Neisseria gonorrhoeae Multivalent Maxibody with a Broad Spectrum of Strain Specificity and Sensitivity for Gonorrhea Diagnosis
Abstract
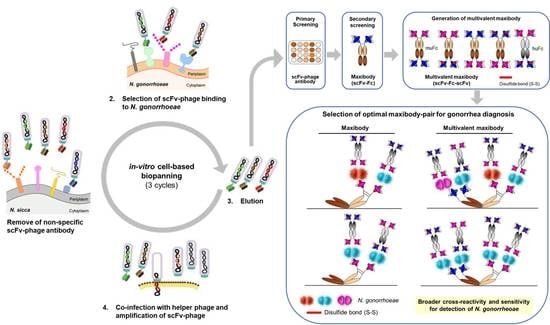
:1. Introduction
2. Materials and Methods
2.1. Neisseria gonorrhoeae Cell Culture
2.2. Characterization of N. gonorrhoeae Clinical Isolates
2.3. In-Vitro Cell-Based Bio-Panning for N. gonorrhoeae
2.4. Screening of scFv-Phage by Phage ELISA
2.5. Conversion of scFv-Phage Antibodies into Maxibodies and Multivalent Maxibodies
2.6. Binding Analysis by ELISA
2.7. Determination of Maxibody Affinity by ELISA
3. Results and Discussion
3.1. Molecular Characterization and Antimicrobial Susceptibility of N. gonorrhoeae Clinical Isolates
3.2. Bio-Panning and Selection of scFv-Phage Antibodies against N. gonorrhoeae Strains
3.3. Generation and Antigen-Binding Characterization of Anti-N. gonorrhoeae Maxibody
3.4. Two Maxibodies Exhibited Binding against a Broad Spectrum of N. gonorrhoeae Strains
3.5. Generation of Multivalent N. gonorrhoeae Maxibody
3.6. Selection of the Optimal Maxibody Pair for Immunodiagnostic Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Genet. 2018, 16, 226–240. [Google Scholar] [CrossRef]
- Unemo, M.; Seifert, H.S.; Hook, E.W.; Hawkes, S.; Ndowa, F.; Dillon, J.-A.R. Gonorrhoea. Nat. Rev. Dis. Prim. 2019, 5, 1–23. [Google Scholar] [CrossRef]
- Unemo, M.; Lahra, M.M.; Cole, M.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.-A.R.; Ramon-Pardo, P.; Bolan, G.; Wi, T. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): Review of new data and evidence to inform international collaborative actions and research efforts. Sex. Health 2019, 16, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.; Matias, R.; Rodrigues, J.C.; Duarte, S.; Vieira, L.; Gonçalves, I.; Gonçalves, M.J.; Ramos, M.H.; Gomes, J.P.; Borrego, M.J. Cephalosporin-Resistant Neisseria gonorrhoeae Isolated in Portugal, 2019. Sex. Transm. Dis. 2020, 47, e54–e56. [Google Scholar] [CrossRef] [PubMed]
- Callander, D.; Guy, R.; Fairley, C.K.; McManus, H.; Prestage, G.; Chow, E.P.F.; Chen, M.; O Connor, C.C.; Grulich, A.E.; Bourne, C.; et al. Gonorrhoea gone wild: Rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics. Sex. Health 2019, 16, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, E.P.; Fairley, C.K. The role of saliva in gonorrhoea and chlamydia transmission to extragenital sites among men who have sex with men: New insights into transmission. J. Int. AIDS Soc. 2019, 22, e25354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexually Transmitted Disease Surveillance 2017; CDC: Atlanta, GA, USA, 2018.
- Ndowa, F.; Lusti-Narasimhan, M.; Unemo, M. The serious threat of multidrug-resistant and untreatable gonorrhoea: The pressing need for global action to control the spread of antimicrobial resistance, and mitigate the impact on sexual and reproductive health. Sex. Transm. Infect. 2012, 88, 317–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaughnessy, J.; Gulati, S.; Agarwal, S.; Unemo, M.; Ohnishi, M.; Su, X.-H.; Monks, B.G.; Visintin, A.; Madico, G.; Lewis, L.A.; et al. A Novel Factor H–Fc Chimeric Immunotherapeutic Molecule againstNeisseria gonorrhoeae. J. Immunol. 2016, 196, 1732–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborti, S.; Lewis, L.A.; Cox, A.D.; Michael, F.S.; Li, J.; Rice, P.A.; Ram, S. Phase-Variable Heptose I Glycan Extensions Modulate Efficacy of 2C7 Vaccine Antibody Directed against Neisseria gonorrhoeae Lipooligosaccharide. J. Immunol. 2016, 196, 4576–4586. [Google Scholar] [CrossRef] [Green Version]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.-A.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef] [Green Version]
- Młynarczyk-Bonikowska, B.; Majewska, A.; Malejczyk, M.; Młynarczyk, G.; Majewski, S. Multiresistant Neisseria gonorrhoeae: A new threat in second decade of the XXI century. Med. Microbiol. Immunol. 2020, 209, 95–108. [Google Scholar] [CrossRef]
- Nakayama, S.-I.; Shimuta, K.; Furubayashi, K.-I.; Kawahata, T.; Unemo, M.; Ohnishi, M. New Ceftriaxone- and Multidrug-Resistant Neisseria gonorrhoeae Strain with a Novel MosaicpenAGene Isolated in Japan. Antimicrob. Agents Chemother. 2016, 60, 4339–4341. [Google Scholar] [CrossRef] [Green Version]
- Eyre, D.W.; Sanderson, N.D.; Lord, E.; Regisford-Reimmer, N.; Chau, K.; Barker, L.; Morgan, M.; Newnham, R.; Golparian, D.; Unemo, M.; et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Eurosurveillance 2018, 23, 1800323. [Google Scholar] [CrossRef] [PubMed]
- Fifer, H.; Hughes, G.; Whiley, D.; Lahra, M.M. Lessons learnt from ceftriaxone-resistant gonorrhoea in the UK and Australia. Lancet Infect. Dis. 2020, 20, 276–278. [Google Scholar] [CrossRef]
- Lefebvre, B.; Martin, I.; Demczuk, W.; Deshaies, L.; Michaud, S.; Labbé, A.-C.; Beaudoin, M.-C.; Longtin, J. Ceftriaxone-Resistant Neisseria gonorrhoeae, Canada, 2017. Emerg. Infect. Dis. 2018, 24, 381–383. [Google Scholar] [CrossRef] [Green Version]
- Golparian, D.; Ohlsson, A.K.; Janson, H.; Lidbrink, P.; Richtner, T.; Ekelund, O.; Fredlund, H.; Unemo, M. Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014. Eurosurveillance 2014, 19, 20862. [Google Scholar] [CrossRef] [Green Version]
- Bodie, M.; Gale-Rowe, M.; Alexandre, S.; Auguste, U.; Tomas, K.; Martin, I. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: There is no time like the present. Can. Commun. Dis. Rep. 2019, 45, 54–62. [Google Scholar] [CrossRef]
- Martin, I.; Sawatzky, P.; Allen, V.; Lefebvre, B.; Hoang, L.; Naidu, P.; Minion, J.; Van Caeseele, P.; Haldane, D.; Gad, R.R.; et al. Multidrug-resistant and extensively drug-resistant Neisseria gonorrhoeae in Canada, 2012–2016. Can. Commun. Dis. Rep. 2019, 45, 45–53. [Google Scholar] [CrossRef]
- Berenger, B.M.; Demczuk, W.; Gratrix, J.; Pabbaraju, K.; Smyczek, P.; Martin, I. Genetic Characterization and Enhanced Surveillance of Ceftriaxone-ResistantNeisseria gonorrhoeaeStrain, Alberta, Canada, 2018. Emerg. Infect. Dis. 2019, 25, 1660–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, L.-K.; E Martin, I. The Laboratory Diagnosis ofNeisseria gonorrhoeae. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, T.; Buder, S. The Laboratory Diagnosis of Neisseria gonorrhoeae: Current Testing and Future Demands. Pathogens 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, R.J.; Causer, L.M.; Klausner, J.D.; Unemo, M.; Toskin, I.; Azzini, A.M.; Peeling, R.W. Performance and operational characteristics of point-of-care tests for the diagnosis of urogenital gonococcal infections. Sex. Transm. Infect. 2017, 93, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, C.A.; Melendez, J.H. Point-by-Point Progress: Gonorrhea Point of Care Tests. Expert Rev. Mol. Diagn. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Samarawickrama, A.; Cheserem, E.; Graver, M.; Wade, J.; Alexander, S.; Ison, C. Pilot study of use of the BioStar Optical ImmunoAssay GC point-of-care test for diagnosing gonorrhoea in men attending a genitourinary medicine clinic. J. Med. Microbiol. 2014, 63, 1111–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaydos, C.; Hardick, J. Point of care diagnostics for sexually transmitted infections: Perspectives and advances. Expert Rev. Anti-Infect. Ther. 2014, 12, 657–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natoli, L.; Maher, L.; Shephard, M.; Hengel, B.; Tangey, A.; Badman, S.G.; Ward, J.; Guy, R.J.; on behalf of the TTANGO Investigators. Point-of-Care Testing for Chlamydia and Gonorrhoea: Implications for Clinical Practice. PLoS ONE 2014, 9, e100518. [Google Scholar] [CrossRef] [Green Version]
- Alary, M.; Gbenafa-Agossa, C.; Aina, G.; Ndour, M.; Labbe, A.C.; Fortin, D.; Steele, M.; Peeling, R.W. Evaluation of a rapid point-of-care test for the detection of gonococcal infection among female sex workers in Benin. Sex. Transm. Infect. 2006, 82, v29–v32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivard, K.R.; Dumkow, L.E.; Draper, H.M.; Brandt, K.L.; Whalen, D.W.; Egwuatu, N.E. Impact of rapid diagnostic testing for chlamydia and gonorrhea on appropriate antimicrobial utilization in the emergency department. Diagn. Microbiol. Infect. Dis. 2017, 87, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Nuñez-Forero, L.; Moyano-Ariza, L.; Gaitán-Duarte, H.; Ángel-Müller, E.; Ruiz-Parra, A.; González, P.; Rodríguez, A.; E Tolosa, J. Diagnostic accuracy of rapid tests for sexually transmitted infections in symptomatic women. Sex. Transm. Infect. 2015, 92, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Benzaken, A.S.; Galban, E.G.; Antunes, W.; Dutra, J.C.; Peeling, R.W.; Mabey, D.; Salama, A. Diagnosis of gonococcal infection in high risk women using a rapid test. Sex. Transm. Infect. 2006, 82, v26–v28. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Suurs, F.V.; Hooge, M.N.L.-D.; de Vries, E.G.; de Groot, D.J.A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Ahamadi-Fesharaki, R.; Fateh, A.; Vaziri, F.; Solgi, G.; Siadat, S.D.; Mahboudi, F.; Jamnani, F.R. Single-Chain Variable Fragment-Based Bispecific Antibodies: Hitting Two Targets with One Sophisticated Arrow. Mol. Ther. Oncolytics 2019, 14, 38–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontermann, R.E.; Brinkmann, U. Bispecific antibodies. Drug Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef] [Green Version]
- McCallion, C.; Peters, A.D.; Booth, A.; Rees-Unwin, K.; Adams, J.; Rahi, R.; Pluen, A.; Hutchinson, C.V.; Webb, S.J.; Burthem, J. Dual-action CXCR4-targeting liposomes in leukemia: Function blocking and drug delivery. Blood Adv. 2019, 3, 2069–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundranda, M.; Gracian, A.; Zafar, S.; Meiri, E.; Bendell, J.; Algül, H.; Rivera, F.; Ahn, E.; Watkins, D.; Pelzer, U.; et al. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann. Oncol. 2020, 31, 79–87. [Google Scholar] [CrossRef]
- Chung, Y.; Han, M.; Park, J.-Y.; Kang, S.; Kim, I.; A Park, J.; Kim, J.-S. Molecular Analysis of Eight American Type Culture Collection Gonococcal Strains by Neisseria gonorrhoeae Multiantigen Sequence Typing and PorB Sequence Typing. J. Lab. Med. Qual. Assur. 2019, 41, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, M.P. Clinical and Laboratory Standards Institute (CLSI) M100-S25 Document, 30th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Unemo, M.; Golparian, D.; Sánchez-Busó, L.; Grad, Y.; Jacobsson, S.; Ohnishi, M.; Lahra, M.M.; Limnios, A.; Sikora, A.E.; Wi, T.; et al. The novel 2016 WHONeisseria gonorrhoeaereference strains for global quality assurance of laboratory investigations: Phenotypic, genetic and reference genome characterization. J. Antimicrob. Chemother. 2016, 71, 3096–3108. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.G.; Jeong, J.; Lee, J.; Jung, H.; Kim, M.; Zhao, Y.; Yi, E.C.; Kim, K.M. Rapid Evaluation of Antibody Fragment Endocytosis for Antibody Fragment–Drug Conjugates. Biomolecules 2020, 10, 955. [Google Scholar] [CrossRef]
- Barbas, C.F.; Burton, D.R.; Scott, J.K.; Silverman, G.J. Phage Display: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2004. [Google Scholar]
- Kontermann, R.E.; Dübel, S. Antibody Engineering; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- McCafferty, J.; Hoogenboom, H.; Chiswell, D. Antibody Engineering: A practical Approach; IRL Press at Oxford University Press: Oxford, NY, USA, 1996; Volume xxiii, 352p. [Google Scholar]
- Schanzer, J.; Jekle, A.; Nezu, J.; Lochner, A.; Croasdale, R.; Dioszegi, M.; Zhang, J.; Hoffmann, E.; Dormeyer, W.; Stracke, J.O.; et al. Development of Tetravalent, Bispecific CCR5 Antibodies with Antiviral Activity against CCR5 Monoclonal Antibody-Resistant HIV-1 Strains. Antimicrob. Agents Chemother. 2011, 55, 2369–2378. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.C.; Von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- MedCalc Software. Available online: https://www.medcalc.org/calc/diagnostic_test.php (accessed on 20 February 2020).
- Caugant, D.A.; Brynildsrud, O.B. Neisseria meningitidis: Using genomics to understand diversity, evolution and pathogenesis. Nat. Rev. Genet. 2020, 18, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Dorey, R.B.; Theodosiou, A.A.; Read, R.C.; Jones, C.E. The nonpathogenic commensal Neisseria. Curr. Opin. Infect. Dis. 2019, 32, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K. Immunodiagnostics: An Emerging Opportunity in Biotechnology. Available online: http://adamasuniversity.ac.in/immunodiagnostics-an-emerging-opportunity-in-biotechnology/ (accessed on 20 May 2020).
- Songsivilai, S.; Lachmann, P.J. Bispecific antibody: A tool for diagnosis and treatment of disease. Clin. Exp. Immunol. 2008, 79, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Takeuchi, R.; Takeda, K.; Takamori, M.; Ito, K.; Igarashi, Y.; Hayashi, E.; Iguchi, M.; Ono, M.; Kashiyama, T.; et al. Ultrasensitive enzyme-linked immunosorbent assay for the detection of MPT64 secretory antigen to evaluate Mycobacterium tuberculosis viability in sputum. Int. J. Infect. Dis. 2020, 96, 244–253. [Google Scholar] [CrossRef] [PubMed]





| NG Clinical Isolate # | NG-MAST 1 | PubMLST 2 | ||
|---|---|---|---|---|
| Allele | Sequence Type (ST) | Allele | ||
| porB | tbpB | porB | ||
| 744 | 543 | 899 | 4278 | 12 |
| 832 | 4016 | 33 | 6734 | 18 |
| 834 | 1785 | 2422 | 15,525 | 8 |
| 840 | 4623 | 455 | 7693 | 632 |
| 850 | 8061 | 33 | 13,973 | 18 |
| 1167 | 4016 | 33 | 6734 | 18 |
| 1363 | 2514 | 455 | 11,361 | 8 |
| 1442 | 8521 | 60 | 14,668 | 11 |
| 1446 | 3764 | 110 | 12,402 | 8 |
| 1471 | 785 | 60 | 3611 | 12 |
| 1481 | 1659 | 1058 | New 3 | 8 |
| Round of Screening | Input (CFU) a | Output (CFU) b | Enrichment Fold | Total Enrichment Fold |
|---|---|---|---|---|
| 1 | 1 × 1011 | 6.6 × 104 | 1 | N/A |
| 2 | 1 × 1011 | 3.25 × 105 | 4.9 | 4.9 |
| 3 | 1 × 1011 | 5 × 109 | 15,384 | 75,382 |
| Ab | No. of Positive Isolates Detected (% Sensitivity) 1 | 95% CI | Cross Reactivity with Control Neisseria spp. | Clinical Isolate No. | ||
|---|---|---|---|---|---|---|
| Maxibody | Group 1 | Clone 1 | 10/11 (90.9%) | 59 to 100 | No 2 | 744, 832, 834, 840, 850, 1167, 1363, 1442, 1446, 1471 |
| Group 2 | Clone 4 | 9/11 (81.8%) | 48 to 98 | No | 744, 832, 840, 850, 1167, 1363, 1442, 1471, 1481 | |
| Group 3 | Clone 3 | 6/8 (75%) | 35 to 97 | No | 832, 850, 1363, 1442, 1446, 1471 | |
| Group 4 | Clones 2, 6, 8, 11, 12 | 4/8 (50%) | 16 to 84 | No | 832, 850, 1442, 1471 | |
| Group 5 | Clone 9 | 2/8 (25%) | 3 to 65 | No | 850, 1363 | |
| Multivalent maxibody | Clone-1/4 | 19/19 (100%) | 82 to 100 | No | 744, 832, 833, 834, 840, 850, 1059, 1167,1247, 1362, 1363, 1442, 1446, 1471, 1481, 1539, 1590, 1700, 2466 | |
| Clone-1/4(ds) | 19/19 (100%) | 82 to 100 | No | 744, 832, 833, 834, 840, 850, 1059, 1167, 1247, 1362, 1363, 1442, 1446, 1471, 1481, 1539, 1590, 1700, 2466 | ||
| Commercial Ab (Artron BioResearch) | A30-Ab1 | 11/11 (100%) | 72 to 100 | N. sicca and N. meningitidis | 744, 832, 834, 840, 850, 1167, 1363, 1442, 1446, 1471, 1481 | |
| A30-Ab2 | 3/11 (27.3%) | 6 to 10 | N. sicca and N. meningitidis | 834, 840, 1167 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Kim, J.-S.; Lee, J.; Seo, Y.R.; Yi, E.C.; Kim, K.M. Neisseria gonorrhoeae Multivalent Maxibody with a Broad Spectrum of Strain Specificity and Sensitivity for Gonorrhea Diagnosis. Biomolecules 2021, 11, 484. https://doi.org/10.3390/biom11030484
Jeong J, Kim J-S, Lee J, Seo YR, Yi EC, Kim KM. Neisseria gonorrhoeae Multivalent Maxibody with a Broad Spectrum of Strain Specificity and Sensitivity for Gonorrhea Diagnosis. Biomolecules. 2021; 11(3):484. https://doi.org/10.3390/biom11030484
Chicago/Turabian StyleJeong, Jieun, Jae-Seok Kim, Junghyeon Lee, Yu Ri Seo, Eugene C. Yi, and Kristine M. Kim. 2021. "Neisseria gonorrhoeae Multivalent Maxibody with a Broad Spectrum of Strain Specificity and Sensitivity for Gonorrhea Diagnosis" Biomolecules 11, no. 3: 484. https://doi.org/10.3390/biom11030484