Scholar]
- Khoi, N.M.; Dat, N.T.; Na, M.K.; Thuong, P.T.; Min, B.S.; Bae, K.H. Cytotoxic activity of parthenin, a sesquiterpene isolated from a Crinum ensifolium. Nat. Prod. Sci. 2011, 17, 100–103. [Google Scholar]
- Abd El Hafiz, M.A.; Ramadan, M.A.; Jung, M.L.; Beck, J.P.; Anton, R. Cytotoxic activity of Amaryllidaceae alkaloids from Crinum augustum and Crinum bulbispermum. Planta Med. 1991, 57, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, O.B.; Marzouk, A.M.; Mothana, R.; Awadh, N. A new tyrosinase inhibitor from Crinum yemense as potential treatment for hyperpigmentation. Die Pharm. Int. J. Pharm. Sci. 2008, 63, 405–407. [Google Scholar]
- Masi, M.; Mubaiwa, B.; Cimmino, A.; Van Otterlo, W.A.L.; Green, I.R.; Evidente, A. First isolation of acetovanillone and piceol from Crinum buphanoides and Crinum graminicola (I. Verd.) Amaryllidaceae. South Afric. J. Bot. 2018, 114, 37–39. [Google Scholar] [CrossRef]
- Burkill, H.M. The Useful Plants of West Tropical Africa; Royal Botanic Gardens: London, UK, 1985; Volume 1, p. 163. [Google Scholar]
- Kianfé, B.Y.; Teponno, R.B.; Kühlborn, J.; Tchuenguem, R.T.; Ponou, B.K.; Helaly, S.E.; Dzoyemd, J.P.; Opatz, T.; Tapondjou, L.A. Flavans and other chemical constituents of Crinum biflorum (Amaryllidaceae). Biochem. Syst. Ecol. 2019, 87, 103953. [Google Scholar] [CrossRef]
- Silayo, A.; Ngadjui, B.T.; Abegaz, B.M. Homoisoflavonoids and stilbenes from the bulbs of Scilla nervosa subsp. rigidifolia. Phytochemistry 1999, 52, 947–955. [Google Scholar] [CrossRef]
- Dai, Y.; Harinantenaina, L.; Brodie, P.J.; Goetz, M.; Shen, Y.; TenDyke, K.; Kingston, D.G. Antiproliferative homoisoflavonoids and bufatrienolides from Urginea depressa. J. Nat. Prod. 2013, 76, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Sidwell, W.T.L.; Tamm, C. The homo-isoflavones II1). Isolation and structure of 4′-O-methyl-punctatin, autumnalin and 3,9-dihydro-autumnalin. Tetrahedron Lett. 1970, 11, 475–478. [Google Scholar] [CrossRef]
- Bhatti, M.K.; Akhtar, F.; Choudhary, M.I. Alkaloids of Fumaria indica. Phytochemistry 1992, 31, 2869–2872. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cacarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Amoresano, A.; Di Costanzo, A.; Leo, G.; Di Cunto, F.; La Mantia, G.; Guerrini, L.; Calabrò, V. Identification of ΔNp63α protein interactions by mass spectrometry. J. Proteome Res. 2010, 9, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Vivo, M.; Fontana, R.; Ranieri, M.; Capasso, G.; Angrisano, T.; Pollice, A.; Calabrò, V.; La Mantia, G. p14ARF interacts with the focal adhesion kinase and protects cells from anoikis. Oncogene 2017, 36, 4913–4928. [Google Scholar] [CrossRef] [Green Version]
- Sangermano, F.; Masi, M.; Vivo, M.; Ravindra, P.; Cimmino, A.; Pollice, A.; Evidente, A.; Calabrò, V. Higginsianins A and B, two fungal diterpenoid α-pyrones with cytotoxic activity against human cancer cells. Toxicol. In Vitro 2019, 61, 104614. [Google Scholar] [CrossRef]
- di Martino, O.; Troiano, A.; Guarino, A.M.; Pollice, A.; Vivo, M.; La Mantia, G.; Calabrò, V. DNp63a controls YB-1 protein stability: Evidence on YB-1 as a new player in keratinocyte differentiation. Genes Cells 2016, 21, 648–660. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, S.; Yin, P.; Yan, L.; Han, J.; Shi, L.; Zhou, X.; Liu, Y.; Ma, C. α-Glucosidase inhibitory activity of polyphenols from the burs of Castanea mollissima Blume. Molecules 2014, 19, 8373–8386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Borgonovo, G.; Caimi, S.; Morini, G.; Scaglioni, L.; Bassoli, A. Taste-active compounds in a traditional Italian food: ‘Lampascioni’. Chem. Biodiver. 2008, 5, 1184–1194. [Google Scholar] [CrossRef]
- Okuyama, T.; Shibata, S.; Hoson, M.; Kawada, T.; Osada, H.; Noguchi, T. Effect of oriental plant drugs on platelet aggregation; III1. Effect of Chines drug “Xiebai” on human platelet aggregation. Planta Med. 1986, 52, 171–175. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA doublestranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [Green Version]
- Dickey, J.S.; Redon, C.E.; Nakamura, A.J.; Baird, B.J.; Sedelnikova, O.A.; Bonner, W.M. H2AX: Functional roles and potential applications. Chromosoma 2009, 118, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, T.J.; Watanabe, J.; Kawabata, J.R.; Niki, I. Isolation and activity of N-p-coumaroyltyramine, an α-glucosidase inhibitor in welsh onion (Allium fistulosum). Biosci. Biotech. Biochem. 1997, 61, 1138–1141. [Google Scholar] [CrossRef]
- Pasetto, S.; Pardi, V.; Murata, R.M. Anti-HIV-1 activity of flavonoid myricetin on HIV-1 infection in a dual-chamber in vitro model. PLoS ONE 2014, 9, e115323. [Google Scholar] [CrossRef] [PubMed]
- Koagne, R.R.; Annang, F.; de la Cruz, M.; Bitchagno, G.T.M.; Perez-Victoria, I.; Konga, I.S.; Vincente, F.; Reyes, F.; Tane, P. Antibacterial activity of flavans from Crinum distichum. Nat. Prod. Commun. 2018, 13, 1637–1638. [Google Scholar] [CrossRef] [Green Version]
- Barone, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M. Homoisoflavanones from Muscari neglectum. Phytochemistry 1988, 27, 921–923. [Google Scholar] [CrossRef]
- Evidente, A.; Iasiello, I.; Randazzo, G. An improved method for the large-scale preparation of lycorine. Chem. Ind. 1984, 9, 348–349. [Google Scholar]
- Giordano, F.; Lanzetta, R. Structure and absolute configuration of (–)-(3R)-5,7-dimethoxy-3,9-dihydro-4′-eucomnalinyl p-bromobenzoate: An uncommon case. Acta Crystallogr. Sect. C Struct. Chem. 1989, 45, 1603–1606. [Google Scholar] [CrossRef]
- Zask, A.; Ellestad, G. Biomimetic syntheses of race-mic natural products. Chirality 2018, 30, 157–164. [Google Scholar] [CrossRef]
- Di Lecce, R.; Masi, M.; Linaldeddu, B.T.; Pescitelli, G.; Maddau, L.; Evidente, A. Bioactive secondary metabolites produced by the emerging pathogen Diplodia olivarum. Phytopathol. Mediterr. 2021, 60, 129–138. [Google Scholar] [CrossRef]
- Fitch, R.W.; Snider, B.B.; Zhou, Q.; Foxman, B.M.; Pandya, A.A.; Yakel, J.L.; Olson, T.T.; Al-Muhtasib, N.; Xiao, Y.; Welch, K.D.; et al. Absolute configuration and pharmacology of the poison frog alkaloid phantasmidine. J. Nat. Prod. 2018, 81, 1029–1035. [Google Scholar] [CrossRef]
- Murray, A.F.; Moore, A.J.; Munafo, J.P., Jr. Key odorants from the american matsutake, Tricholoma magnivelare. J. Agric. Food Chem. 2020, 68, 9768–9775. [Google Scholar] [CrossRef] [PubMed]
- Tran-Cong, N.M.; Mándi, A.; Király, S.B.; Kurtán, T.; Lin, W.; Liu, Z.; Proksch, P. Furoic acid derivatives from the endophytic fungus Coniothyrium sp. Chirality 2020, 32, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Deng, A.J.; Zhang, H.J.; Li, Q.; Li, Z.H.; Zhang, Z.H.; Wu, L.Q.; Li, L.; Qin, H.L. Six scalemic mixtures of 6-monosubstituted dihydrobenzophenanthridine alkaloids from Chelidonium majus and optically active structures of enantiomers. Phytochemistry 2017, 144, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Takamatsu, S.; Sakamura, S. Three new phenolic amides from the roots of eggplant (Solanum melongena L.). Agric. Biol. Chem. 1978, 42, 623–627. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.C.; Chang, G.Y.; Ko, F.N.; Teng, C.M. Bioactive constitutents from the stems of Annona montana. Planta Med. 1995, 61, 146–149. [Google Scholar] [CrossRef]
- Barbalho, S.M.; de Alvares Goulart, R.; Vasques Farinazzi-Machado, F.M.; da Silva Soares de Souza, M.; Cincotto dos Santos Bueno, P.; Landgraf Guiguer, E.; Araújo, A.C.; Groppo, M. Annona sp: Plants with multiple applications as alternative medicine-A review. Curr. Bioact. Compd. 2012, 8, 277–286. [Google Scholar] [CrossRef]
- Park, J.B. Identification and quantification of a major anti-oxidant and anti-inflammatory phenolic compound found in basil, lemon thyme, mint, oregano, rosemary, sage, and thyme. Int. J. Food Sci. Nutr. 2011, 62, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y. Natural alkamides: Pharmacology, chemistry, and distribution. In Drug Discovery: Research in Pharmacognosy; Books on Demand: Norderstedt, Germany, 2012; pp. 107–144. [Google Scholar]
- Morimoto, N.; Ueno, K.; Teraishi, M.; Okumoto, Y.; Mori, N.; Ishihara, A. Induced phenylamide accumulation in response to pathogen infection and hormone treatment in rice (Oryza sativa). Biosci. Biotech. Bioch. 2018, 82, 407–416. [Google Scholar] [CrossRef]
- Castelli, M.V.; López, S.N. Homoisoflavonoids: Occurrence, biosynthesis, and biological activity. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 315–354. [Google Scholar]
- Abegaz, B.M.; Kinfe, H.H. Naturally occurring homoisoflavonoids: Phytochemistry, biological activities, and synthesis (Part II). Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J. Global burden of metabolic risk factors of chronic diseases collaborating, G. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 3140. [Google Scholar]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switherland, 2006; pp. 1–50. [Google Scholar]
- Mohan, S.; Nandhakumar, L. Role of various flavonoids: Hypotheses on novel approach to treat diabetes. J. Med. Hypot. Ideas 2014, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The possible role of flavonoids in the prevention of diabetic complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef] [Green Version]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qiang, X.; Luo, L.; Yang, X.; Xiao, G.; Zheng, Y.; Cao, Z.; Sang, Z.; Su, F.; Deng, Y. Multitarget drug design strategy against Alzheimer’s disease: Homoisoflavonoid Mannich base derivatives serve as acetylcholinesterase and monoamine oxidase B dual inhibitors with multifunctional properties. Bioorg. Med. Chem. 2017, 25, 714–726. [Google Scholar] [CrossRef]
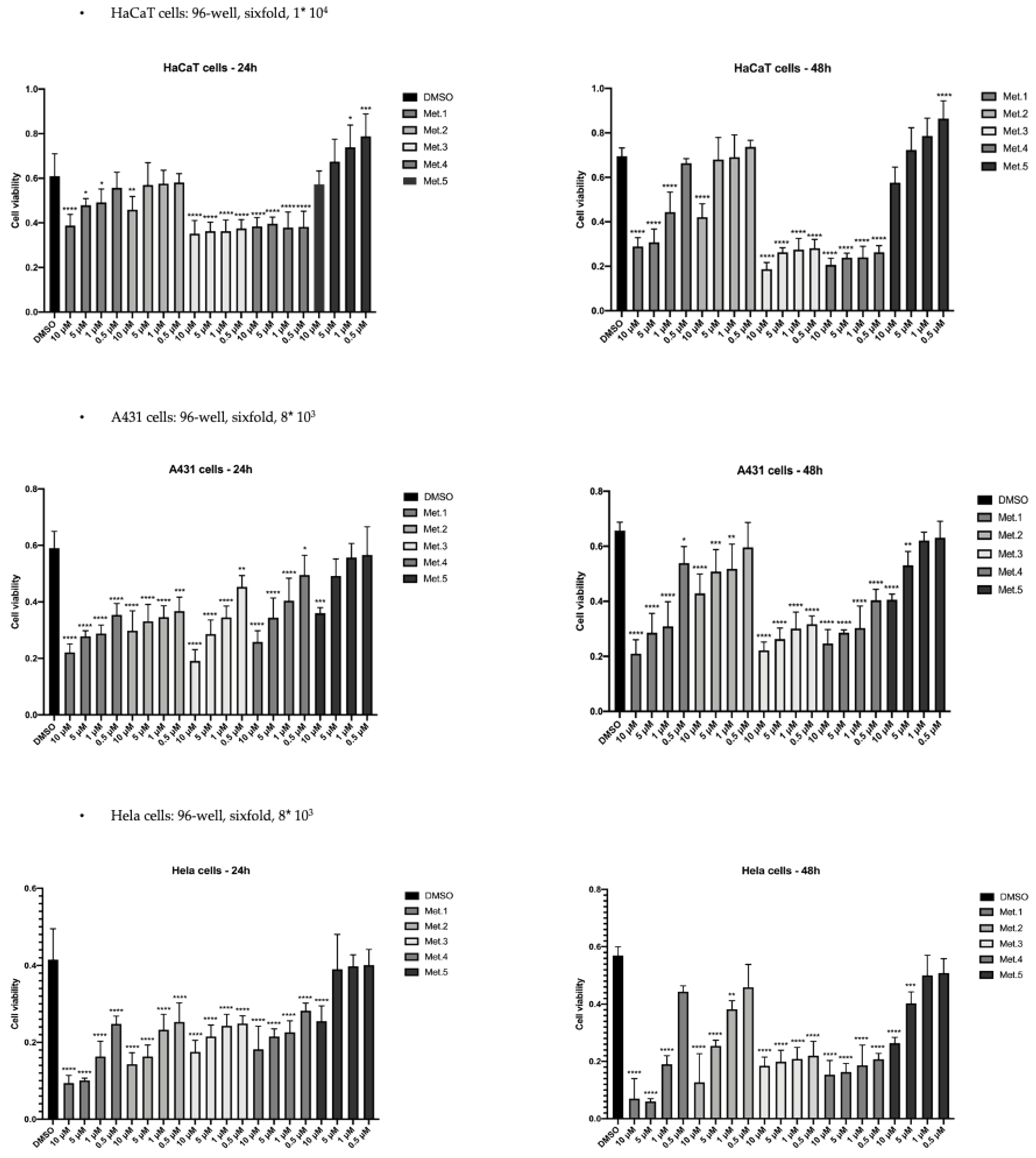
| Compound | HaCaT | A431 | HeLa |
|---|---|---|---|
| 1 | >10 | 6.0 | 1.0 |
| 2 | >10 | >10 | 4.0 |
| 3 | >10 | 4.0 | 4.5 |
| 4 | >10 | 6.5 | 5.5 |
| 5 | Not detected | >10 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Koirala, M.; Delicato, A.; Di Lecce, R.; Merindol, N.; Ka, S.; Seck, M.; Tuzi, A.; Desgagne-Penix, I.; Calabrò, V.; et al. Isolation and Biological Characterization of Homoisoflavanoids and the Alkylamide N-p-Coumaroyltyramine from Crinum biflorum Rottb., an Amaryllidaceae Species Collected in Senegal. Biomolecules 2021, 11, 1298. https://doi.org/10.3390/biom11091298
Masi M, Koirala M, Delicato A, Di Lecce R, Merindol N, Ka S, Seck M, Tuzi A, Desgagne-Penix I, Calabrò V, et al. Isolation and Biological Characterization of Homoisoflavanoids and the Alkylamide N-p-Coumaroyltyramine from Crinum biflorum Rottb., an Amaryllidaceae Species Collected in Senegal. Biomolecules. 2021; 11(9):1298. https://doi.org/10.3390/biom11091298
Chicago/Turabian StyleMasi, Marco, Manoj Koirala, Antonella Delicato, Roberta Di Lecce, Natacha Merindol, Seydou Ka, Matar Seck, Angela Tuzi, Isabel Desgagne-Penix, Viola Calabrò, and et al. 2021. "Isolation and Biological Characterization of Homoisoflavanoids and the Alkylamide N-p-Coumaroyltyramine from Crinum biflorum Rottb., an Amaryllidaceae Species Collected in Senegal" Biomolecules 11, no. 9: 1298. https://doi.org/10.3390/biom11091298






