Combined Analytical Approaches to Standardize and Characterize Biomaterials Formulations: Application to Chitosan-Gelatin Cross-Linked Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
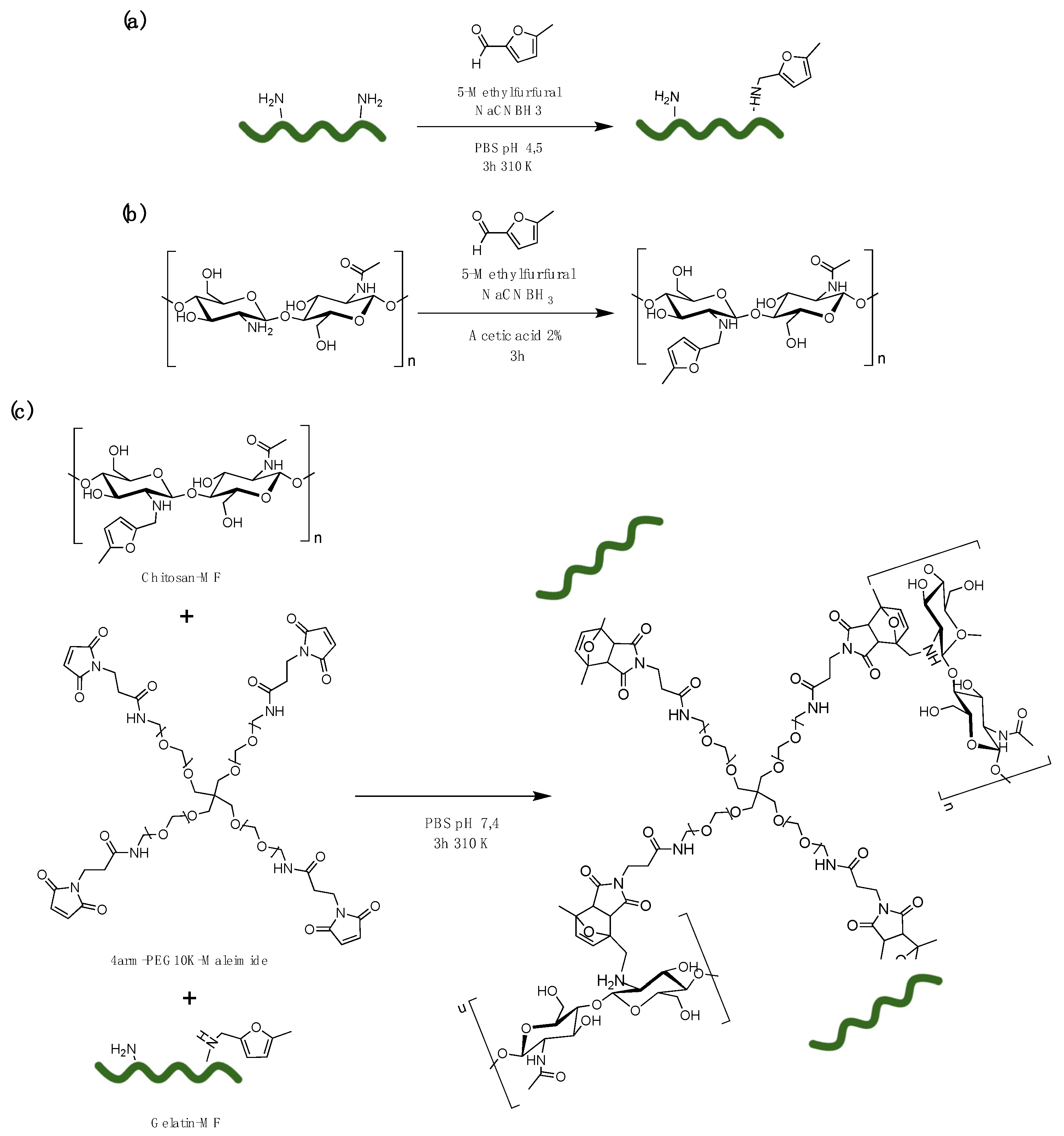
2.1. Sample Preparations
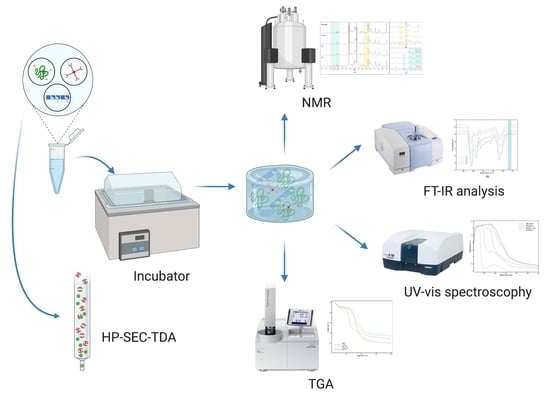
2.2. Analytical Methods
3. Results
3.1. Molecular Weight Distribution by Size Exclusion Chromatography with Triple Detector Array (HP-SEC-TDA) Analysis
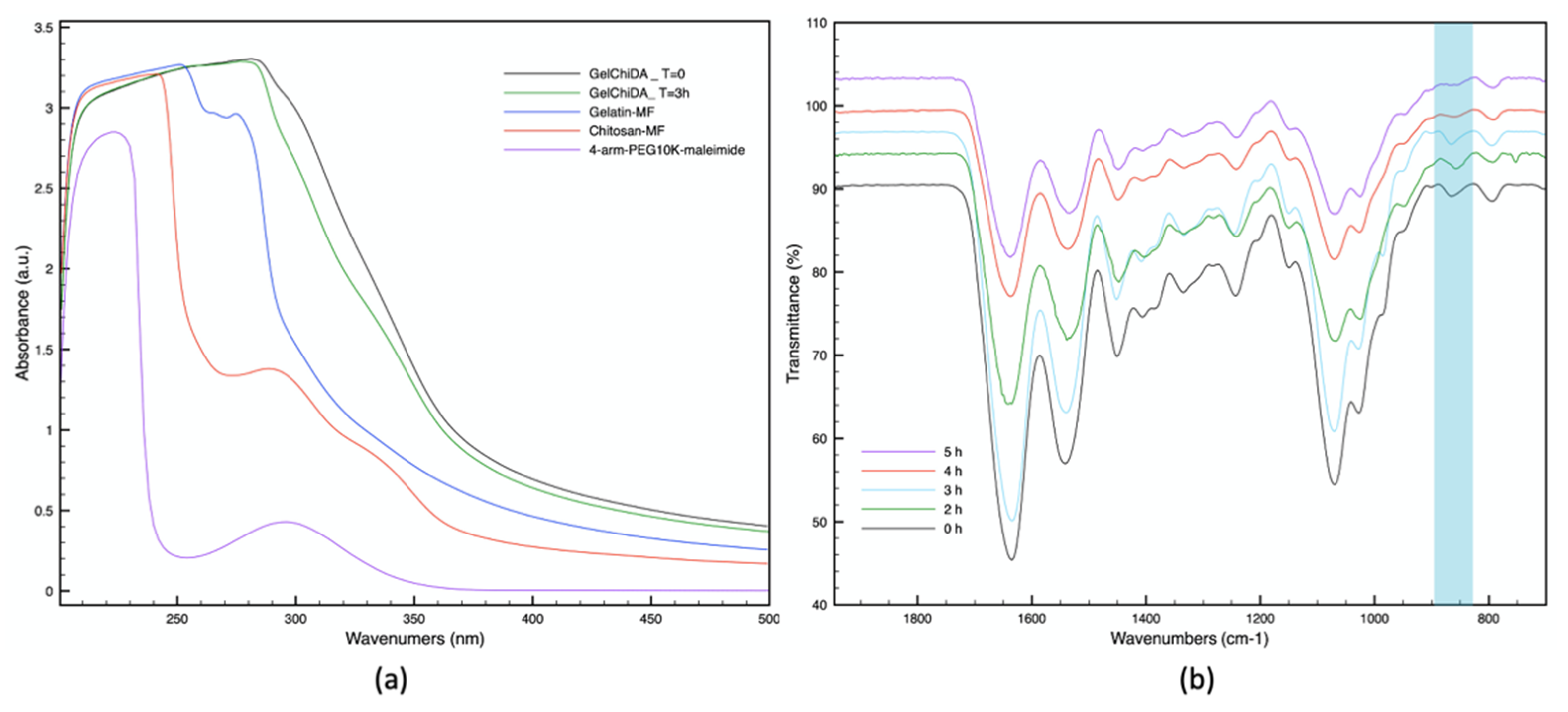
3.2. UV and FT-IR Analysis
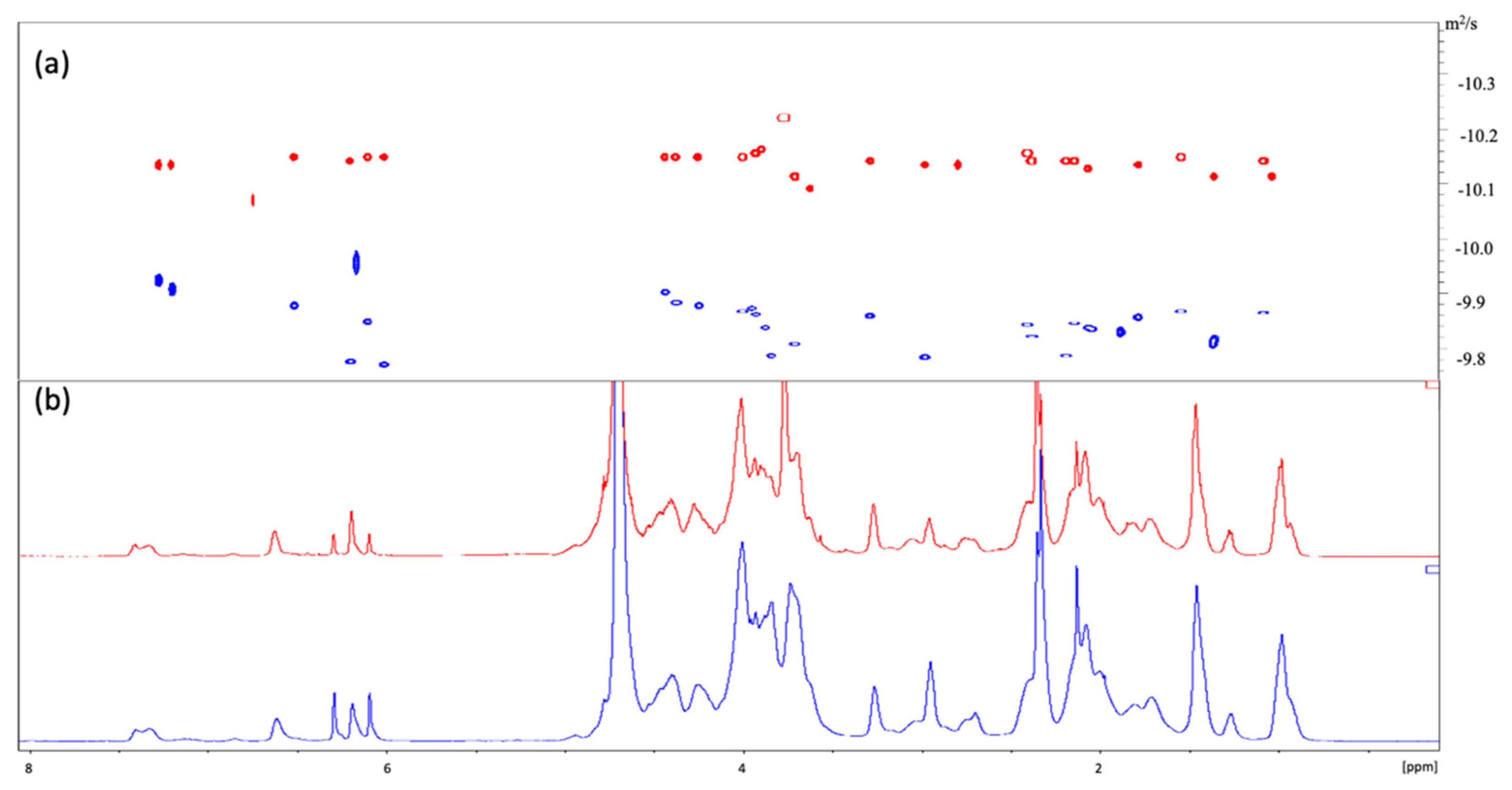
3.3. 1H-NMR Characterization
3.4. Thermogravimetric Analysis (TGA)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| MF | Methylfuran |
| GelChiDA | Gelatin-chitosan Diels Alder hybrid hydrogel |
| HP-SEC-TDA | High-performance size exclusion chromatography on line with a triple detector array |
| NMR | Nuclear magnetic resonance |
| DOSY | Diffusion order spectroscopy NMR |
| FTIR-ATR | Fourier-transform infrared spectroscopy-attenuated total reflection |
| UV-VIS | Ultraviolet–visible spectroscopy |
| TGA | Thermogravimetric analysis |
References
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart Polymeric Hydrogels for Cartilage Tissue Engineering: A Review on the Chemistry and Biological Functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2020. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamadi, N.; Zarrabi, A.; Abasi, S.; Dehghannoudeh, G.; Tamaddondoust, R.N.; Khanbabaei, H.; Mohammadinejad, R.; Thakur, V.K. Chitosan-Based Advanced Materials for Docetaxel and Paclitaxel Delivery: Recent Advances and Future Directions in Cancer Theranostics. Int. J. Biol. Macromol. 2020, 145, 282–300. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New Horizons in Hydrogels for Methotrexate Delivery. Gels 2020, 7, 2. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like Materials—Biomaterials for Applications in Regenerative Medicine and Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and Bioprinting Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From Tissue and Organ Development to in Vitro Models. Chem. Rev. 2020, 120, 10547–10607. [Google Scholar] [CrossRef]
- Wade, R.J.; Burdick, J.A. Engineering ECM Signals into Biomaterials. Mater. Today 2012, 15, 454–459. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable In Vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Zhang, Y.S.; Migliori, B.; Barbani, N.; Lazzeri, L.; Shin, S.R.; Dokmeci, M.R.; Cascone, M.G. Protein/Polysaccharide-Based Scaffolds Mimicking Native Extracellular Matrix for Cardiac Tissue Engineering Applications. J. Biomed. Mater. Res. Part A 2018, 106, 769–781. [Google Scholar] [CrossRef]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef] [Green Version]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Mohn, D.; Stark, W.J.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Accelerated Mineralization of Dense Collagen-Nano Bioactive Glass Hybrid Gels Increases Scaffold Stiffness and Regulates Osteoblastic Function. Biomaterials 2011, 32, 8915–8926. [Google Scholar] [CrossRef]
- Marsano, A.; Maidhof, R.; Wan, L.Q.; Wang, Y.; Gao, J.; Tandon, N.; Vunjak-Novakovic, G. Scaffold Stiffness Affects the Contractile Function of Three-Dimensional Engineered Cardiac Constructs. Biotechnol. Prog. 2010, 26, 1382–1390. [Google Scholar] [CrossRef] [Green Version]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked Hyaluronic Acid Hydrogels: A Strategy to Functionalize and Pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef]
- Nimni, M.E.; Cheung, D.; Strates, B.; Kodama, M.; Sheikh, K. Chemically Modified Collagen: A Natural Biomaterial for Tissue Replacement. J. Biomed. Mater. Res. 1987, 21, 741–771. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B. Thiol-Yne ’Click’/Coupling Chemistry and Recent Applications in Polymer and Materials Synthesis and Modification. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. “Click” Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Cao, Z.; Ni, B.; Wang, X.; Ning, P.; Ma, D.; Wei, H.; Liu, M. Dual Crosslinked Chondroitin Sulfate Injectable Hydrogel Formed via Continuous Diels-Alder (DA) Click Chemistry for Bone Repair. Carbohydr. Polym. 2017, 166, 123–130. [Google Scholar] [CrossRef]
- Cuvellier, A.; Verhelle, R.; Brancart, J.; Vanderborght, B.; Van Assche, G.; Rahier, H. The Influence of Stereochemistry on the Reactivity of the Diels–Alder Cycloaddition and the Implications for Reversible Network Polymerization. Polym. Chem. 2019, 10, 473–485. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C. Rapid Diels-Alder Cross-Linking of Cell Encapsulating Hydrogels. Chem. Mater. 2019, 31, 8035–8043. [Google Scholar] [CrossRef]
- Koehler, K.C.; Anseth, K.S.; Bowman, C.N.; Nimmo, C.M.; Owen, S.C.; Shoichet, M.S.; Madl, C.M.; Heilshorn, S.C.; Hall, D.J.; Van Den Berghe, H.M.; et al. Cross-Linked Hydrogels Formed through Diels–Alder Coupling of Furan- and Maleimide-Modified Poly(Methyl Vinyl Ether-Alt-Maleic Acid). Biomacromolecules 2013, 14, 1–29. [Google Scholar]
- Montiel-Herrera, M.; Gandini, A.; Goycoolea, F.M.; Jacobsen, N.E.; Lizardi-Mendoza, J.; Recillas-Mota, M.; Argüelles-Monal, W.M. N-(Furfural) Chitosan Hydrogels Based on Diels-Alder Cycloadditions and Application as Microspheres for Controlled Drug Release. Carbohydr. Polym. 2015, 128, 220–227. [Google Scholar] [CrossRef]
- Gandini, A. The Furan/Maleimide Diels-Alder Reaction: A Versatile Click-Unclick Tool in Macromolecular Synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Polgar, L.M.; Kingma, A.; Roelfs, M.; van Essen, M.; van Duin, M.; Picchioni, F. Kinetics of Cross-Linking and de-Cross-Linking of EPM Rubber with Thermoreversible Diels-Alder Chemistry. Eur. Polym. J. 2017, 90, 150–161. [Google Scholar] [CrossRef]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.G.; Binth Mohammad, N.; Trant, J.F.; Shoichet, M.S. Diels-Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Gregoritza, M.; Brandl, F.P. The Diels-Alder Reaction: A Powerful Tool for the Design of Drug Delivery Systems and Biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels–Alder Crosslinking. Eur. J. Org. Chem. 2021, 2021, 374–382. [Google Scholar] [CrossRef]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels-Alder Click Cross-Linked Hyaluronic Acid Hydrogels for Tissue Engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Cao, X.; Li, Y.; Zeng, L.; Yuan, B.; Chen, X. An Injectable Hyaluronic Acid/PEG Hydrogel for Cartilage Tissue Engineering Formed by Integrating Enzymatic Crosslinking and Diels-Alder “Click Chemistry”. Polym. Chem. 2014, 5, 1082–1090. [Google Scholar] [CrossRef]
- Bi, B.; Ma, M.; Lv, S.; Zhuo, R.; Jiang, X. In-Situ Forming Thermosensitive Hydroxypropyl Chitin-Based Hydrogel Crosslinked by Diels-Alder Reaction for Three Dimensional Cell Culture. Carbohydr. Polym. 2019, 212, 368–377. [Google Scholar] [CrossRef]
- Bertini, S.; Bisio, A.; Torri, G.; Bensi, D.; Terbojevich, M. Molecular Weight Determination of Heparin and Dermatan Sulfate by Size Exclusion Chromatography with a Triple Detector Array. Biomacromolecules 2005, 6, 168–173. [Google Scholar] [CrossRef]
- Bowman, W.A.; Rubinstein, M.; Tan, J.S. Polyelectrolyte-Gelatin Complexation: Light-Scattering Study. Macromolecules 1997, 30, 3262–3270. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M.; Dung, P. Le Characterization of Chitosan. Influence of Ionic Strength and Degree of Acetylation on Chain Expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandini, A.; Coelho, D.; Gomes, M.; Reis, B.; Silvestre, A. Materials from Renewable Resources Based on Furan Monomers and Furan Chemistry: Work in Progress. J. Mater. Chem. 2009, 19, 8656–8664. [Google Scholar] [CrossRef] [Green Version]
- García-Astrain, C.; Gandini, A.; Peña, C.; Algar, I.; Eceiza, A.; Corcuera, M.; Gabilondo, N. Diels-Alder “Click” Chemistry for the Cross-Linking of Furfuryl-Gelatin-Polyetheramine Hydrogels. RSC Adv. 2014, 4, 35578–35587. [Google Scholar] [CrossRef]
- Cui, J.; Wang, S.; Huang, K.; Li, Y.; Zhao, W.; Shi, J.; Gu, J. Conjugation-Induced Fluorescence Labelling of Mesoporous Silica Nanoparticles for the Sensitive and Selective Detection of Copper Ions in Aqueous Solution. New J. Chem. 2014, 38, 6017. [Google Scholar] [CrossRef]
- González, R.; Figueroa, J.M.; González, H. Furfuryl Alcohol Polymerisation by Iodine in Methylene Chloride. Eur. Polym. J. 2002, 38, 287–297. [Google Scholar] [CrossRef]
- Durand, P.-L.; Grau, E.; Cramail, H. Bio-Based Thermo-Reversible Aliphatic Polycarbonate Network. Molecules 2019, 25, 74. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.G.B.; Muniz, E.C.; Hsieh, Y. Lo 1H NMR and 1H-13C HSQC Surface Characterization of Chitosan-Chitin Sheath-Core Nanowhiskers. Carbohydr. Polym. 2015, 123, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Alves, P.; Santos, M.; Mendes, S.; Miguel, S.P.; de Sá, K.D.; Cabral, C.S.D.; Correia, I.J.; Ferreira, P. Photocrosslinkable Nanofibrous Asymmetric Membrane Designed for Wound Dressing. Polymers 2019, 11, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claaßen, C.; Claaßen, M.H.; Truffault, V.; Sewald, L.; Tovar, G.E.M.; Borchers, K.; Southan, A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018, 19, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Sun, J.; Lian, H.; Xu, C.; Liu, X.; Zheng, S.; Zhang, D.; Han, X.; Liu, Y.; Chen, X.; et al. The Influence of Shuttle-Shape Emodin Nanoparticles on the Streptococcus Suis Biofilm. Front. Pharmacol. 2018, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhof, S.; Brandl, F.P.; Hammer, N.; Goepferich, A.M. Investigation of the Diels-Alder Reaction as a Cross-Linking Mechanism for Degradable Poly(Ethylene Glycol) Based Hydrogels. J. Mater. Chem. B 2013, 1, 4855–4864. [Google Scholar] [CrossRef] [PubMed]
- Ghanian, M.H.; Mirzadeh, H.; Baharvand, H. In Situ Forming Hydrogels Based on Clickable Star-PEG for Biomedical Applications. In Eco-friendly and Smart Polymer Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 92–95. [Google Scholar]
- Mirzadeh, H.; Katbab, A.A. Eco-Friendly and Smart Polymer Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Ladiè, R.; Cosentino, C.; Tagliaro, I.; Antonini, C.; Bianchini, G.; Bertini, S. Supramolecular Structuring of Hyaluronan-Lactose-Modified Chitosan Matrix: Towards High-Performance Biopolymers with Excellent Biodegradation. Biomolecules 2021, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Fractionation of Carboxylate Anions from Aqueous Solution Using Chitosan Cross-Linked Sorbent Materials. RSC Adv. 2015, 5, 82065–82077. [Google Scholar] [CrossRef]
- Kakkar, P.; Verma, S.; Manjubala, I.; Madhan, B. Development of Keratin-Chitosan-Gelatin Composite Scaffold for Soft Tissue Engineering. Mater. Sci. Eng. C 2014, 45, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.G.; Popa, M.I.; Lisa, G.; Vereştiuc, L. Thermal Behavior of Hydrophobically Modified Hydrogels Using TGA/FTIR/MS Analysis Technique. Thermochim. Acta 2015, 613, 28–40. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.H.; Lee, J.H.; Kim, G.H. 3D Bioprinting and Its In Vivo Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication Strategies for 3D In Vitro Models and Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
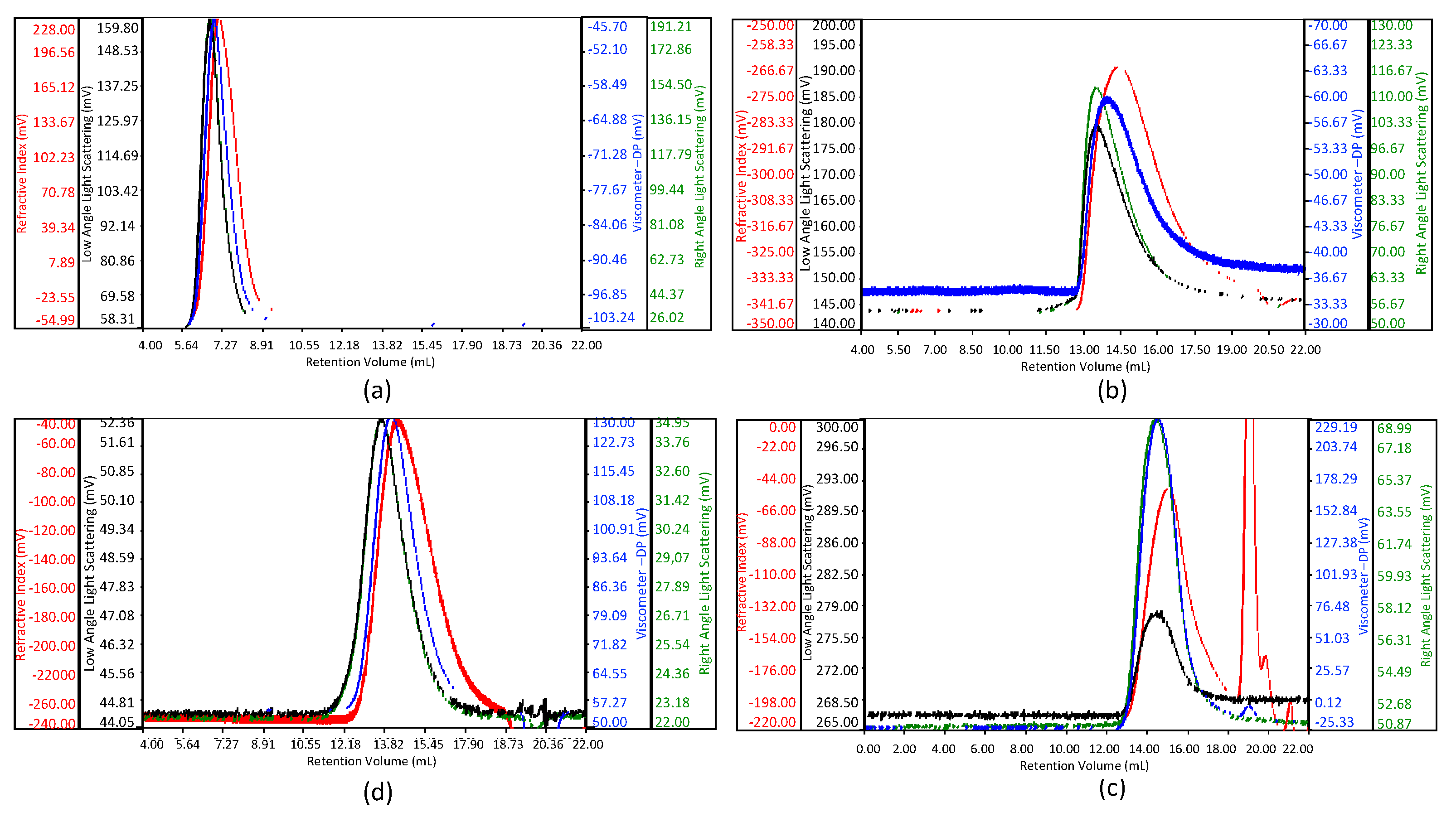


| Sample | Mw kDa | Mn kDa | D (Mw/Mn) | (η) dL/g |
|---|---|---|---|---|
| Gelatin | 210 | 115 | 1.9 | 0.51 |
| Gelatin-MF | 196 | 140 | 1.4 | 0.46 |
| Sample | Mw kDa | Mn kDa | D (Mw/Mn) | (μ) dL/g |
|---|---|---|---|---|
| Chitosan | 47.5 | 28.7 | 1.7 | 1.5 |
| Chitosan-MF | 57.0 | 30.0 | 1.9 | 1.7 |
| Sample | Water Desorption | Degradation | ||||
|---|---|---|---|---|---|---|
| Peak (K) | Range (K) | Weight Loss (%) | Peak (K) | Range (K) | Weight Loss (%) | |
| Gelatin | 348 | 300–429 | 10.6 | 592 | 457–879 | 67.4 |
| Gelatin-MF | 340 | 300–421 | 8.6 | 597 | 443–877 | 68.1 |
| Chitosan | 361 | 300–428 | 12.9 | 499 | 453–634 | 44.7 |
| Chitosan-MF | 349 | 300–423 | 9.3 | 508 | 434–616 | 40 |
| GelChiDa | 348 | 300–429 | 8.2 | 582 | 440–923 | 55.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magli, S.; Rossi, L.; Consentino, C.; Bertini, S.; Nicotra, F.; Russo, L. Combined Analytical Approaches to Standardize and Characterize Biomaterials Formulations: Application to Chitosan-Gelatin Cross-Linked Hydrogels. Biomolecules 2021, 11, 683. https://doi.org/10.3390/biom11050683
Magli S, Rossi L, Consentino C, Bertini S, Nicotra F, Russo L. Combined Analytical Approaches to Standardize and Characterize Biomaterials Formulations: Application to Chitosan-Gelatin Cross-Linked Hydrogels. Biomolecules. 2021; 11(5):683. https://doi.org/10.3390/biom11050683
Chicago/Turabian StyleMagli, Sofia, Lorenzo Rossi, Cesare Consentino, Sabrina Bertini, Francesco Nicotra, and Laura Russo. 2021. "Combined Analytical Approaches to Standardize and Characterize Biomaterials Formulations: Application to Chitosan-Gelatin Cross-Linked Hydrogels" Biomolecules 11, no. 5: 683. https://doi.org/10.3390/biom11050683