Chalcones: Synthetic Chemistry Follows Where Nature Leads
Abstract
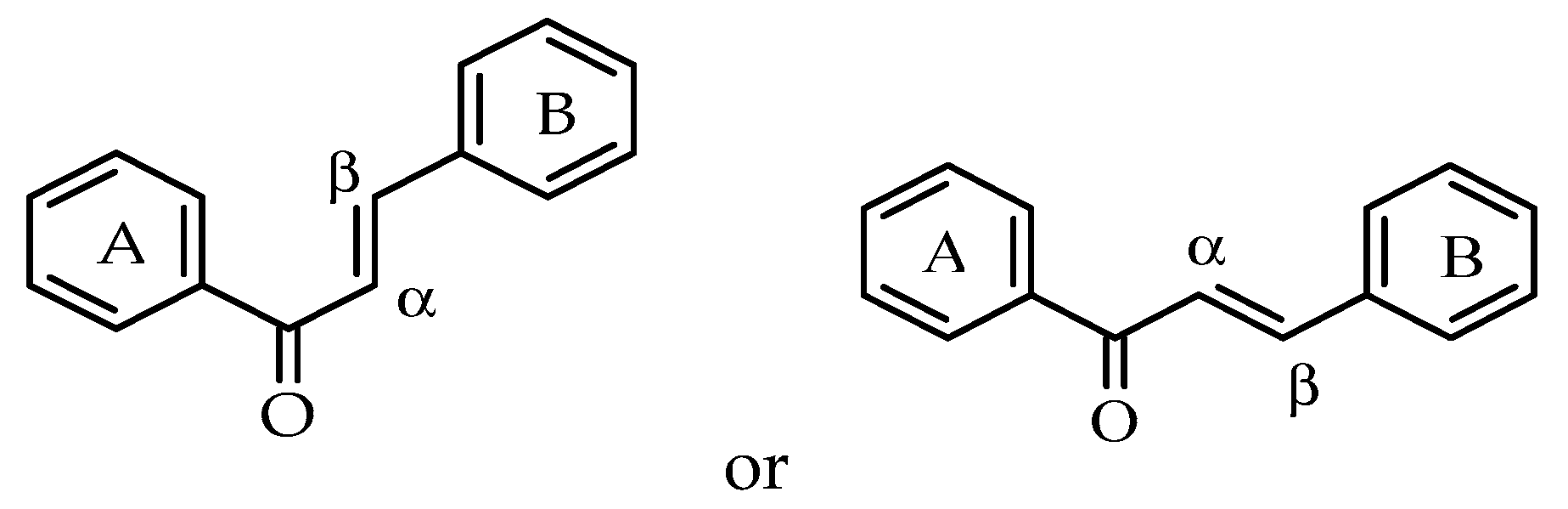
:1. Introduction
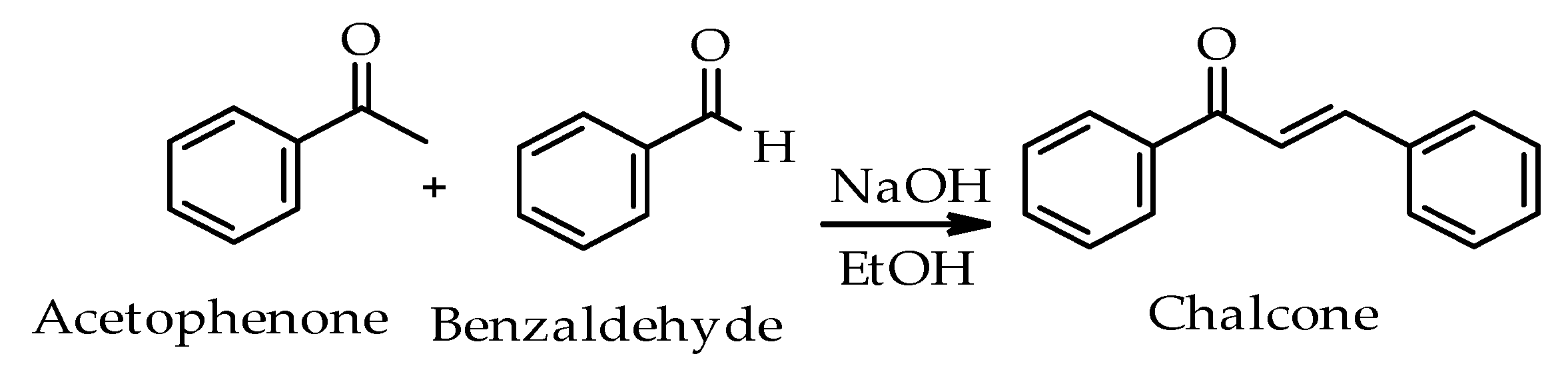
2. Chalcone Synthesis
3. Molecular Mechanisms of Chalcone Actions
3.1. Activation of Nuclear Factor-Erythroid 2 p45 Subunit-Related Factor 2 Pathway and Cellular Defence Genes
3.2. Activation of the Nuclear Factor-Kappa B (NF-κB) PI3K/Akt Signaling Pathway
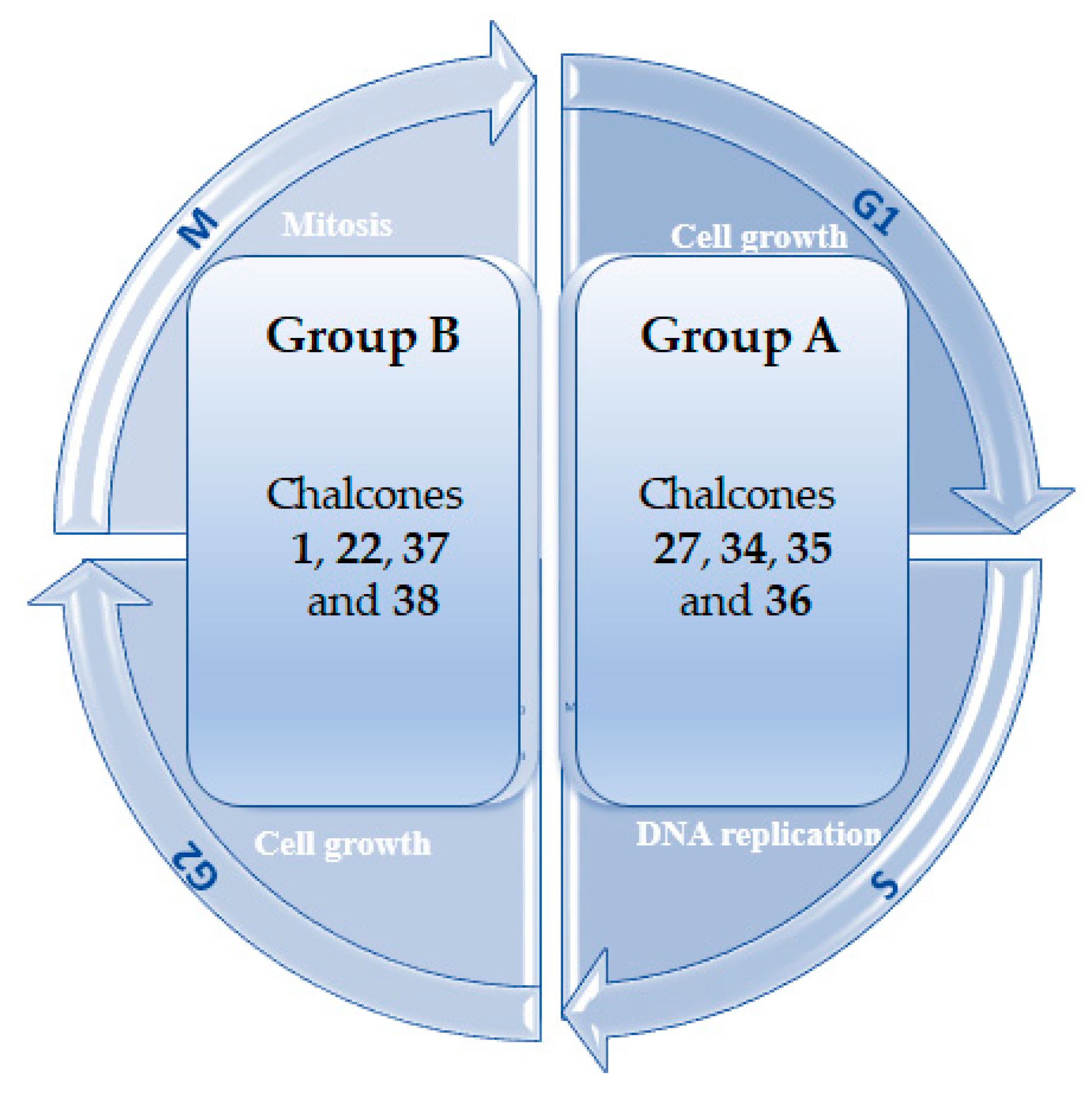
3.3. Effects on the Cell Cycle
3.4. Effects on Apoptosis
3.5. Binding with the Estrogenic Receptor (ER)
4. Bioactivity of Chalcones
4.1. Anticancer and Cancer Chemopreventive Activity
4.2. Antidiabetic Activity
4.3. Anti-Inflammatory Activity
4.4. Antimicrobial Activity
4.4.1. Antibacterial Activity
4.4.2. Antifungal Activity
4.4.3. Antiviral Activity
4.5. Antioxidant Activity
4.6. Antiparasitic Activity
4.7. Immunoregulatory Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nahar, L.; Sarker, S.D. Chemistry for Pharmacy Students: General, Organic and Natural Product Chemistry, 2nd ed.; Wiley and Sons: Chichester, UK, 2019. [Google Scholar]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T. Plant polyphenols: Recent advances in epidemiological research and other studies on cancer prevention. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, T.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. 269–295. [Google Scholar]
- Gupta, D.; Jain, D.; Trivedi, P. Recent advances in chalcones as anti-infective agents. Int. J. Chem. Sci. 2010, 8, 649–654. [Google Scholar]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. J. Med. Chem. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar] [CrossRef]
- Hutchins, W.A.; Wheeler, T.S. Chalkones: A new synthesis of chrysin, apigenin and luteolin. J. Chem. Soc. 1939, 91–94. [Google Scholar] [CrossRef]
- Bohm, B.A. Chalcones and aurones-7. Methods Plant. Biochem. 1989, 1, 237–282. [Google Scholar]
- Cazarolli, L.H.; Kappel, V.D.; Zanatta, A.P.; Suzuki, D.O.H.; Yunes, R.A.; Nunes, R.J.; Pizzolatti, M.G.; Silva, F.R.M.B. Natural and synthetic chalcones: Tools for the study of targets of action—Insulin secretagogue or insulin mimetic? In Studies in Natural Products Chemistry; Atta-Ur-Rahman, T.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. 47–89. [Google Scholar]
- Watson, R.R. Nutrition and Functional Foods for Healthy Aging, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2017; pp. 1–386. [Google Scholar]
- Higgins, L.G. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Pharmacol. Toxicol. Appl. Pharmacol. 2008, 226, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Thurston, D.E. Chemistry and Pharmacology of Anticancer Drugs; CRC Press Inc.: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zhang, Y.; Hou, Y.; Liu, C.; Li, Y.; Guo, W.; Wu, J.-L.; Xu, D.; You, X.; Pan, Y.; Chen, Y. Identification of an adaptor protein that facilitates Nrf2-Keap1 complex formation and modulates antioxidant response. Free Radic. Biol. Med. 2016, 97, 38–49. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, B.K.; Park, J.-H.; Choi, J.W.; Park, S.J.; Byeon, S.R.; Pae, A.N.; Lee, Y.S.; Cheong, E.; Park, K.D. A novel chalcone derivatives as Nrf2 activator attenuates learning and memory impairment in a scopolamine-induced mouse model. J. Med. Chem. Eur. J. Med. Chem. 2020, 185, 111777. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 signaling pathway by natural and synthetic chalcones: A therapeutic road map for oxidative stress. Expert Opin. Clin. Pharmacol. 2021, 14, 465–480. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Yakubu, M.T.; Oladiji, A.T. Electrophilic and reactive oxygen species detoxification potentials of chalcone dimers is mediated by redox transcription factor Nrf-2. J. Biochem. Mol. Toxicol. 2014, 28, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Meng, D.; Pan, Y.; Cui, Q.; Li, G.; Ni, H.; Sun, Y.; Qing, D.; Jia, X.; Pan, Y. Anti-neuroinflammatory and NQO1 inducing activity of natural phytochemicals from Coreopsis tinctoria. J. Funct. Foods 2015, 17, 837–846. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Vale, D.L.; Steffen, V.S.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Trans.-chalcone added in topical formulation inhibits skin inflammation and oxidative stress in a model of ultraviolet B radiation skin damage in hairless mice. J. Photochem. Photobiol. 2017, 171, 139–146. [Google Scholar] [CrossRef]
- Kachadourian, R.; Day, B.J.; Pugazhenti, S.; Franklin, C.C.; Genoux-Bastide, E.; Mahaffey, G.; Gauthier, C.; Di Pietro, A.; Boumendjel, A.N. A synthetic chalcone as a potent inducer of glutathione biosynthesis. J. Med. Chem. 2012, 55, 1382–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basar, N.; Nahar, L.; Oridupa, O.A.; Ritchie, K.J.; Talukdar, A.D.; Stafford, A.; Kushiev, H.; Kan, A.; Sarker, S.D. Utilization of the ability to induce activation of the nuclear factor (erythroid-derived 2)-like factor 2 (Nrf2) to assess potential cancer chemopreventive activity of liquorice samples. Phytochem. Anal. 2016, 27, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Plopper, G.; Sharp, D.; Sikorski, E.; Lewin, B. Lewin’s Cells, 3rd ed.; Plopper, G., Sharp, D., Sikorski, E., Eds.; Jones & Bartlett Learning: Burlington, MA, USA, 2015. [Google Scholar]
- Orlikova, B.; Schnekenburger, M.; Zloh, M.; Golais, F.; Diederich, M.; Tasdemir, D. Natural chalcones as dual inhibitors of HDACs and NF-κB. Oncol. Rep. 2012, 28, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Jeon, S.-H.; Kim, S.H.; Kim, C.; Lee, S.-J.; Koh, D.; Lim, Y.; Ha, K.; Shin, S.Y. A new synthetic chalcone derivative, 2-hydroxy-3′, 5, 5′-trimethoxychalcone (DK-139), suppresses the Toll-like receptor 4-mediated inflammatory response through inhibition of the Akt/NF-κB pathway in BV2 microglial cells. Exp. Mol. Med. 2012, 44, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Zhong, P.; Wu, L.; Qian, Y.; Fang, Q.; Liang, D.; Wang, J.; Zeng, C.; Wang, Y.; Liang, G. Blockage of ROS and NF-κB-mediated inflammation by a new chalcone L6H9 protects cardiomyocytes from hyperglycemia-induced injuries. BBA Mol. Basis Dis. 2015, 1852, 1230–1241. [Google Scholar] [CrossRef] [Green Version]
- Rajajendram, R.; Tham, C.L.; Akhtar, M.N.; Sulaiman, M.R.; Israf, D.A. Inhibition of epithelial CC-family chemokine synthesis by the synthetic chalcone DMPF-1 via disruption of NF-[kappa]B nuclear translocation and suppression of experimental asthma in mice. Mediat. Inflamm. 2015, 2015, 176926. [Google Scholar] [CrossRef] [Green Version]
- Kuruc, T.; Kello, M.; Petrova, K.; Kudlickova, Z.; Kubatka, P. The newly synthesized chalcone L1 is involved in the cell growth inhibition, induction of apoptosis and suppression of epithelial-to-mesenchymal transition of HeLa cells. Molecules 2021, 26, 1356. [Google Scholar] [CrossRef]
- Bortolotto, L.F.B.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of trans-chalcone and lichochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 2017, 85, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, M.; Liu, Y.; Zhang, M.; Yang, G. Tetramethoxychalcone, a chalcone derivative, suppresses proliferation, block cell cycle progression, and induces apoptosis of human ovarian cancer cells. PLoS ONE 2014, 9, e106206. [Google Scholar] [CrossRef] [Green Version]
- Hseu, Y.-C.; Lee, M.-S.; Wu, C.-R.; Cho, H.-J.; Lin, K.-Y.; Lai, G.-H.; Wang, S.-Y.; Kuo, Y.-H.; Kumar, K.J.S.; Yang, H.-L. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAOK signaling pathway. J. Agric. Food Chem. 2012, 60, 2385–2397. [Google Scholar] [CrossRef]
- Ko, H.; Kim, Y.; Park, J.; Amor, E.C.; Lee, J.W.; Yang, H. Dimethyl cardamonin induces G(1)-phase cell cycle arrest, apoptosis, and autophagy in HCT116 cells. Cancer Res. 2010, 70, 780. [Google Scholar]
- Maioral, M.F.; Gaspar, P.C.; Souza, G.R.R.; Mascarello, A.; Chiaradia, L.D.; Licínio, M.A.; Moraes, A.C.R.; Yunes, R.A.; Nunes, R.J.; Santos-Silva, M.C. Apoptotic events induced by synthetic naphthylchalcones in human acute leukemia cell lines. Biochimie 2013, 95, 866–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kello, M.; Drutovic, D.; Pilatova, M.B.; Tischlerova, V.; Perjesi, P.; Mojzis, J. Chalcone derivatives cause accumulation of colon cancer cells in the G2/M phase and induce apoptosis. Life Sci. 2016, 150, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Novilla, A.; Astuti, I.; Suwito, H. Molecular Mechanism of synthesized potential anticancer agent chalcone in leukemia cell line K562. J. Med. Sci. 2017, 49, 23–28. [Google Scholar]
- Rao, Y.K.; Kao, T.-Y.; Ko, J.-L.; Tzeng, Y.-M. Chalcone HTMC causes in vitro selective cytotoxicity, cell-cycle G 1 phase arrest through p53-dependent pathway in human lung adenocarcinoma A549 cells, and in vivo tumor growth suppression. Bioorg. Med. Chem. Lett. 2010, 20, 6508–6512. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.; Hassaneen, H.M.; Abdelhamid, I.A. Cytotoxicity, molecular modeling, cell cycle arrest, and apoptotic induction induced by novel tetrahydro-[1,2,4]triazolo[3,4-a]isoquinoline chalcones. J. Med. Chem. Eur. J. Med. Chem. 2018, 143, 532–541. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, Y.; Li, Y.; Shu, N.; Wang, Z.; Wang, Y.; Li, Y.; Chen, Z. Antineoplastic activity of isoliquiritigenin, a chalcone compound, in androgen-independent human prostate cancer cells linked to G2/M cell cycle arrest and cell apoptosis. Eur. J. Pharmacol. 2018, 821, 57–67. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Pilatova, M.B.; Kudlickova, Z.; Vilkova, M.; Slepcikova, P.; Petik, P.; Mojzis, J. New chalcone derivative exhibits antiproliferative potential by inducing G2/M cell cycle arrest, mitochondrial-mediated apoptosis and modulation of MAPK signalling pathway. Chem. Interact. 2018, 292, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wani, Z.A.; Pathania, A.S.; Mahajan, G.; Behl, A.; Mintoo, M.J.; Guru, S.K.; Viswanath, A.; Malik, F.; Kamal, A.; Mondhe, D.M. Anticancer activity of a novel quinazolinone-chalcone derivative through cell cycle arrest in pancreatic cancer cell line. J. Solid Tumors 2015, 5, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Kuo, P.; Tzeng, W.; Lin, C. Chalcone inhibits the proliferation of human breast cancer cell by blocking cell cycle progression and inducing apoptosis. Food Chem. Toxicol. 2006, 44, 704–713. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, D.; Li, X.-Z.; Jiang, Z.; Tan, C.; Wei, X.-Y.; Ling, J.; Jing, J.; Liu, F.; Li, N. A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay. Sci. Rep. 2017, 7, 10729. [Google Scholar] [CrossRef] [Green Version]
- Pedrini, F.S.; Licínio, M.A.; De Moraes, A.C.R.; Curta, J.C.; Costa, A.; Santos-Silva, M.C.; Chiaradia, L.D.; Mascarello, A.; Nunes, R.J.; Yunes, R.A.; et al. Induction of apoptosis and cell cycle arrest in L-1210 murine lymphoblastic leukaemia cells by (2E)-3-(2-naphthyl)-1-(3′-methoxy-4′-hydroxy- phenyl)-2-propen-1-one. J. Pharm. Pharmacol. 2010, 62, 1128–1136. [Google Scholar] [CrossRef]
- Zhang, Y.; Srinivasan, B.; Xing, C.; Lü, J. A new chalcone derivative (E)-3-(4-methoxyphenyl)-2-methyl-1-(3,4,5-trimethoxyphenyl) prop-2-en-1-one suppresses prostate cancer involving p53-mediated cell cycle arrests and apoptosis. Anticancer Res. 2012, 32, 3689–3698. [Google Scholar]
- Maggiolini, M.; Statti, G.; Vivacqua, A.; Gabriele, S.; Rago, V.; Loizzo, M.; Menichini, F.; Amdo, S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 82, 315–322. [Google Scholar] [CrossRef]
- Mutchtaridi, M.; Syahidah, H.N.; Subarans, A.; Yusuf, M.; Bryant, S.D.; Langer, T. Molecular docking and 3D-pharmacophore modeling to study the interactions of chalcone derivatives with estrogen receptor alpha. Pharmaceutical 2017, 10, 81. [Google Scholar]
- Prasetiawati, R.; Zamri, A.; Barliana, M.I.; Mutchtaridi, M. In silico predictive for modification of chalcone with pyrazole derivatives as a novel therapeutic compound for targeted breast cancer treatment. J. Appl. Pharm. Sci. 2019, 9, 20–28. [Google Scholar]
- Herber, C.B.; Quirti, J.G.; Firestone, G.; Krois, C. 2′,3′,4′-Trihydroxychalcone is an estrogen receptor ligand which modulates the activity of 17β-estradiol. bioRxiv 2019, 607275. [Google Scholar] [CrossRef]
- Branham, W.S.; Dial, S.L.; Moland, C.L.; Hass, B.S.; Blair, R.M.; Fang, H.; Shi, L.; Tong, W.; Perkins, R.G.; Sheehan, D.M. Phytoestrogens and mycoestrogens bind to the rat uterine estrogen receptor. J. Nutr. 2002, 132, 658–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dube, P.N.; Thombare, Y.B.; Chatpalliwar, V.A. Design and Synthesis of Novel chalcone-phenylpyranone derivatives as estrogen receptor modulators. Proceedings 2018, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Ghribia, L.; Ghouilaa, H.; Omrib, A.; Besbesb, M.; Janneta, H.B. Antioxidant and anti–acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac. J. Trop. Biomed. 2014, 4, S417–S423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voon, F.-L.; Sulaiman, M.R.; Akhtar, M.N.; Idris, M.F.; Akira, A.; Perimal, E.K.; Israf, D.A.; Ming-Tatt, L. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur. J. Pharmacol. 2017, 794, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Kim, M.K.; Choi, Y.K.; Shin, Y.C.; Cho, S.-G.; Ko, S.-G. Rhus verniciflua Stokes (RVS) and butein induce apoptosis of paclitaxel-resistant SKOV-3/PAX ovarian cancer cells through inhibition of AKT phosphorylation. BMC Complement. Altern. Med. 2016, 16, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozmer, Z.; Perjesi, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Hasegawa, D.; Ukiya, M.; Kimura, Y.; Enjo, F.; Suzuki, T.; Nishino, H. Chalcones and other compounds from the exudates of Angelica keiskei and their cancer chemopreventive effects. Indian J. Nat. Prod. 2006, 69, 38–42. [Google Scholar] [CrossRef]
- Memon, A.H.; Ismail, Z.; Aisha, A.F.; Al-Suede, F.S.R.; Hamil, M.S.R.; Hashim, S.; Saeed, M.A.A.; Laghari, M.; Majid, A.; Shah, A.M. Isolation, characterization, crystal structure elucidation, and anticancer study of dimethyl cardamonin, isolated from Syzygium campanulatum Korth. Evid. Based Complement. Alternat. Med. 2014, 2014, 470179. [Google Scholar] [CrossRef] [Green Version]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Abdullah, M.P.; Ho, C.L.; Omar, A.R.; Ismail, J.; Alitheen, N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases In vitro. BMC Complement. Altern. Med. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-F.; Su, Y.-Z.; Tseng, Y.-H.; Wang, S.-Y.; Wang, H.-M.; Chueh, P.J. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic. Biol. Med. 2010, 49, 214–226. [Google Scholar] [CrossRef]
- Yang, L.; Su, L.; Cao, C.; Xu, L.; Zhong, D.; Xu, L.; Liu, X. The chalcone 2′-hydroxy-4′, 5′-dimethoxychalcone activates death receptor 5 pathway and leads to apoptosis in human nonsmall cell lung cancer cells. IUBMB Life 2013, 65, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Taniguchi, M.; Baba, K.; Kimura, Y. Antitumor and antimetastatic actions of xanthoangelol and 4-hydroxyderricin isolated from Angelica keiskei roots through the inhibited activation and differentiation of M2 macrophages. Phytomedicine 2015, 22, 759–767. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, W.-Z.; Huo, A.; Zhou, W.; Jin, X.-H. Isobavachalcone inhibits the proliferation and invasion of tongue squamous cell carcinoma cells. Oncol. Lett. 2017, 14, 2852–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagura, T.; Motomiya, T.; Ito, M.; Honda, G.; Iida, A.; Kiuchi, F.; Tokuda, H.; Nishino, H. Anticarcinogenic compounds in the Uzbek medicinal plant, Helichrysum maracandicum. J. Nat. Med. 2008, 62, 174–177. [Google Scholar] [CrossRef]
- Ramirez-Tagle, R.; Escobar, C.A.; Romero, V.; Montorfano, I.; Armisén, R.; Borgna, V.; Jeldes, E.; Pizarro, L.; Simon, F.; Echeverria, C. Chalcone-induced apoptosis through caspase-dependent intrinsic pathways in human hepatocellular carcinoma cells. Int. J. Mol. Sci. 2016, 17, 260–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.; Pereira, S.; Paiva, R.; Cavalcante, C.; Sudo, S.; Tinoco, L.; Moreira, D.L.; Guimaraes, E.; Sudo, R.; Kaplan, M.; et al. Hypoglycemic effect of the methanol flower extract of piper claussenianum and the major constituent 2′,6′-dihydroxy-4′-methoxychalcone in streptozotocin diabetic rats. Indian J. Pharm. Sci. 2015, 77, 237–243. [Google Scholar]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef]
- Lin, C.-T.; Senthil Kumar, K.J.; Tseng, Y.-H.; Wang, Z.-J.; Pan, M.-Y.; Xiao, J.-H.; Chien, S.-C.; Wang, S.-Y. Anti-inflammatory activity of flavokawain B from Alpinia pricei Hayata. J. Agric. Food Chem. 2009, 57, 6060–6065. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kuang, Y.; Li, K.; Wang, S.; Song, W.; Qiao, X.; Sabir, G.; Ye, M. Screening for bioactive natural products from a 67-compound library of Glycyrrhiza inflata. Bioorg. Med. Chem. 2017, 25, 3706–3713. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Vinciguerra, I.; Ferrone, A.; Riccioni, H.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Licocalchone-C extracted from Glycyrrhiza glabra inhibits lipopolysaccharide-interferon-γ inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression. Molecules 2011, 16, 5720–5734. [Google Scholar] [CrossRef] [PubMed]
- Daikonya, A.; Kitanaka, S.; Katsuki, S. Antiallergic agents from natural sources 9. Inhibition of nitric oxide production by novel chalcone derivatives from Mallotus philippinensis (Euphorbiaceae). Chem. Pharm. Bull. 2004, 52, 1326–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampini, I.C.; Vattuone, M.A.; Isla, M.I. Antibacterial activity of Zuccagnia punctata Cav. ethanolic extracts. J. Ethnopharmacol. 2005, 102, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Endo, E.H.; Cortez, D.; Nakamura, T.; Nakamura, C.; Filho, B.D. Antimicrobial effects of Piper hispidum extract, fractions and chalcones against Candida albicans and Staphylococcus aureus. J. Med. Mycol. 2016, 26, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, T.L.C.; Cabral, I.S.; d’Arce, M.A.R.; Rosalen, P.L.; Ikegaki, M.; Nascimento, A.M.; Alencar, S.M. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Tupe, S.G.; Gample, S.P.; Chandgude, M.G.; Sarkar, D.; Deshpande, M.V.; Joshi, S.P. Antifungal dimeric chalcone derivative kamalachalcone E from Mallotus philippinensis. Nat. Prod. Res. 2014, 28, 245–250. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Balasooriya, B.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef]
- Mohammed, M.M.; Hamdy, A.-H.A.; El-Fiky, N.M.; Mettwally, W.S.; El-Beih, A.A.; Kobayashi, N. Anti-influenza A virus activity of a new dihydrochalcone diglycoside isolated from the Egyptian seagrass Thalassodendron ciliatum (Forsk.) den Hartog. Nat. Prod. Res. 2014, 28, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, T.T.; Nguyen, P.H.; Lee, H.S.; Kim, E.; Park, J.; Lim, S.I.; Oh, W.K. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg. Med. Chem. Lett. 2011, 21, 294–298. [Google Scholar] [CrossRef]
- Uchiumi, F.; Hatano, T.; Ito, H.; Yoshida, T.; Tanuma, S.-I. Transcriptional suppression of the HIV promoter by natural compounds. Antivir. Res. 2003, 58, 89–98. [Google Scholar] [CrossRef]
- Park, J.-Y.; Ko, J.-A.; Kim, D.W.; Kim, Y.M.; Kwon, H.-J.; Jeong, H.J.; Kim, C.Y.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem. 2016, 31, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Borges-Argáez, R.; Balnbury, L.; Flowers, A.; Giménez-Turba, A.; Ruiz, G.; Waterman, P.G.; Peña-Rodríguez, L.M. Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine 2007, 14, 530–533. [Google Scholar] [CrossRef]
- Rodrigues, D.; Maniscalco, D.; Silva, F.; Chiari, B.; Castelli, M.; Isaac, V.; Cicarelli, R.; Lopez, S. Trypanocidal activity of flavokawin B, a component of Polygonum ferrugineum Wedd. Planta Med. 2017, 83, 239–244. [Google Scholar] [CrossRef]
- Chen, M.; Theander, T.G.; Christensen, S.B.; Hviid, L.; Zhai, L.; Kharazmi, A. Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrob. Agents Chemother. 1994, 38, 1470–1476. [Google Scholar] [CrossRef] [Green Version]
- Yenesew, A.; Induli, M.; Derese, S.; Midiwo, J.O.; Heydenreich, M.; Peter, M.G.; Akala, H.; Wangui, J.; Liyala, P.; Waters, N.C. Anti-plasmodial flavonoids from the stem bark of Erythrina abyssinica. Phytochemistry 2004, 65, 3029–3032. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Masuda, Y.; Tomomura, M.; Yokose, S.; Uesawa, Y.; Ikezoe, N.; Asahara, D.; Takao, K.; Kanamoto, T.; Terakubo, S. Quantitative structure–cytotoxicity relationship of chalcones. Anticancer Res. 2017, 37, 1091–1098. [Google Scholar] [PubMed] [Green Version]
- da Silva Lima, D.C.; do Vale, C.R.; Véras, J.H.; Bernardes, A.; Pérez, C.N.; Chen-Chen, L. Absence of genotoxic effects of the chalcone (E)-1-(2-hydroxyphenyl)-3-(4-methylphenyl)-prop-2-en-1-one) and its potential chemoprevention against DNA damage using in vitro and in vivo assays. PLoS ONE 2017, 12, e0171224. [Google Scholar]
- Emayavaramban, M.; Santhi, N.; Gopi, C.; Manivannan, C.; Raguraman, A. Synthesis, Characterization and anti-diabetic activity of 1, 3, 5-triaryl-2-pyrazolines in acetic acid solution under ultrasound irradiation. Int. Lett. Chem. Phys. Astron. 2003, 9, 172–185. [Google Scholar] [CrossRef]
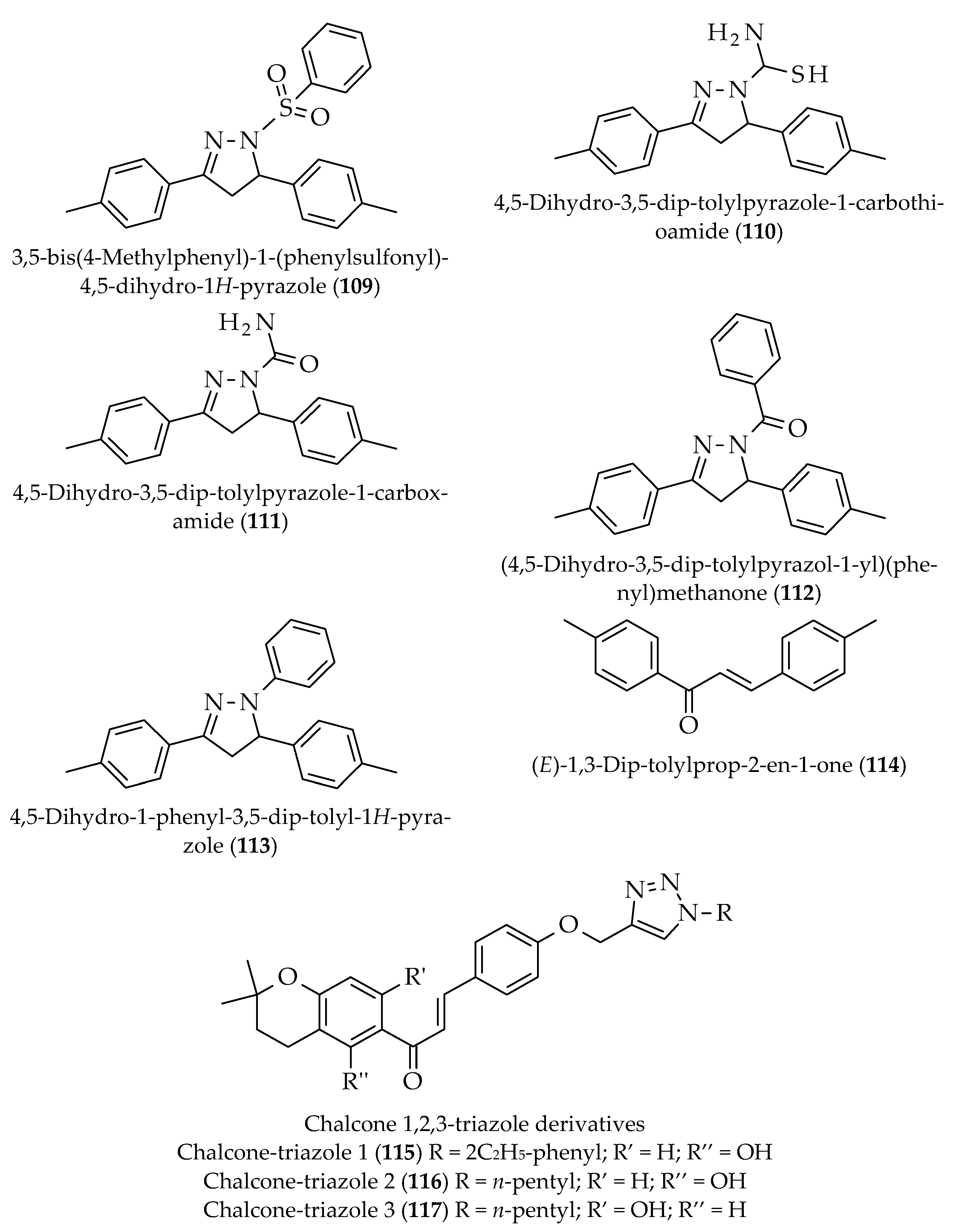
- Chinthala, Y.; Thakur, S.; Tirunagari, S.; Chinde, S.; Domatti, A.K.; Arigari, N.K.; Srinivas, K.; Alam, S.; Jonnala, K.K.; Khan, F. Synthesis, docking and ADMET studies of novel chalcone triazoles for anti-cancer and anti-diabetic activity. J. Med. Chem. Eur. 2015, 93, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-K.; Tsao, L.-T.; Wang, J.-P.; Lin, C.-N. Synthesis and anti-inflammatory effect of chalcones. J. Pharm. Pharmacol. 2000, 52, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-J.; Liu, C.-T.; Tsao, L.-T.; Weng, J.-R.; Ko, H.-H.; Wang, J.-P.; Lin, C.-N. Synthetic chalcones as potential anti-inflammatory and cancer chemopreventive agents. J. Med. Chem. Eur. 2005, 40, 103–112. [Google Scholar] [CrossRef]
- Jantan, I.; Bukhari, S.N.A.; Adekoya, O.A.; Sylte, I. Studies of synthetic chalcone derivatives as potential inhibitors of secretory phospholipase A2, cyclooxygenases, lipoxyugenase and pro-inflammatory cytokines. Drug Des. Dev. Ther. 2014, 8, 1405–1418. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-F.; Wu, S.-N.; Gao, J.-M.; Liao, Z.-Y.; Tseng, Y.-T.; Fulop, F.; Chang, F.-R.; Lo, Y.-C. The antioxidant, anti-inflammatory, and neuroprotective properties of the synthetic chalcone derivative AN07. Molecules 2020, 25, 2907. [Google Scholar] [CrossRef] [PubMed]
- Solankee, A.; Kapadia, K.; Ana Ćirić, M.; Soković, I.; Doytchinova, A.; Geronikaki, A. Synthesis of some new S-triazine based chalcones and their derivatives as potent antimicrobial agents. J. Med. Chem. Eur. J. Med. Chem. 2010, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
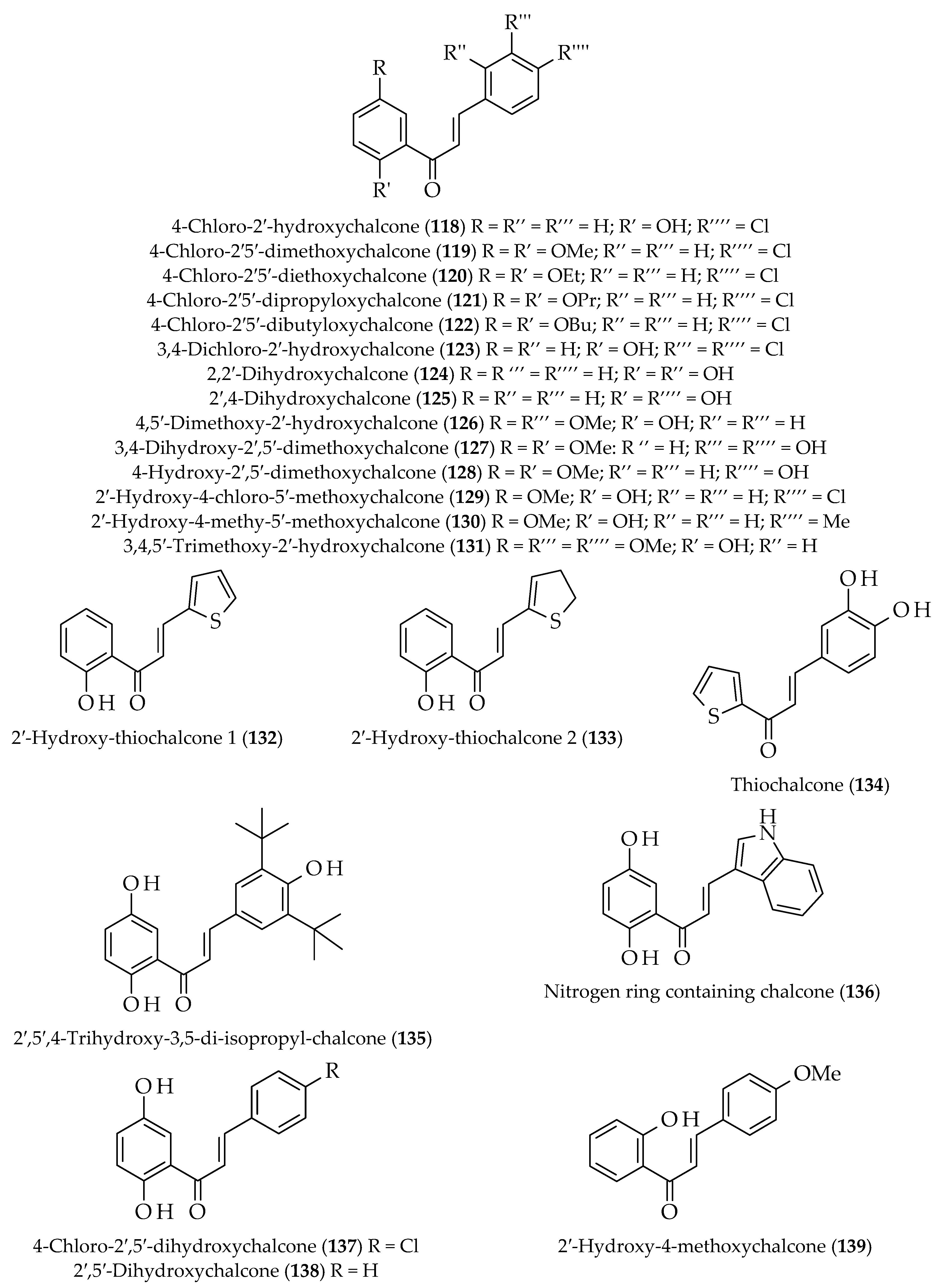
- Chu, W.-C.; Bai, P.-Y.; Yang, Z.-Q.; Cui, D.-Y.; Hua, Y.-G.; Yang, Y.; Yang, Q.-Q.; Zhang, E.; Qin, S. Synthesis and antibacterial evaluation of novel cationic chalcone derivatives possessing broad spectrum antibacterial activity. J. Med. Chem. Eur. 2018, 143, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Arif, R.; Rana, M.; Yasmeen, S.; Amaduddin, M.; Khan, M.S.; Abid, M.; Khan, M.S.; Rahisuddin, M. Facile synthesis of chalcone derivatives as antibacterial agents: Synthesis, DNA binding, molecular docking, DFT and antioxidant studies. J. Mol. Struct. 2020, 1208, 127905. [Google Scholar] [CrossRef]
- Morao, L.G.; Lorenzoni, A.S.G.; Chakraborty, P.; Ayusso, G.M.; Cavalca, L.B.; Santos, M.B.; Marques, B.C.; Dilarri, G.; Zamuner, C.; Regasini, L.O.; et al. Investigating the modes of action of the antimicrobial chalcones BC1 and T9A. Molecules 2020, 25, 4596. [Google Scholar] [CrossRef]
- Amole, K.L.; Bello, I.A.; Oyewale, A.O. Synthesis, characterization and antibacterial activities of new fluorinated chalcones. Chem. Afr. 2019, 2, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K.P. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 2019, 91, 103133. [Google Scholar] [CrossRef]
- Zhang, M.; Prior, A.M.; Maddox, M.M.; Shen, W.-J.; Hevener, K.E.; Bruhn, D.F.; Lee, R.B.; Singh, A.P.; Reinicke, J.; Simmons, C.J.; et al. Pharmacophore modeling, synthesis, and antibacterial evaluation of chalcones and derivatives. ACS Omega 2018, 3, 18343–18360. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, C.; Suresj, J.; Valarmathi, N.; Sumathi, S.; Karthikeyan, A.; Arun, A. Synthesis, characterization of acrylate polymer having chalcone moiety: Evaluation of antimicrobial, anticancer and drug release study. J. Biomater. Sci. Polym. Ed. 2020, 32, 438–453. [Google Scholar] [CrossRef]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Domínguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortés, J.C.; Ribas, J.C.; Devia, C.; Rodríguez, A.M. In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Tailor, N.K. Synthesis and antifungal activity of certain chalcones and their reduction. Indo Glob. J. Pharm. Sci. 2014, 4, 25–28. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D.K. Chalcone derivatives as potential antifungal agents: Synthesis and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114–117. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Gao, S.; Ma, M.; Ren, G.; Liu, H. Synthesis and antifungal activity of chalcone derivatives. Nat. Prod. Res. 2015, 29, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Espinoza, L.; Madrid, A.; Mella, J.; Chavez-Weisser, E.; Diaz, K.; Cuellar, M. Design, synthesis, antifungal activity, and structure-activity relationship studies of chalcones and hybrid dihydrochromane-chalcones. Mol. Div. 2020, 24, 603–615. [Google Scholar] [CrossRef]
- Andrade, J.T.; Santos, F.R.S.; Lima, W.G.; Sousa, C.D.F.; Oliveira, L.S.F.M.; Ribeiro, R.I.M.A.; Gomes, A.J.P.S.; Araujo, M.G.F.; Villar, J.A.F.P.; Ferreira, J.M.S. Design, synthesis, biological activity and structure activity relationship studies of chalcone derivatives as potential anti-Candida agents. J. Antibiot. 2018, 71, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Lagu, S.B.; Yejella, R.P.; Bhandare, R.R.; Shaik, A.B. Design, synthesis, and antibacterial and antifungal activities of novel trifluoromethyl and trifluoromethoxy substituted chalcone derivatives. Pharmaceuticals 2020, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.L.; Hossain, S.; Cole, A.M.; Phanstiel, O. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg. Med. Chem. 2016, 24, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Mateeva, N.; Eyunni, S.V.; Redda, K.K.; Ononuju, U.; Hansberry II, T.D.; Aikens, C.; Nag, A. Functional evaluation of synthetic flavonoids and chalcones for potential antiviral and anticancer properties. Bioorg. Med. Chem. Lett. 2017, 27, 2350–2356. [Google Scholar] [CrossRef]
- Fu, Y.; Zeng, L.H.; Ren, X.; Song, B.; Hu, D.; Gan, X. New chalcone derivatives: Synthesis, antiviral activity and mechanism of action. RSC Adv. 2020, 10, 24483. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Al-Hashimi, I.; Al Moustafa, A.-E.; Khalil, A. A comprehensive review on the antiviral activities of chalcones. J. Drug Target. 2021, 29, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Marinov, R.; Markova, N.; Krumova, S.; Yotovska, K.; Zaharieva, M.M.; Genova-Kalou, P. Antiviral properties of chalcones and their synthetic derivatives: A mini review. Pharmacia 2020, 67, 325–337. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, D.; He, F.; Song, B.; Hu, D. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorg. Med. Chem. Lett. 2018, 28, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Cheng, C.; Li, G.; Xie, J.; Shen, M.; Chen, Q.; Li, W.; He, W.; Qiu, P.; et al. Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharm. Sin. B 2019, 9, 335–350. [Google Scholar] [CrossRef]
- Stepanic, V.; Matijasic, M.; Horvat, T.; Verbanac, D.; Kucerova-Chlupacova, M.; Saso, L.; Zarkovic, N. Antioxidant activities of alkyl substiyuted pyrazine derivatives of chalcones—In vitro and in silico study. Antioxidants 2019, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Lahsasni, A.S.; Al Korbi, F.H.; Aljaber, N.A.-A. Synthesis, characterization and evaluation of antioxidant activities of some novel chalcones analogues. Chem. Cent. J. 2014, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Elaasser, M.M.; Asiri, A.M. Synthesis of novel chalcone-based phenothiazine derivatives as antioxidant and anticancer agents. Molecules 2020, 25, 4566. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Cheng, C.-C.; Shen, L.-L.; Wang, Z.-K.; Wu, S.-B.; Li, W.-L.; Chen, S.-H.; Zhou, R.-P.; Qiu, P.-H. Synthetic chalcones with potent antioxidant ability on H2O2-induced apoptosis in PC12 cells. Int. J. Mol. Sci. 2014, 15, 18525–18539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatachalam, H.; Nayak, Y.; Jayashree, B.S. Synthesis, characterization and antioxidant activities of synthetic chalcones and flavones. APCBEE Procedia 2012, 3, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Padhye, S.; Ahmad, A.; Oswa, N.; Sarkar, F.H. Emerging role of garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J. Hematol. Oncol. 2009, 2, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, H.; Prabhakar, V.; Sangith, A.; Chandrika, B.; Balasubramanian, R. Synthesis, anti-inflammatory and antioxidant activity of ring-A-monosubstituted chalcone derivatives. Med. Chem. Res. 2014, 23, 4383–4394. [Google Scholar] [CrossRef]
- Ugwu, D.I.; Ezema, B.E.; Eze, F.U.; Onoabedje, E.A.; Ezema, C.G.; Ekoh, O.C.; Ayogu, J.I. Synthesis and antimalarial activities of chalcone derivatives. Int. J. ChemTech Res. 2015, 7, 1966–1984. [Google Scholar]
- Yadav, N.; Dixit, S.K.; Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Antimalarial activity of newly synthesized chalcone derivatives in vitro. Chem. Biol. Drug Des. 2012, 80, 340–347. [Google Scholar] [CrossRef]
- Dominguez, J.N.; de Dominguez, N.G.; Rodrigues, J.; Acosta, E.M.; Caraballo, N.; Leon, C. Synthesis and antimalarial activity of uranyl Bis-chalcone in vitro and in vivo. J. Enzyme Inhib. Med. Chem. 2013, 28, 1267–1273. [Google Scholar] [CrossRef] [Green Version]
- Roussaki, M.; Hall, B.; Lima, S.C.; da Silva, A.C.; Wilkinson, S.; Detsi, A. Synthesis and anti-parasitic activity of a novel quinolinone-chalcone series. Bioorg. Med. Chem. Lett. 2013, 23, 6436–6441. [Google Scholar] [CrossRef]
- Sinha, S.; Radotra, B.D.; Medhi, B.; Batovska, D.I.; Markova, N.; Sehgal, R. Ultrastructural alterations in Plasmodium falciparum induced by chalcone derivatives. BMC Res. Notes 2020, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.A.; Upegui, Y.A.; Rivas, L.; Echeverri, F.; Escobar, G.; Robledo, S.M.; Quinones, W. Effect of substituents in the A and B rings of chalcones on antiparasitic activity. Arch. Pharm. 2020, 353, 2000157. [Google Scholar] [CrossRef] [PubMed]
- Bhoj, P.; Togre, N.; Bahekar, S.; Goswami, K.; Chandak, H.; Patil, M. Immunomodulatory activity of sulfonamide chalcone compounds in mice infected with filarial parasite Brugia malayi. Indian J. Clin. Biochem. 2019, 34, 225–229. [Google Scholar] [CrossRef]
- Lee, J.S.; Bukhari, S.N.A.; Fauzi, N.M. Effects of chalcone derivatives on players of the immune system. Drug Des. Dev. Ther. 2015, 9, 4761–4778. [Google Scholar]
- Arshad, L.; Jantan, I.; Bukhari, S.N.A.; Haque, M.A. Immunosuppressive effects of natural α,β-unsaturated carbonyl-based compounds and their analogs and derivatives on immune cells: A review. Front. Pharmacol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]





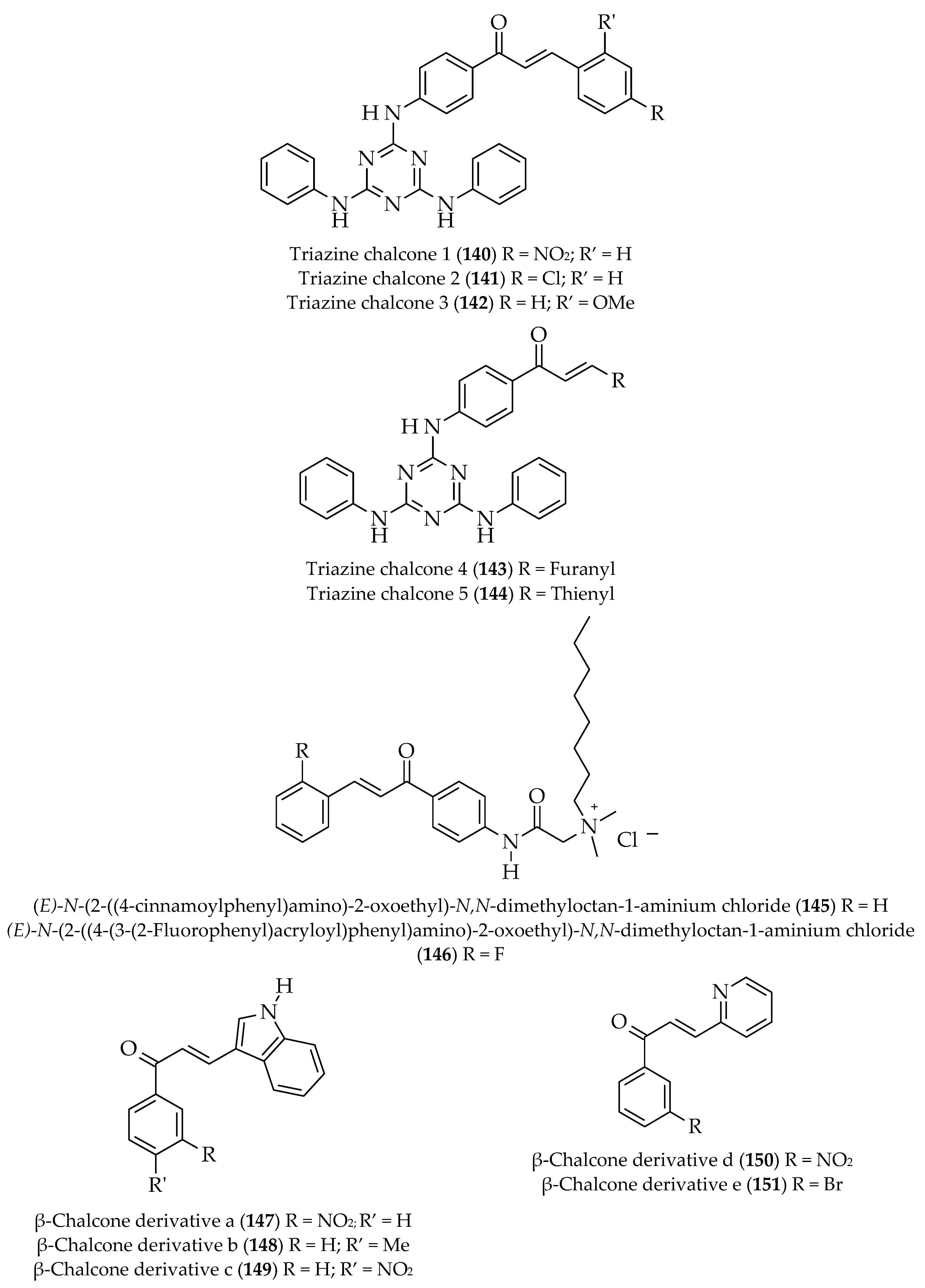
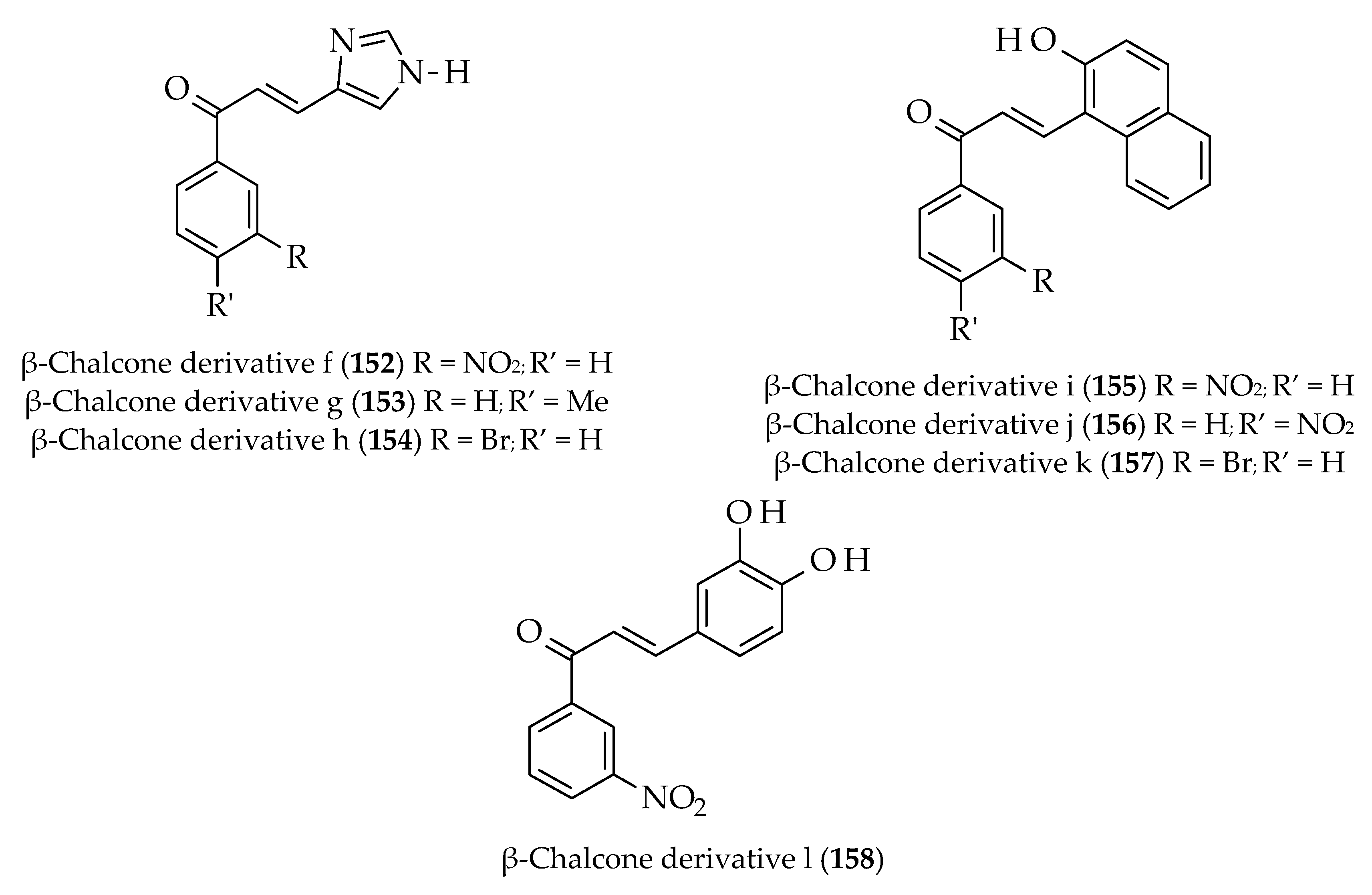
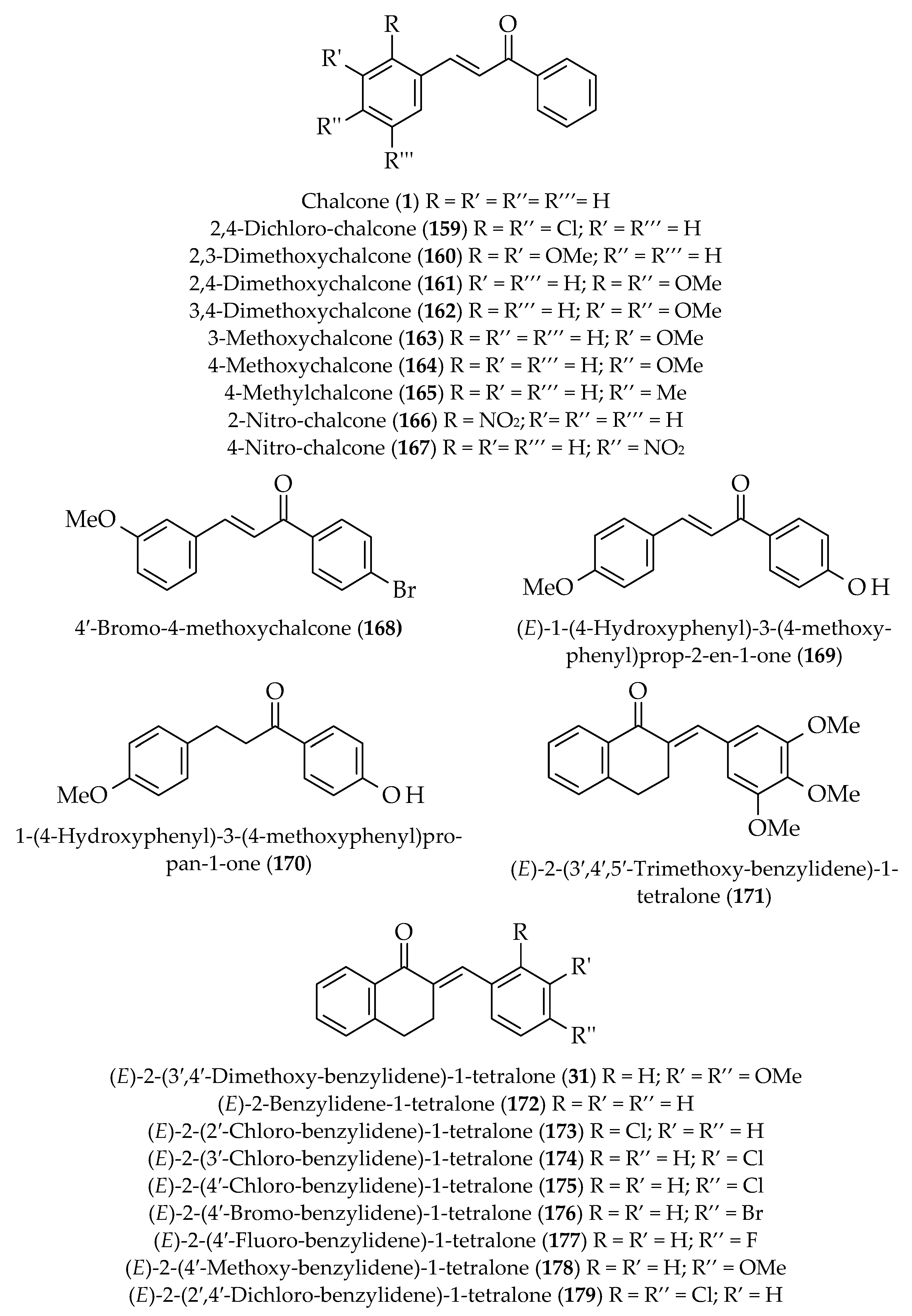
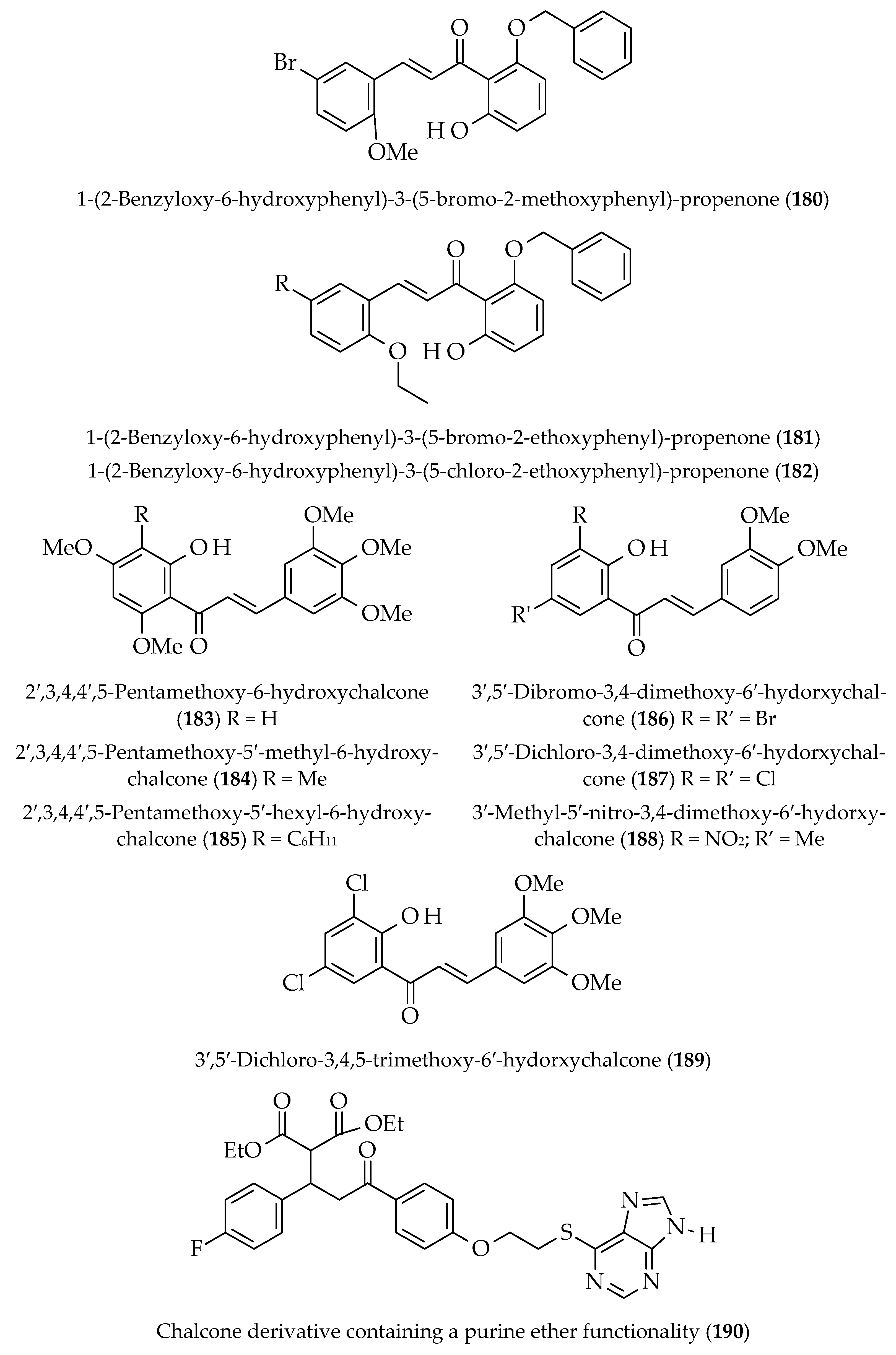
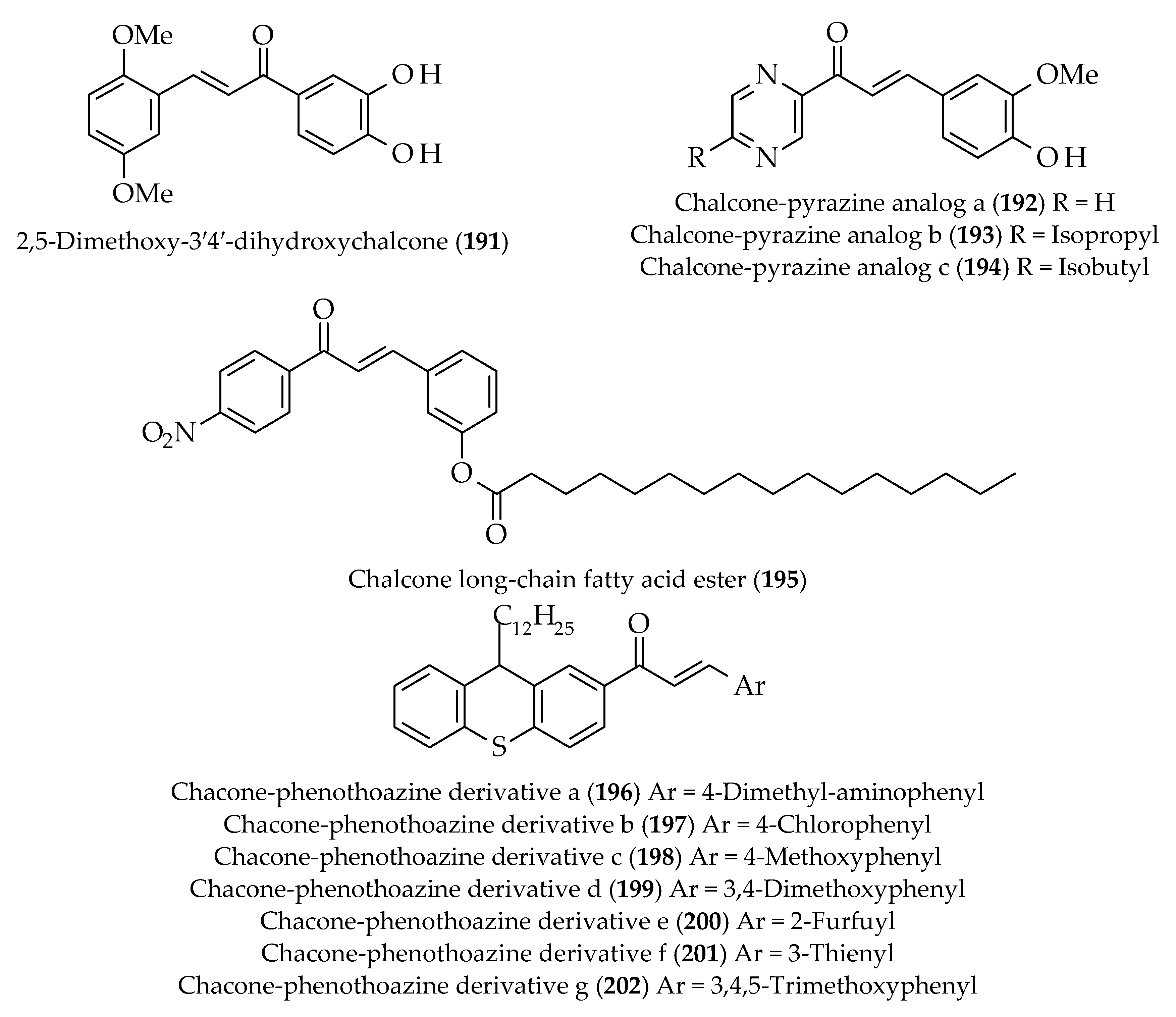

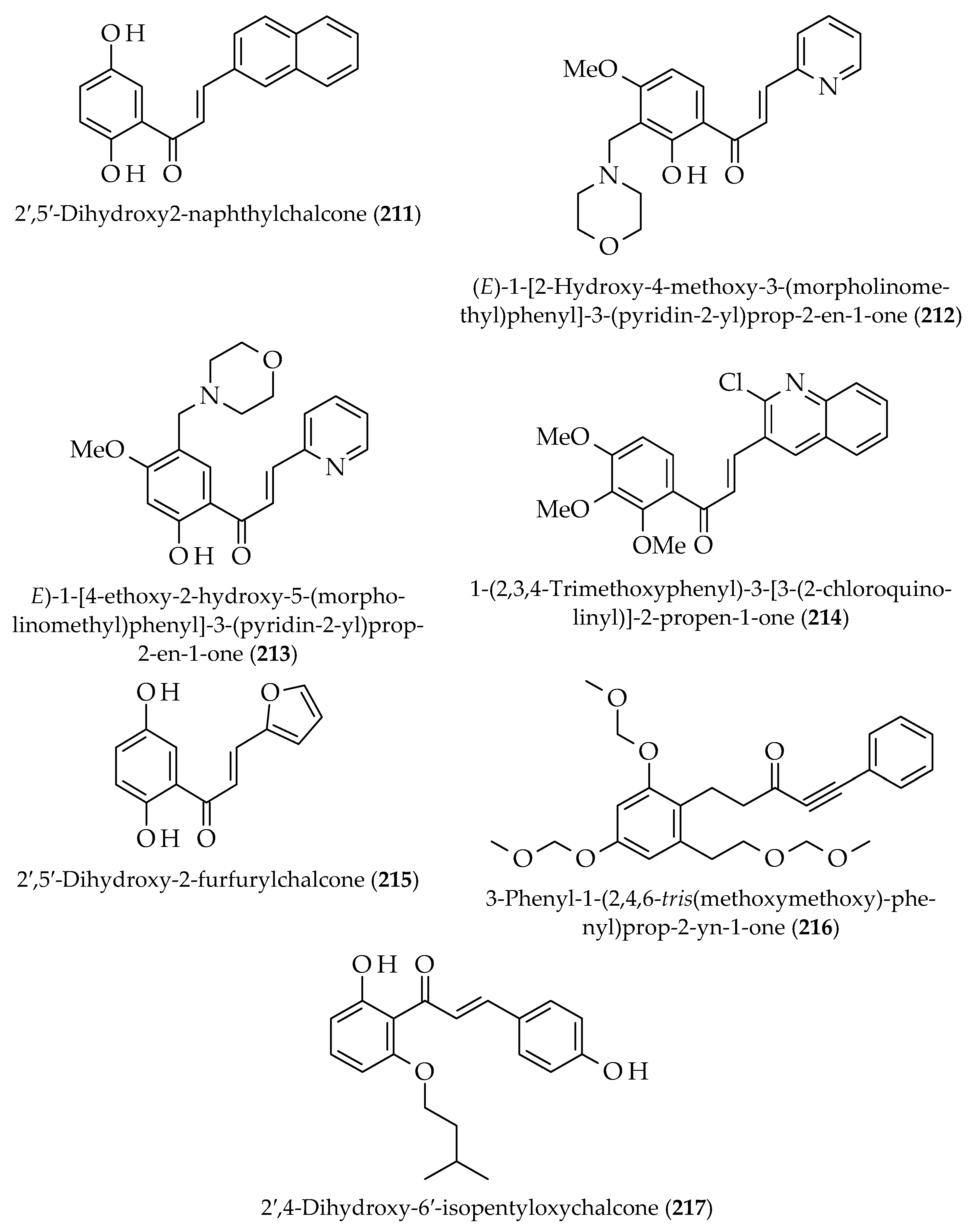
| Approach | Compound 1 | Compound 2 | Reaction Conditions | Synthesize Chalcone and Its Derivatives |
|---|---|---|---|---|
| Microwave |  | 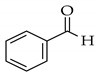 | NaOH/MeOH, microwave radiation at 110 W, 55 °C | 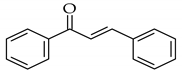 (1) |
| I2-Al2O3, microwave radiation for 80 s | ||||
| Ionic liquids | 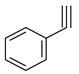 |  | HBr, Bmim(OTs), 12 h, 100 °C | |
| Claisen-Schmidt | 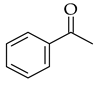 |  | Base | |
| Using SOCl2 | 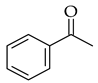 | 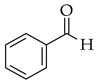 | SOCl2/EtOH | |
| Alkynes and aldehydes | 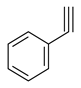 | 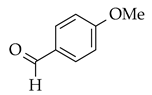 | Amberlyst-15, DCM, at r.t. | 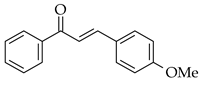 (2) |
| Sonogashira coupling |  | 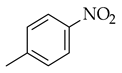 | [PdCl2(PPh3)2], Et3N, THF, heat | 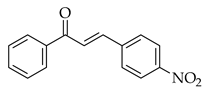 (3) |
| Cross-coupling | 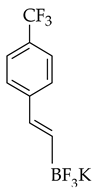 |  | [PdCl2(dtbpf)], K2CO3, 1,4-dioxane, microwave radiation for 30 min at 140 °C | 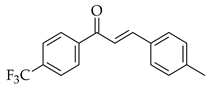 (4) |
| Wittig reaction | 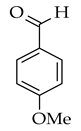 | 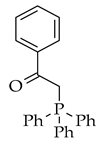 | Br-, NaOEt, toluene, microwave radiation for 5 min |  (2) |
| Claisen-Schmidt | 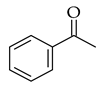 |  | Zeolite ultrasound | 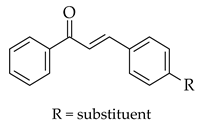 (5) |
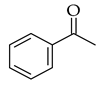 |  | NaOH base | 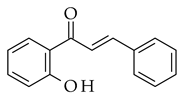 (6) | |
| Solvent free |  | 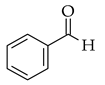 | SiO2, H2SO4, 80 °C |  (7) |
| Suzuki reaction | 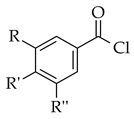 | 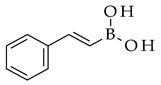 | (PPh3)4Pd(0), Cs2CO3, toluene | 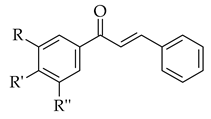 (8) |
 |  | PdCl2, Na2CO3, water/acetone | 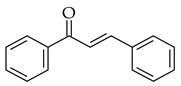 (1) | |
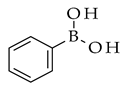 |  | Pd | 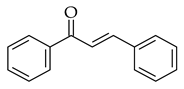 (1) | |
| Base-driven | 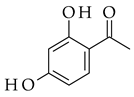 |  | KOH/EtOH, heat, 1–5 h | 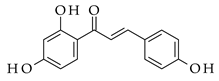 (9) |
 |  | NaOH | 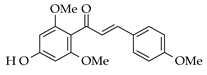 (10) |
| Bioactivities | Chalcones | Sources | References | |
|---|---|---|---|---|
| Anti-acetylcholinesterase | Isosalipurposide (66) Naringenin chalcone (78) | Acacia cyanophylla | [50] | |
| Anti-arthritic | Cardamonin (48) | Boesenbergia rotunda | [51] | |
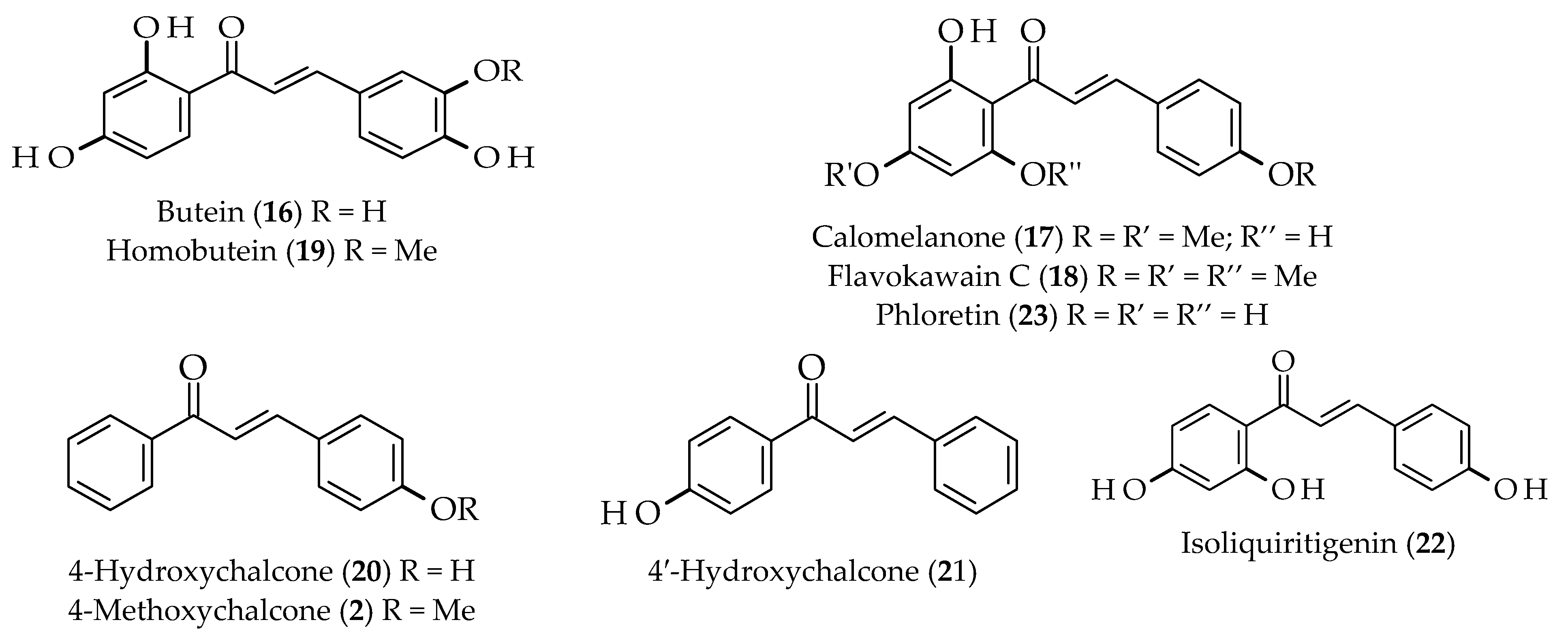
| Anticancer | Butein (16) | Toxicodendron vernicifluum | [52] | |
| Butea dahlia | [22,53] | |||
| Calomelanone (17) | Stevia lucida | |||
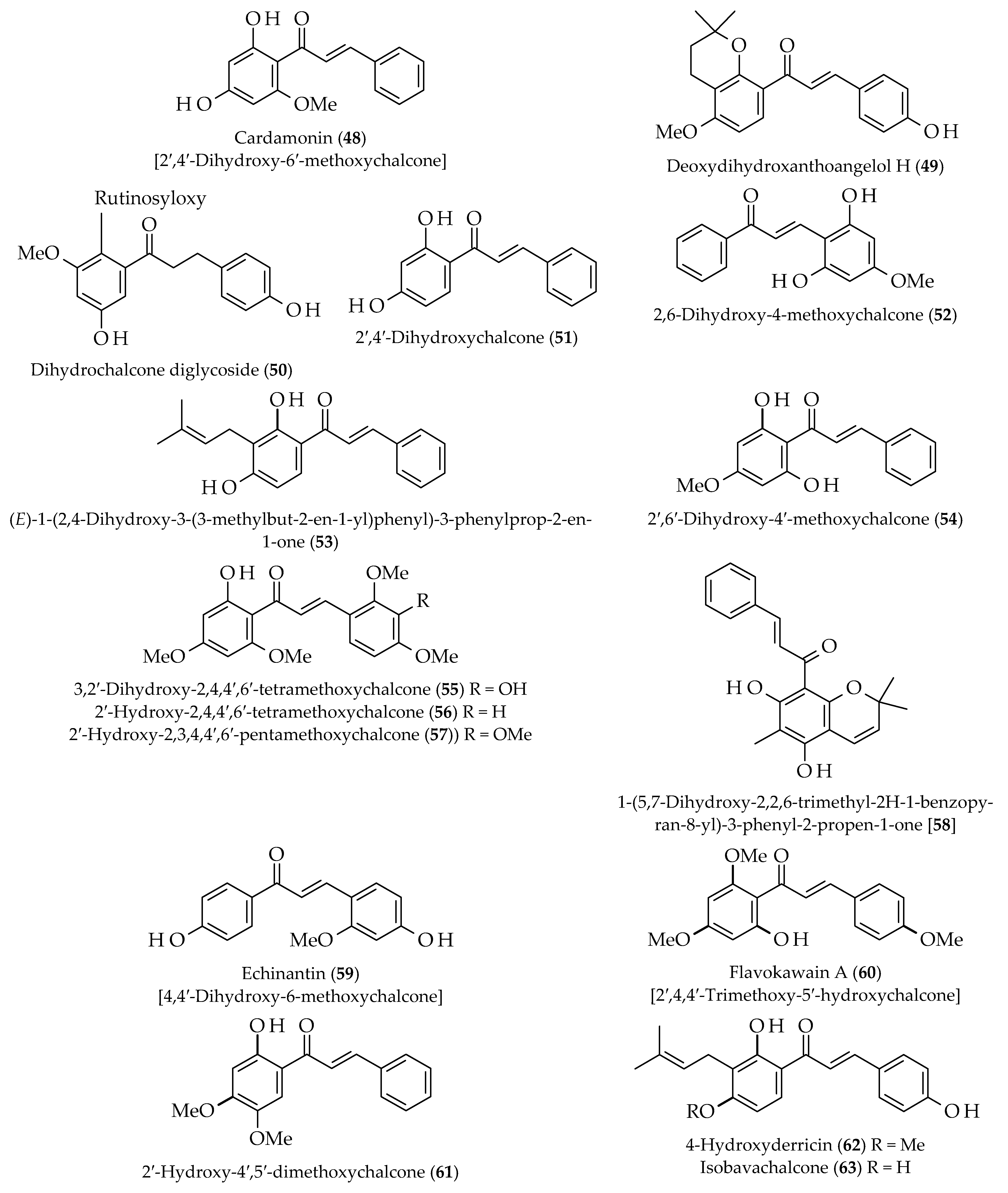
| Deoxydihydroxanthoangelol H (49) | Angelica keiskei | [54] | ||
| 2,6-Dihydroxy-4-methoxychalcone (52) | ||||
| Dimethyl-cardamonin (27) | Syzygium campanulatum | [55] | ||
| Echinantin (59) | Piper methysticum & Alpinia pricei | [56] | ||
| Flavokawain A (60) | Piper methysticum | |||
| Flavokawain B (28) | ||||
| Flavokawain B (28) | Alpinia pricei | [57] | ||
| Flavokawain C (18) | Piper methysticum | [22,53] | ||
| Homobutein (19) | Butea frondosa | |||
| 2′-Hydroxy-4′,5′-dimethoxychalcone (61) | Sarcandra hainanensis | [58] | ||
| 4-Hydroxychalcone (20) | Glycyrrhiza glabra | [22,53] | ||
| 4′-Hydroxychalcone (21) | ||||
| 4-Hydroxyderricin (62) | Angelica keiskei | [59] | ||
| Isobavachalcone (63) | [54] | |||
| Isobavachalcone (63) | Psoralea corylifolia | [60] | ||
| Isoliquiritigenin (22) | Piper methysticum | [57] | ||
| Alpinia pricei | ||||
| Glycyrrhiza glabra | [22,53] | |||
| Isosalipurposide (66) | Helichrysum maracandicum | [61] | ||
| Lonchocarpin (41) | Pongamia pinnata | [41] | ||
| Marein (77) | Glycyrrhiza glabra | [22,53] | ||
| 4-Methoxychalcone (2) | ||||
| Phloretin (23) | Stevia lucida | [22,53] | ||
| 2,3,4-Trimethoxy-2′-hydroxychalcone (82) | Piper methysticum | [62] | ||
| Xanthoangelol (83) | Angelica keiskei | [59] | ||
| Xanthoangelol I (91) | [54] | |||
| Xanthoangelol J (92) | ||||
| Antidiabetic | 2′,6′-Dihydroxy-4′-methoxychalcone (54) | Piper claussenianum | [63] | |
| 4-Hydroxyderricin (62) | Angelica keiskei | [64] | ||
| Xanthoangelol (83) | ||||
| Anti-inflammatory | Flavokawain B (28) | Alpinia pricei | [65] | |
| Isoliquiritigenin (22) | ||||
| Licoagrochalcone A (70) | Glycyrrhiza inflata | [66] | ||
| Licochalcone B (68) | ||||
| Licochalcone C (71) | Glycyrrhiza glabra | [67] | ||
| Mallotophilippens C (74) | Mallotus philippinensis | [68] | ||
| Mallotophilippens D (75) | ||||
| Mallotophilippens E (76) | ||||
| Antimicrobial | ||||
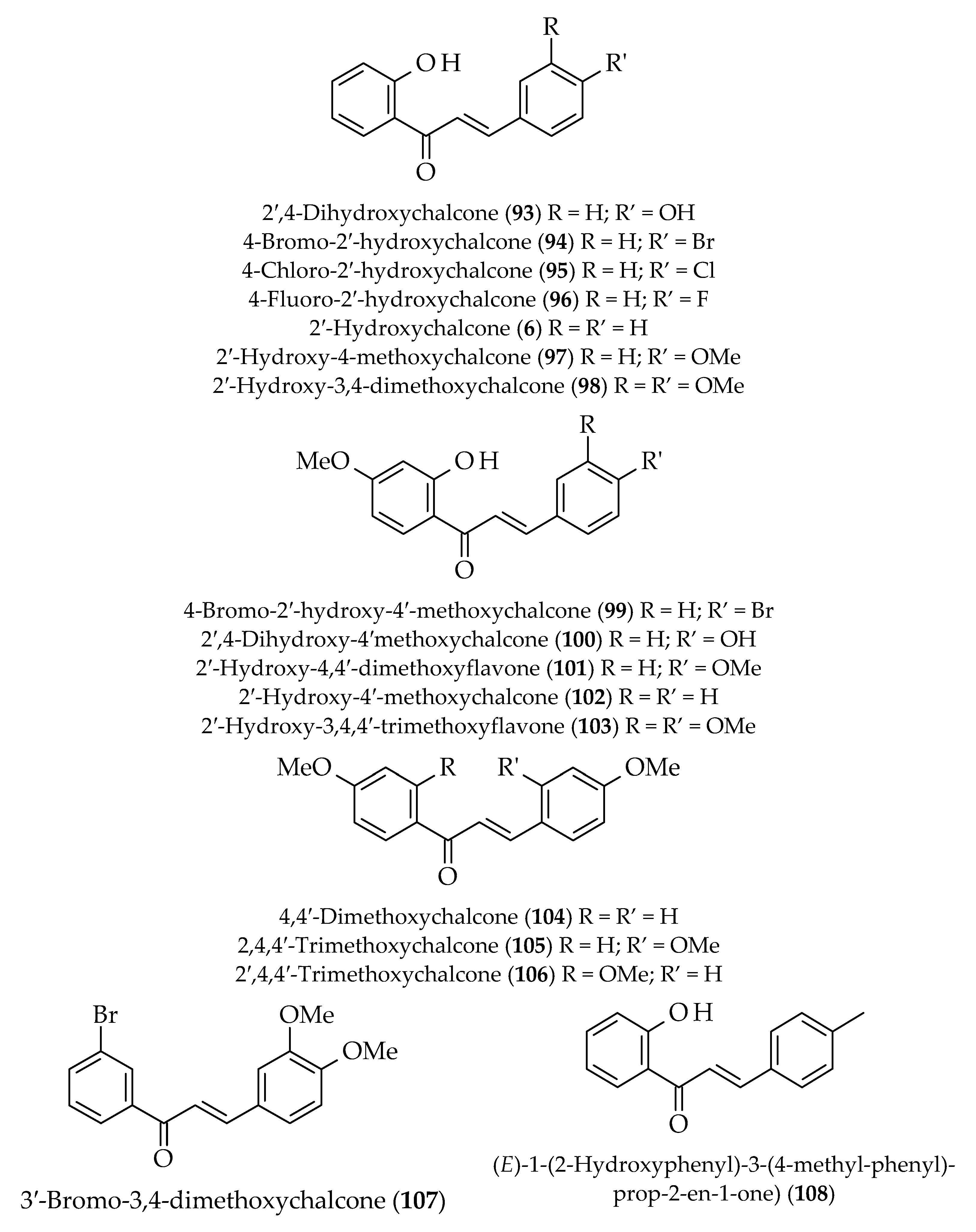
| Antibacterial | 2′,4′-Dihydroxychalcone (51) | Zuccagnia punctata | [69] | |
| 3,2′-Dihydroxy-2,4,4′,6′-tetramethoxychalcone (55) | Piper hispidum | [70] | ||
| 2′-Hydroxy-2,4,4′,6′-tetramethoxychalcone (56) | ||||
| 2′-Hydroxy-2,3,4,4′,6′-pentamethoxychalcone (57) | ||||
| Isoliquiritigenin (22) | Apis mellifera | [71] | ||
| Antifungal | 1-(5,7-Dihydroxy-2,2,6-trimethyl-2H-1-benzopyran-8-yl)-3-phenyl-2-propen-1-one [58] | Mallotus philippinensis | [72] | |
| 3,2′-Dihydroxy-2,4,4′,6′-tetramethoxychalcone (55) | Piper hispidum | [70] | ||
| 2′-Hydroxy-2,4,4′,6′-tetramethoxychalcone (56) | ||||
| 2′-Hydroxy-2,3,4,4′,6′-pentamethoxychalcone (57) | ||||
| 4′-Hydroxyrottlerin (64) | Mallotus philippinensis | [72] | ||
| Isoliquiritigenin (22) | Apis mellifera | [71] | ||
| Kamalachalcone E (67) | Mallotus philippinensis | [72] | ||
| Rottlerin (65) | ||||
| 2′,3,4,4′-Tetrahydroxy-3′-geranylchalcone (85) | Artocarpus nobilis | [73] | ||
| 2′,3,4,4′-Tetrahydroxy-3′-(6-hydroxy-3,7-dimethyl-7-octadienyl)chalcone (79) | ||||
| 2′,4′,4-Trihydroxy-3′-geranylchalcone (83) [xanthoangelol] | ||||
| 2′,4′,4-Trihydroxy-3′-(6-hydroxy-3,7-dimethyl-7-octadienyl)chalcone (80) | ||||
| 2′,4′,4-Trihydroxy-3′-[2-hydroxy-7-methyl-3-methylene-6-octenyl-chalcone] (81) | ||||
| Antiviral | Dihydrochalcone diglycoside (50) | Thalassodendrin ciliatum | [74] | |
| Echinantin (59) | Glycyrrhiza inflata | [75] | ||
| Isoliquiritigenin (22) | ||||
| Licochalcone A (40) | Quercus coccifera | [76] | ||
| Glycyrrhiza inflata | [75] | |||
| Licochalcone B (68) | Quercus coccifera | [76] | ||
| Licochalcone D (72) | Glycyrrhiza inflata Quercus coccifera | [76] | ||
| Licochalcone G (73) | ||||
| Tetrahydroxy-methoxychalcone (69) | [76] | |||
| Xanthoangelol (83) | Angelica keiskei | [77] | ||
| Xanthoangelol F (84) | ||||
| Xanthoangelol D (89) | ||||
| Xanthoangelol E (90) | ||||
| Xanthoangelol B (86) | ||||
| Xanthoangelol G (87) | ||||
| Xanthodeistal A (88) | ||||
| Antioxidant | Licochalcone C (71) | Glycyrrhiza glabra | [67] | |
| Isosalipurposide (66) Naringenin chalcone (78) | Acacia cyanophylla | [50] | ||
| Antiparasitic | (E)-1-(2,4-Dihydroxy-3-(3-methylbut-2-en-1-yl)phenyl)-3-phenylprop-2-en-1-one (53) | Lonchocarpus sp. | [78] | |
| Flavokawain B (28) | Polygonum ferrugineum | [79] | ||
| Licochalcone C (71) | Glycyrrhiza glabra | [80] | ||
| Licoagrochalcone A (70) | Erythrina abyssinica | [81] | ||
| Immunoregulatory | Mallotophilippens C (74) | Mallotus philippinensis | [68] | |
| Mallotophilippens D (75) | ||||
| Mallotophilippens E (76) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. https://doi.org/10.3390/biom11081203
Jasim HA, Nahar L, Jasim MA, Moore SA, Ritchie KJ, Sarker SD. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules. 2021; 11(8):1203. https://doi.org/10.3390/biom11081203
Chicago/Turabian StyleJasim, Hiba A., Lutfun Nahar, Mohammad A. Jasim, Sharon A. Moore, Kenneth J. Ritchie, and Satyajit D. Sarker. 2021. "Chalcones: Synthetic Chemistry Follows Where Nature Leads" Biomolecules 11, no. 8: 1203. https://doi.org/10.3390/biom11081203









