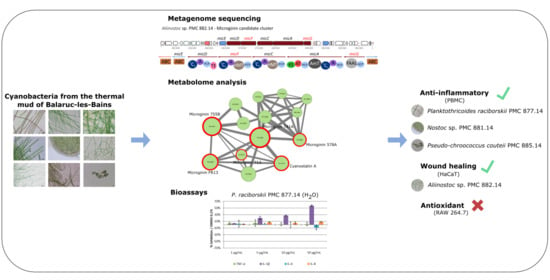
Anti-Inflammatory, Antioxidant, and Wound-Healing Properties of Cyanobacteria from Thermal Mud of Balaruc-Les-Bains, France: A Multi-Approach Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Genome Sequencing and Assembly
2.3. In Silico Analyses
2.4. Pigment Composition Analysis
2.5. Metabolite Extraction, Analysis by Mass Spectrometry, and Annotation
2.6. Molecular Networking
2.7. Bioassays
3. Results
3.1. Characteristics of the Cyanobacterial Genomes
3.2. Pigment Composition
3.3. Specialized Metabolites
3.4. Bioactivity Assessment
4. Discussion
4.1. Genome Quality, Genome Mining, and Molecular Network Analysis
4.2. Cytotoxic Properties
4.3. Antioxidant Properties
4.4. Wound-Healing Properties
4.5. Anti-Inflammatory Properties
4.6. General Comments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural products from cyanobacteria: Focus on beneficial activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Shah, S.A.; Akhter, N.; Auckloo, B.N.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.W. Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Mar. Drugs 2017, 15, 354. [Google Scholar] [CrossRef] [Green Version]
- Calteau, A.; Fewer, D.P.; Latifi, A.; Coursin, T.; Laurent, T.; Jokela, J.; Kerfeld, C.A.; Sivonen, K.; Piel, J.; Gugger, M. Phylum-wide comparative genomics unravel the diversity of secondary metabolism in Cyanobacteria. BMC Genom. 2014, 15, 977. [Google Scholar] [CrossRef] [Green Version]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.D.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; Tandeau de Marsac, N.; Rippka, R.; et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef] [Green Version]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef]
- Leao, T.; Castelão, G.; Korobeynikov, A.; Monroe, E.A.; Podell, S.; Glukhov, E.; Allen, E.E.; Gerwick, W.H.; Gerwick, L. Comparative genomics uncovers the prolific and distinctive metabolic potential of the cyanobacterial genus Moorea. Proc. Natl. Acad. Sci. USA 2017, 114, 3198–3203. [Google Scholar] [CrossRef] [Green Version]
- Nivina, A.; Yuet, K.P.; Hsu, J.; Khosla, C. Evolution and Diversity of Assembly-Line Polyketide Synthases. Chem. Rev. 2019, 119, 12524–12547. [Google Scholar] [CrossRef]
- Martins, J.; Vasconcelos, V. Cyanobactins from cyanobacteria: Current genetic and chemical state of knowledge. Mar. Drugs 2015, 13, 6910–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.T. Biomedical potential of marine cyanobacteria. J. Coast. Dev. 2006, 9, 129–136. [Google Scholar]
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria—A review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Cantoral Uriza, E.A.; Asencio, A.D.; Aboal, M. Are we underestimating benthic cyanotoxins? extensive sampling results from Spain. Toxins 2017, 9, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Rivera, E.; Paul, V.J. Chemical deterrence of a cyanobacterial metabolite against generalized and specialized grazers. J. Chem. Ecol. 2007, 33, 213–217. [Google Scholar] [CrossRef]
- Sneed, J.M.; Meickle, T.; Engene, N.; Reed, S.; Gunasekera, S.; Paul, V.J. Bloom dynamics and chemical defenses of benthic cyanobacteria in the Indian River Lagoon, Florida. Harmful Algae 2017, 69, 75–82. [Google Scholar] [CrossRef]
- Baudinat, C. Contribution à l’Etude de la Maturation de Péloïdes: Application aux Stations Thermales de Balaruc-les-Bains (34) et Cransac (12). Ph.D. Thesis, Université de Montpellier, Montpellier, France, 1986. [Google Scholar]
- Dupuis, E. Pré-Etude Relative à l’Evaluation de la Production Algale des Eaux Thermales de Balruc-les-Bain; Science Sorbonne Universite: Montpellier, France, 1987. [Google Scholar]
- Counilh, P.; Gibert, J.-L. Process for Preparing Thermal Muds, Muds Thus Obtained and Their Uses. European Patent EP0646647A1, 5 April 1995. [Google Scholar]
- Lalli, A.; Andreoli, C.; Ceschi-Berrini, C.; De Appolonia, F.; Marcolongo, G. Anti-Inflammatory Active Principles in Euganean Thermal Mud. European Patent PAT-EP1571203 05100038.8, 1 May 2005. [Google Scholar]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef] [Green Version]
- Hamlaoui, S. Isolement, Culture et Analyses Toxicologiques de Souches de Micro-Algues et de Cyanobactéries des Eaux Thermales de Balaruc-les-Bains; Science Sorbonne Universite: Paris, France, 2014. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [Green Version]
- Ziemert, N.; Alanjary, M.; Weber, T. The evolution of genome mining in microbes-a review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, C.; Hamlaoui, S.; Piquet, B.; Toutirais, G.; Yéprémian, C.; Reinhardt, A.; Duperron, S.; Marie, B.; Demay, J.; Bernard, C. Characterization of cyanobacteria isolated from thermal muds of Balaruc-Les-Bains (France) and description of a new genus and species Pseudo-chroococcus couteii. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive κ-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Meijenfeldt, F.A.B.; Arkhipova, K.; Cambuy, D.D.; Coutinho, F.H.; Dutilh, B.E. Robust taxonomic classification of uncharted microbial sequences and bins with CAT and BAT. Genome Biol. 2019, 20, 217. [Google Scholar] [CrossRef] [Green Version]
- Vallenet, D.; Calteau, A.; Dubois, M.; Amours, P.; Bazin, A.; Beuvin, M.; Burlot, L.; Bussell, X.; Fouteau, S.; Gautreau, G.; et al. MicroScope: An integrated platform for the annotation and exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. Nucleic Acids Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; Van Der Hooft, J.J.J.; Van Santen, J.A.; Tracanna, V.; Suarez Duran, H.G.; Pascal Andreu, V.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, D454–D458. [Google Scholar] [CrossRef] [Green Version]
- Ras, J.; Claustre, H.; Uitz, J. Spatial variability of phytoplankton pigment distributions in the Subtropical South Pacific Ocean: Comparison between in situ and predicted data. Biogeosciences 2008, 5, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Yéprémian, C.; Catherine, A.; Bernard, C.; Congestri, R.; Elersek, T.; Pilkaityte, R. Phycocyanin Extraction and Determination. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 335–338. [Google Scholar]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. Comprehensive database of secondary metabolites from cyanobacteria. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Olivon, F.; Elie, N.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MetGem Software for the Generation of Molecular Networks Based on the t-SNE Algorithm. Anal. Chem. 2018, 90, 13900–13908. [Google Scholar] [CrossRef] [PubMed]
- Houël, E.; Nardella, F.; Jullian, V.; Valentin, A.; Vonthron-Sénécheau, C.; Villa, P.; Obrecht, A.; Kaiser, M.; Bourreau, E.; Odonne, G.; et al. Wayanin and guaijaverin, two active metabolites found in a Psidium acutangulum Mart. ex DC (syn. P. persoonii McVaugh) (Myrtaceae) antimalarial decoction from the Wayana Amerindians. J. Ethnopharmacol. 2016, 187, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abboud, D.; Daubeuf, F.; Do, Q.T.; Utard, V.; Villa, P.; Haiech, J.; Bonnet, D.; Hibert, M.; Bernard, P.; Galzi, J.L.; et al. A strategy to discover decoy chemokine ligands with an anti-inflammatory activity. Sci. Rep. 2015, 5, 14746. [Google Scholar] [CrossRef] [Green Version]
- Calas, A.G.; Hanak, A.S.; Jaffré, N.; Nervo, A.; Dias, J.; Rousseau, C.; Courageux, C.; Brazzolotto, X.; Villa, P.; Obrecht, A.; et al. Efficacy assessment of an uncharged reactivator of nop-inhibited acetylcholinesterase based on tetrahydroacridine pyridine-aldoxime hybrid in mouse compared to pralidoxime. Biomolecules 2020, 10, 858. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Cornet, L.; Meunier, L.; Van Vlierberghe, M.; Léonard, R.R.; Durieu, B.; Lara, Y.; Misztak, A.; Sirjacobs, D.; Javaux, E.J.; Philippe, H.; et al. Consensus assessment of the contamination level of publicly available cyanobacterial genomes. PLoS ONE 2018, 13, e0200323. [Google Scholar] [CrossRef] [Green Version]
- NCBI Genome: Cyanobacteria Overview. Available online: https://www.ncbi.nlm.nih.gov/genome/?term=txid1117 (accessed on 16 June 2020).
- Alvarenga, D.O.; Fiore, M.F.; Varani, A.M. A metagenomic approach to cyanobacterial genomics. Front. Microbiol. 2017, 8, 809. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Donia, M.S.; Ravel, J.; Schmidt, E.W. A global assembly line for cyanobactins. Nat. Chem. Biol. 2008, 4, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Degnan, B.M.; Hawkins, C.J.; Lavin, M.F.; McCaffrey, E.J.; Parry, D.L.; Van Den Brenk, A.L.; Watters, D.J. New Cyclic Peptides with Cytotoxic Activity from the Ascidian Lissoclinum patella. J. Med. Chem. 1989, 32, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Banker, R.; Carmeli, S. Tenuecyclamides A-D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue. J. Nat. Prod. 1998, 61, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [Green Version]
- Kosourov, S.; Murukesan, G.; Jokela, J.; Allahverdiyeva, Y. Carotenoid biosynthesis in calothrix sp. 336/3: Composition of carotenoids on full medium, during diazotrophic growth and after long-term H2photoproduction. Plant Cell Physiol. 2016, 57, 2269–2282. [Google Scholar] [CrossRef] [Green Version]
- Hirschberg, J.; Chamovitz, D. Carotenoids in Cyanobacteria BT—The Molecular Biology of Cyanobacteria. In The Molecular Biology of Cyanobacteria; Springer: Dordrecht, The Netherlands, 1994; pp. 559–579. ISBN 978-94-011-0227-8. [Google Scholar]
- Liang, C.; Zhao, F.; Wei, W.; Wen, Z.; Qin, S. Carotenoid biosynthesis in cyanobacteria: Structural and evolutionary scenarios based on comparative genomics. Int. J. Biol. Sci. 2006, 2, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Rajneesh; Singh, S.P.; Häder, D.P.; Sinha, R.P. Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-Like Amino Acids as Multifunctional Secondary Metabolites in Cyanobacteria: From Biochemical to Application Aspects, 1st ed.; Elsevier, B.V.: Amsterdam, The Netherlands, 2018; Volume 59, ISBN 9780444641793. [Google Scholar]
- Katoch, M.; Mazmouz, R.; Chau, R.; Pearson, L.A.; Pickford, R.; Neilan, B.A. Heterologous production of cyanobacterial mycosporine-like amino acids mycosporine-ornithine and mycosporine-lysine in Escherichia coli. Appl. Environ. Microbiol. 2016, 82, 6167–6173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durai, P.; Batool, M.; Choi, S. Structure and effects of cyanobacterial lipopolysaccharides. Mar. Drugs 2015, 13, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; Schluter, P.J.; Shaw, G.R. Cyanobacterial lipopolysaccharides and human health—A review. Environ. Health Glob. Access Sci. Source 2006, 5, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Sevimli Gur, C.; Kiraz Erdogan, D.; Onbasılar, I.; Atilla, P.; Cakar, N.; Gurhan, I.D. In vitro and in vivo investigations of the wound healing effect of crude Spirulina extract and C-phycocyanin. J. Med. Plants Res. 2013, 7, 425–433. [Google Scholar]
- Sarkar, P.; Stefi, R.V.; Pasupuleti, M.; Paray, B.A.; Al-Sadoon, M.K.; Arockiaraj, J. Antioxidant molecular mechanism of adenosyl homocysteinase from cyanobacteria and its wound healing process in fibroblast cells. Mol. Biol. Rep. 2020, 47, 1821–1834. [Google Scholar] [CrossRef]
- Yin, H.; Chen, C.Y.; Liu, Y.W.; Tan, Y.J.; Deng, Z.L.; Yang, F.; Huang, F.Y.; Wen, C.; Rao, S.S.; Luo, M.J.; et al. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics 2019, 9, 2678–2693. [Google Scholar] [CrossRef]
- Cai, W.; Salvador-Reyes, L.A.; Zhang, W.; Chen, Q.Y.; Matthew, S.; Ratnayake, R.; Seo, S.J.; Dolles, S.; Gibson, D.J.; Paul, V.J.; et al. Apratyramide, a Marine-Derived Peptidic Stimulator of VEGF-A and Other Growth Factors with Potential Application in Wound Healing. ACS Chem. Biol. 2018, 13, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zervou, S.K.; Gkelis, S.; Kaloudis, T.; Hiskia, A.; Mazur-Marzec, H. New microginins from cyanobacteria of Greek freshwaters. Chemosphere 2020, 248, 125961. [Google Scholar] [CrossRef] [PubMed]
- Reshef, V.; Carmeli, S. Protease inhibitors from a water bloom of the cyanobacterium Microcystis aeruginosa. Tetrahedron 2001, 57, 2885–2894. [Google Scholar] [CrossRef]
- Lifshits, M.; Zafrir-Ilan, E.; Raveh, A.; Carmeli, S. Protease inhibitors from three fishpond water blooms of Microcystis spp. Tetrahedron 2011, 67, 4017–4024. [Google Scholar] [CrossRef]
- Ishida, K.; Kato, T.; Murakami, M.; Watanabe, M.; Watanabe, M.F. Microginins, zinc metalloproteases inhibitors cyanobacterium Microcystis aeruginosa. Tetrahedron 2000, 56, 8643–8656. [Google Scholar] [CrossRef]
- Okino, T.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Microginin, an angiotensin-converting enzyme inhibitor from the blue-green alga Microcystis aeruginosa. Tetrahedron Lett. 1993, 34, 501–504. [Google Scholar] [CrossRef]
- Nath Bagchi, S.; Sondhia, S.; Kumar Agrawal, M.; Banerjee, S. An angiotensin-converting enzyme-inhibitory metabolite with partial structure of microginin in a cyanobacterium Anabaena fertilissima CCC597, producing fibrinolytic protease. J. Appl. Phycol. 2016, 28, 177–180. [Google Scholar] [CrossRef]
- Hanke, T.; Merk, D.; Steinhilber, D.; Geisslinger, G.; Schubert-Zsilavecz, M. Small molecules with anti-inflammatory properties in clinical development. Pharmacol. Ther. 2016, 157, 163–187. [Google Scholar] [CrossRef]
- Ridker, P.M.; Lüscher, T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014, 35, 1782–1791. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial Sunscreen Scytonemin: Role in Photoprotection and Biomedical Research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563. [Google Scholar] [CrossRef]
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in vivo anti-inflammatory action of the galactolipid monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Tateo, F.; Ravaglioli, A.; Andreoli, C.; Bonina, F.; Coiro, V.; Degetto, S.; Giaretta, A.; Menconi Orsini, A.; Puglia, C.; Summa, V. The in-vitro percutaneous migration of chemical elements from a thermal mud for healing use. Appl. Clay Sci. 2009, 44, 83–94. [Google Scholar] [CrossRef]
- Tolomio, C.; Ceschi-Berrini, C.; Moschin, E.; Galzigna, L. Colonization by diatoms and antirheumatic activity of thermal mud. Cell Biochem. Funct. 1999, 17, 29–33. [Google Scholar] [CrossRef]
- Centini, M.; Tredici, M.R.; Biondi, N.; Buonocore, A.; Maffei Facino, R.; Anselmi, C. Thermal mud maturation: Organic matter and biological activity. Int. J. Cosmet. Sci. 2015, 37, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Carraturo, F.; Aliberti, F.; De Bonis, S.; Libralato, G.; Morra, M.; Guida, M. Characterization of microflora composition and antimicrobial activity of algal extracts from Italian thermal muds. J. Nat. Sci. Biol. Med. 2018, 9, 150–158. [Google Scholar]
- Beliaev, A.S.; Romine, M.F.; Serres, M.; Bernstein, H.C.; Linggi, B.E.; Markillie, L.M.; Isern, N.G.; Chrisler, W.B.; Kucek, L.A.; Hill, E.A.; et al. Inference of interactions in cyanobacterial-heterotrophic co-cultures via transcriptome sequencing. ISME J. 2014, 8, 2243–2255. [Google Scholar] [CrossRef] [Green Version]
- Briand, E.; Reubrecht, S.; Mondeguer, F.; Sibat, M.; Hess, P.; Amzil, Z.; Bormans, M. Chemically mediated interactions between Microcystis and Planktothrix: Impact on their growth, morphology and metabolic profiles. Environ. Microbiol. 2019, 21, 1552–1566. [Google Scholar] [CrossRef] [Green Version]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef]
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef] [Green Version]
- Shishido, T.K.; Jokela, J.; Kolehmainen, C.-T.; Fewer, D.P.; Wahlsten, M.; Wang, H.; Rouhiainen, L.; Rizzi, E.; De Bellis, G.; Permi, P.; et al. Antifungal activity improved by coproduction of cyclodextrins and anabaenolysins in Cyanobacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 13669–13674. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Order | Species | Strain Number | BioProject Accession Numbers |
|---|---|---|---|
| Chroococcales | Pseudo-chroococcus couteii | PMC 885.14 | PRJNA686263 |
| Synechococcales | Leptolyngbya boryana | PMC 883.14 | PRJNA686260 |
| Oscillatoriales | Planktothricoides raciborskii | PMC 877.14 | PRJNA686238 |
| Laspinema sp. | PMC 878.14 | PRJNA686242 | |
| Microcoleus vaginatus | PMC 879.14 | PRJNA686244 | |
| Lyngbya martensiana | PMC 880.14 | PRJNA686257 | |
| Nostocales | Nostoc sp. | PMC 881.14 | PRJNA686258 |
| Aliinostoc sp. | PMC 882.14 | PRJNA686259 | |
| Calothrix sp. | PMC 884.14 | PRJNA686262 |
| Genus/Species | Strain | Genome Length (bp) | GC Content | Number of Contigs | Number of CDS | Number of rRNA | Average CDS Length (bp) | CheckM Completeness | CheckM Contamination |
|---|---|---|---|---|---|---|---|---|---|
| P. raciborskii | PMC 877.14 | 7,392,957 | 44% | 15 | 7130 | 12 | 841.8 | 99.9 % | 0.89 % |
| Laspinema sp. | PMC 878.14 | 7,343,542 | 47% | 69 | 5829 | 9 | 1 022.4 | 99.3 % | 1.16 % |
| M. vaginatus | PMC 879.14 | 6,904,242 | 46% | 34 | 6400 | 6 | 904.5 | 99.6 % | 0.22 % |
| L. martensiana | PMC 880.14 | 6,457,204 | 40% | 132 | 5904 | 3 | 937.8 | 97.1 % | 1.19 % |
| Nostoc sp. | PMC 881.14 | 8,003,053 | 42% | 648 | 7711 | 3 | 845.9 | 99.5 % | 1.19 % |
| Aliinostoc sp. | PMC 882.14 | 8,136,653 | 41% | 46 | 7582 | 12 | 883.9 | 98.2 % | 0.22 % |
| L. boryana | PMC 883.14 | 6,695,177 | 47% | 5 | 6313 | 9 | 929.1 | 99.4 % | 1.02 % |
| Calothrix sp. | PMC 884.14 | 13,247,652 | 39% | 46 | 11,176 | 15 | 891.3 | 99.3 % | 1.35 % |
| P. couteii | PMC 885.14 | 5,871,606 | 35% | 137 | 5328 | 6 | 946.5 | 98.5 % | 1.31 % |
| Pigments | P. raciborskii PMC 877.14 | Laspinema sp. PMC 878.14 | M. vaginatus PMC 879.14 | L. martensiana PMC 880.14 | Nostoc sp. PMC 881.14 | Aliinostoc sp. PMC 882.14 | L. boryana PMC 883.14 | Calothrix sp. PMC 884.14 | P. couteii PMC 885.14 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phycobiliproteins | C-Phycocyanin | + | + | + | + | + | + | + | + | + |
| Allophycocyanin | + | + | + | + | + | + | + | + | + | |
| Phycoerythrin | + | - | - | - | + | - | - | + | + | |
| Scytonemin | - | - | - | - | + | + | - | + | - | |
| Hydroxylated carotenoids | Cryptoxanthin | + | + | + | + | + | + | + | + | + |
| Zeaxanthin | + | + | + | + | + | + | + | + | + | |
| Caloxanthin | - | + | - | + | - | + | + | + | - | |
| Nostoxanthin | - | + | - | + | - | + | + | + | - | |
| Myxoxanthophylls | 1-hydroxylycopene | + | + | + | + | + | + | + | + | + |
| 1′-Hydroxy-γ-carotene | + | + | + | + | + | + | + | + | + | |
| Myxol 2′-pentoside | + | + | + | + | + | + | + | + | + | |
| 2-Hydroxymyxol 2′-methylpentoside | - | + | - | + | - | + | + | + | - | |
| Echinenone | - | + | + | + | + | + | + | + | + | |
| Ketocarotenoids | Canthaxanthin | - | + | + | + | + | + | + | + | + |
| Adonixanthin | - | + | + | + | + | + | + | + | + | |
| Pigments | P. raciborskii PMC 877.14 | Laspinema sp. PMC 878.14 | M. vaginatus PMC 879.14 | L. martensiana PMC 880.14 | Nostoc sp. PMC 881.14 | Aliinostoc sp. PMC 882.14 | L. boryana PMC 883.14 | Calothrix sp. PMC 884.14 | P. couteii PMC 885.14 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Carotenoids | Zeaxanthin | 1.72 | 0.38 | 0.43 | 0.11 | 0.00 | 0.01 | 0.22 | 0.00 | 0.29 |
| Myxoxanthophyll-like | 0.44 | 0.28 | 0.00 | 0.12 | 0.03 | 0.32 | 0.00 | 0.21 | 0.00 | |
| β-Carotene | 3.65 | 1.43 | 1.06 | 0.46 | 0.15 | 1.29 | 1.31 | 0.46 | 0.78 | |
| Chlorophylls | Bacteriochlorophyll a | 0.54 | 0.04 | 0.12 | 0.04 | 0.06 | 0.08 | 0.07 | 0.07 | 0.02 |
| Chlorophyll a | 35.2 | 9.8 | 11.2 | 4.9 | 1.5 | 12.3 | 17.1 | 9.7 | 6.7 | |
| Phaeophytin a | 0.00 | 0.09 | 0.07 | 0.02 | 0.01 | 0.00 | 0.09 | 0.04 | 0.00 | |
| Phycobiliproteins | Phycocyanin | 32.5 ± 12.8 | 18.4 ± 0.2 | 12.2 ± 2.1 | 6.1 ± 0.5 | 2.1 ± 0.1 | 21.3 ± 4.7 | 24.9 ± 4.4 | 9.9 ± 0.4 | 3.5 ± 0.1 |
| Allophycocyanin | 7.9 ± 3.5 | 6.6 ± 0.5 | 5.8 ± 1.1 | 3.7 ± 0.3 | 1.5 ± 0.2 | 15.6 ± 3.5 | 15.2 ± 3.8 | 6.7 ± 0.3 | 2.7 ± 0.2 | |
| Phycoerythrin | 33.3 ± 12.1 | 0.00 | 0.00 | 0.00 | 2.8 ± 0.2 | 0.00 | 0.00 | 9.9 ± 0.3 | 3.3 ± 0.1 |
| Genus/Species | Strain | Nb of Clusters Predicted | Identified Clusters | Reported Activity | Unknown/Homologous Cluster | Unknown/Unknown Cluster |
|---|---|---|---|---|---|---|
| P. raciborskii | PMC 877.14 | 7 | Phytoene or derivative | Antioxidant | 1 | 5 |
| Laspinema sp. | PMC 878.14 | 7 | Phytoene or derivative Heterocyclic cyanobactin | Antioxidant Cytotoxic | 5 | 0 |
| M. vaginatus | PMC 879.14 | 7 | Phytoene or derivative | Antioxidant | 3 | 3 |
| L. martensiana | PMC 880.14 | 9 | Phytoene or derivative Squalene Schizokinen-like | Antioxidant Antioxidant Iron-chelating activity | 5 | 1 |
| Nostoc sp. | PMC 881.14 | 20 * | Phytoene or derivative (2×) Heterocyst glycolipid | Antioxidant n.d. | 7 | 10 |
| Aliinostoc sp. | PMC 882.14 | 21 | Shinorine-like Microginin-like Amycomicin-like Phytoene or derivative (2×) Heterocyst glycolipid Microviridin-like | Antioxidant ACE inhibition; no cytotoxic Antibiotic Antioxidant n.d. Protease inhibitor | 6 | 8 |
| L. boryana | PMC 883.14 | 16 | Phytoene or derivative (5×) | Antioxidant | 8 | 3 |
| Calothrix sp. | PMC 884.14 | 27 | Cyanobactin Mycosporine-glycine-like Cylindrocyclophane-like Phytoene/Lycopene or derivative (3×) Heterocyst glycolipid | Cytotoxic Antioxidant Cytotoxic; antibacterial Antioxidant n.d. | 14 | 6 |
| P. couteii | PMC 885.14 | 10 | Phytoene or derivative (2×) | Antioxidant | 2 | 6 |
| Total | 124 | 31 | 51 | 42 |
| Class | Compound | P. raciborskii PMC 877.14 | Laspinema sp. PMC 878.14 | M. vaginatus PMC 879.14 | L. martensiana PMC 880.14 | Nostoc sp. PMC 881.14 | Aliinostoc sp. PMC 882.14 | L. boryana PMC 883.14 | Calothrix sp. PMC 884.14 | P. couteii PMC 885.14 |
|---|---|---|---|---|---|---|---|---|---|---|
| MAAs (antioxidant) | Mycosporine-glycine | - | - | - | - | - | + | - | + | - |
| Mycosporine-ornithine | - | - | - | - | + | - | - | - | - | |
| Nostoc-756 | - | - | - | - | + | - | - | - | - | |
| Shinorine | - | - | - | - | - | + | - | - | - | |
| Carotenoids (antioxidant) | Canthaxanthin | - | - | + | + | - | + | + | + | + |
| Myxoxanthophyll-like | + | + | - | + | - | + | - | + | - | |
| Zeaxanthin | + | + | + | + | - | - | + | - | + | |
| β-carotene | + | + | + | + | + | + | + | + | + | |
| Chlorophylls (antioxidant) | Chlorophyll a | + | + | + | + | + | + | + | + | + |
| Bacteriochlorophyll a | + | - | + | - | - | - | - | - | - | |
| Phycobiliproteins (antioxidant) | Phycocyanin (Anti-inflammatory) | + | + | + | + | + | + | + | + | + |
| Allophycocyanin | + | + | + | + | + | + | + | + | + | |
| Phycoerythrin | + | - | - | - | + | - | - | + | + | |
| Phycoerythrobilin | - | - | - | - | - | - | - | + | + | |
| Aeruginosins (anti-inflammatory) | Aeruginosin TR642 | - | - | - | - | - | - | + | - | - |
| Antioxidant | 8 | 6 | 7 | 7 | 7 | 8 | 6 | 9 | 8 | |
| Anti-inflammatory | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| P. raciborskii PMC 877.14 | Laspinema sp. PMC 878.14 | M. vaginatus PMC 879.14 | L. martensiana PMC 880.14 | Nostoc sp. PMC 881.14 | Aliinostoc sp. PMC 882.14 | L. boryana PMC 883.14 | Calothrix sp. PMC 884.14 | P. couteii PMC 885.14 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioassay | µg·mL−1 | MeOH | H2O | MeOH | H2O | MeOH | H2O | MeOH | H2O | MeOH | H2O | MeOH | H2O | MeOH | H2O | MeOH | H2O | MeOH | H2O | |
| NO secretion RAW 264.7 (antioxidant) | 1 | - | - | - | - | - | - | - | - | - | ||||||||||
| 5 | - | - | - | - | - | - | - | - | - | |||||||||||
| 10 | - | - | - | - | - | - | - | - | - | |||||||||||
| 50 | - | - | - | - | - | +20 ± 2 | - | - | - | - | ||||||||||
| Keratinocytes migration HaCaT (wound healing) | 1 | - | - | - | - | - | - | - | - | - | ||||||||||
| 5 | - | - | - | - | - | - | - | - | - | |||||||||||
| 10 | - | - | - | - | - | - | +22 ± 7 | - | - | - | ||||||||||
| 50 | - | - | - | - | - | - | - | - | - | |||||||||||
| Cytokines secretion PBMC (anti-inflammatory) | TNF α | 1 | - | - | - | - | - | - | - | - | - | |||||||||
| IL-1β | - | - | - | - | - | - | - | - | - | |||||||||||
| IL-6 | - | - | - | - | - | - | - | - | - | |||||||||||
| IL-8 | - | - | - | - | - | - | - | - | - | |||||||||||
| TNF α | 5 | - | - | - | - | - | - | - | - | - | ||||||||||
| IL-1β | - | −20 ± 5 | - | - | +34 ± 3 | - | - | +50 ± 8 | - | +26 ± 3 | - | - | - | |||||||
| IL-6 | - | - | - | - | - | - | - | - | −27 ± 7 | - | ||||||||||
| IL-8 | - | - | - | - | - | - | - | - | - | |||||||||||
| TNF α | 10 | - | - | - | - | −18 ± 4 | −20 ± 4 | - | - | - | - | |||||||||
| IL-1β | - | −28 ± 2 | - | +23 ± 4 | +49 ± 6 | - | −26 ± 4 | +52 ± 7 | - | +30 ± 3 | - | - | +31 ± 2 | - | ||||||
| IL-6 | - | - | - | - | - | - | - | - | −27 ± 4 | - | ||||||||||
| IL-8 | - | - | - | - | - | - | - | - | - | |||||||||||
| TNF α | 50 | −19 ± 6 | - | - | - | −20 ± 1 | - | - | - | - | - | - | ||||||||
| IL-1β | −39 ± 9 | −57 ± 3 | - | +29 ± 3 | +61 ± 2 | - | +18 ± 3 | −45 ± 5 | +60 ± 4 | - | +26 ± 5 | - | - | +20 ± 2 | - | −31 ± 2 | ||||
| IL-6 | - | - | −26 ± 5 | - | - | - | - | −23 ± 4 | - | - | −29 ± 3 | - | −24 ± 3 | |||||||
| IL-8 | - | - | - | - | - | - | - | - | - | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demay, J.; Halary, S.; Knittel-Obrecht, A.; Villa, P.; Duval, C.; Hamlaoui, S.; Roussel, T.; Yéprémian, C.; Reinhardt, A.; Bernard, C.; et al. Anti-Inflammatory, Antioxidant, and Wound-Healing Properties of Cyanobacteria from Thermal Mud of Balaruc-Les-Bains, France: A Multi-Approach Study. Biomolecules 2021, 11, 28. https://doi.org/10.3390/biom11010028
Demay J, Halary S, Knittel-Obrecht A, Villa P, Duval C, Hamlaoui S, Roussel T, Yéprémian C, Reinhardt A, Bernard C, et al. Anti-Inflammatory, Antioxidant, and Wound-Healing Properties of Cyanobacteria from Thermal Mud of Balaruc-Les-Bains, France: A Multi-Approach Study. Biomolecules. 2021; 11(1):28. https://doi.org/10.3390/biom11010028
Chicago/Turabian StyleDemay, Justine, Sébastien Halary, Adeline Knittel-Obrecht, Pascal Villa, Charlotte Duval, Sahima Hamlaoui, Théotime Roussel, Claude Yéprémian, Anita Reinhardt, Cécile Bernard, and et al. 2021. "Anti-Inflammatory, Antioxidant, and Wound-Healing Properties of Cyanobacteria from Thermal Mud of Balaruc-Les-Bains, France: A Multi-Approach Study" Biomolecules 11, no. 1: 28. https://doi.org/10.3390/biom11010028