Fish-Derived Antifreeze Proteins and Antifreeze Glycoprotein Exhibit a Different Ice-Binding Property with Increasing Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Antifreeze Proteins and Antifreeze Glycoprotein
2.2. Evaluation of Protein Concentration
2.3. Analysis of Fluorescence-Based Ice Plane Affinity
2.4. Ethical Approval
3. Results and Discussion
3.1. Solubility Limit Evaluation for Native Antifreeze Proteins and Antifreeze Glycoprotein
3.2. Thermal Hysteresis Measurement for the Native Samples
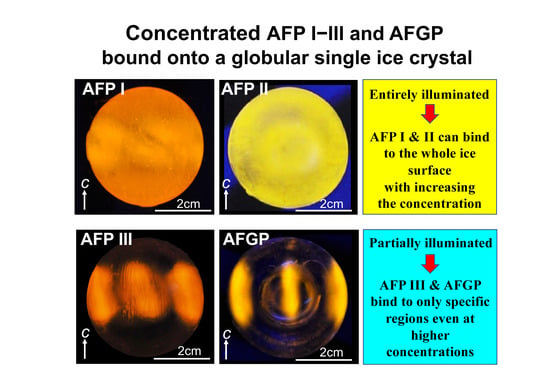
3.3. Analysis of Fluorescence-Based Ice Plane Affinity (FIPA) for the Native Samples
- (a)
- Ca2+-dependent AFP II, AFP III, β-helical AFPs—First prism plane;
- (b)
- Sculpin AFPI, Ca2+-independent AFP II, β-helical AFPs—Second prism plane;
- (c)
- Flounder AFP I, AFP III, β-helical AFPs—Pyramidal plane;
- (d)
- Flounder AFP I dimer (Maxi), β-helical AFPs—Basal plane.
3.4. Oligomerization Hypothesis for Type II Antifreeze Protein
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pace, C.N.; Treviño, S.; Prabhakaran, E.; Scholtz, J.M. Protein structure, stability and solubility in water and other solvents. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1225–1234. [Google Scholar] [CrossRef] [Green Version]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, T. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1996. [Google Scholar] [CrossRef] [Green Version]
- Voets, I.K. From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter 2017, 13, 4808–4823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.-H.; Koo, B.-W. Marine antifreeze proteins: Structure, Function, and application to cryopreservation as a potential cryoprotectant. Mar. Drugs 2017, 15, E27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahatabuddin, S.; Hanada, Y.; Nishimiya, Y.; Miura, A.; Kondo, H.; Davies, P.L.; Tsuda, S. Concentration-dependent oligomerization of an alpha-helical antifreeze polypeptide makes it hyperactive. Sci. Rep. 2017, 7, 42501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimiya, Y.; Kondo, H.; Takamichi, M.; Sugimoto, H.; Suzuki, M.; Miura, A.; Tsuda, S. Crystal structure and mutational analysis of Ca2+-independent type II antifreeze protein from Longsnout poacher, Brachyopsis rostratus. J. Mol. Biol. 2008, 382, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, Y.; Sato, R.; Takamichi, M.; Miura, A.; Tsuda, S. Co-operative effect of the isoforms of type III antifreeze protein expressed in Notched-fin eelpout, Zoarces elongatus Kner. FEBS J. 2005, 272, 482–492. [Google Scholar] [CrossRef]
- Mahatabuddin, S.; Tsuda, S. Applications of Antifreeze proteins: Practical use of the quality products from Japanese fishes. Adv. Exp. Med. Biol. 2018, 1081, 321–327. [Google Scholar] [CrossRef]
- Nishimiya, Y.; Mie, Y.; Hirano, Y.; Kondo, H.; Miura, A.; Tsuda, S. Mass preparation and technological development of an antifreeze protein. Synthesiology 2008, 1, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Harding, M.M.; Anderberg, P.I.; Haymet, A.D.J. ‘Antifreeze’ glycoproteins from polar fish. Eur. J. Biochem. 2003, 270, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Burcham, T.S.; Osuga, D.T.; Narasinga Rao, B.N.; Bush, A.; Feeney, R.E. Purification and primary sequences of the major arginine-containing antifreeze glycopeptides from the Fish Eleginus gracilis. J. Biol. Chem. 1986, 261, 6384–6389. [Google Scholar] [PubMed]
- Tachibana, Y.; Fletcher, G.L.; Fujitani, N.; Tsuda, S.; Monde, K.; Nishimura, S.-I. Antifreeze glycoproteins: Elucidation of the structural motifs that are essential for antifreeze activity. Angnew. Chem. Int. Ed. 2004, 43, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef] [Green Version]
- Inada, T.; Lu., S.-S. Thermal hysteresis caused by non-equilibrium antifreeze activity of poly(vinyl alcohol). Chem. Phys. Lett. 2004, 394, 361–365. [Google Scholar] [CrossRef]
- Wen, D.; Laursen, R.A. Structure-function relationships in an antifreeze polypeptide: The role of neutral, polar amino acids. J. Biol. Chem. 1992, 267, 14102–14108. [Google Scholar] [PubMed]
- Sönnichsen, F.D.; Sykes, B.D.; Chao, H.; Davies, P.L. The nonhelical structure of antifreeze protein type III. Science 1993, 259, 1154–1157. [Google Scholar] [CrossRef]
- Smolin, N.; Daggett, V. Formation of ice-like water structure on the surface of an antifreeze protein. J. Phys. Chem. B 2008, 112, 6193–6202. [Google Scholar] [CrossRef]
- Sharp, K. The remarkable hydration of the antifreeze protein Maxi: A computational study. J. Chem. Phys. 2014, 141, 22D510. [Google Scholar] [CrossRef]
- Hudait, A.; Odendahl, N.; Qiu, Y.; Paesani, F.; Molinero, V. Ice-Nucleating and Antifreeze Proteins Recognize Ice through a Diversity of Anchored Clathrate and Ice-like Motifs. J. Am. Chem. Soc. 2018, 140, 4905–4912. [Google Scholar] [CrossRef]
- Garnham, C.P.; Campbell, R.L.; Davies, P.L. Anchored clathrate waters bind antifreeze proteins to ice. Proc. Natl. Acad. Sci. USA 2011, 108, 7363–7367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, H.; Hanada, Y.; Sugimoto, H.; Hoshino, T.; Garnham, C.P.; Davies, P.L.; Tsuda, S. Ice-binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc. Natl. Acad. Sci. USA 2012, 109, 9360–9365. [Google Scholar] [CrossRef] [Green Version]
- Mahatabuddin, S.; Fukami, D.; Arai, T.; Nishimiya, Y.; Shimizu, R.; Shibazaki, C.; Kondo, H.; Adachi, M.; Tsuda, S. Polypentagonal ice-like water networks emerge solely in an activity-improved variant of ice-binding protein. Proc. Natl. Acad. Sci. USA 2018, 115, 5456–5461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudait, A.; Moberg, D.R.; Qiu, Y.; Odendahl, N.; Paesani, F.; Molinero, V. Preordering of water is not needed for ice recognition by hyperactive antifreeze proteins. Proc. Natl. Acad. Sci. USA 1028, 115, 8266–8271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Lin, F.-H.; Campbell, R.L.; Allingham, J.S.; Davies, P.L. An antifreeze protein folds with an interior network of more than 400 semi-clathrate waters. Science 2014, 343, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Garnham, C.P.; Natarajan, A.; Middleton, A.J.; Kuiper, M.J.; Braslavsky, I.; Davies, P.L. Compound Ice-Binding Site of an Antifreeze Protein Revealed by Mutagenesis and Fluorescent Tagging. Biochemistry 2010, 49, 9063–9071. [Google Scholar] [CrossRef]
- Basu, K.; Garnham, C.P.; Nishimiya, Y.; Tsuda, S.; Braslavsky, I.; Davies, P. Determining the ice-binding planes of antifreeze proteins by fluorescence-based ice plane affinity. JoVE 2014, 83, e51185. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.T.; Arai, T.; Yamauchi, A.; Miura, A.; Kondo, H.; Ohyama, Y.; Tsuda, S. Ice recrystallization is strongly inhibited when antifreeze proteins bind to multiple ice planes. Sci. Rep. 2019, 9, 2212. [Google Scholar] [CrossRef]
- Davies, P.L.; Baardsnes, J.; Kuiper, M.J.; Walker, V.K. Structure and function of antifreeze proteins. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2002, 357, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Mahatabuddin, S.; Nishimiya, Y.; Miura, A.; Kondo, H.; Tsuda, S. Critical ice shaping concentration (CISC): A new parameter to evaluate the activity of antifreeze proteins. Cryobio. Cryotech. 2016, 62, 95–103. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Freed, K.F. Polymer viscosity in concentrated solutions. J. Chem. Phys. 1974, 61, 3626–3633. [Google Scholar] [CrossRef]
- Celik, Y.; Graham, L.A.; Mok, Y.F.; Bar, M.; Davies, P.L.; Braslavsky, I. Superheating of ice crystals in antifreeze protein solutions. Proc. Natl. Acad. Sci. USA 2010, 107, 5423–5428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Amomwittawat, N.; Wen, X. Thermodynamic analysis of thermal hysteresis: Mechanistic insights into biological antifreezes. J. Chem. Thermodyn. 2012, 1, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olijve, L.L.; Meister, K.; DeVries, A.L.; Duman, J.G.; Guo, S.; Bakker, H.J.; Voets, I.K. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 3740–3745. [Google Scholar] [CrossRef] [Green Version]
- Bar-Dolev, M.; Celik, Y.; Wettlaufer, J.S.; Davies, P.L.; Braslavsky, I. New insights into ice growth and melting modifications by antifreeze proteins. J. R. Soc. Interface 2012, 9, 3249–3259. [Google Scholar] [CrossRef]
- Takamichi, M.; Nishimiya, Y.; Miura, A.; Tsuda, S. Effect of annealing time of an ice crystal on the activity of type III antifreeze protein. FEBS J. 2007, 274, 6469–6476. [Google Scholar] [CrossRef]
- Hartel, R.W. Crystallization in Foods, Handbook of Industrial Crystallization, 2nd ed.; University of Wisconsin-Madison: Madison, WI, USA, 2002; pp. 287–304. [Google Scholar] [CrossRef]
- Burcham, T.S.; Osuga, D.T.; Yeh, Y.; Feeney, R.E. A kinetic description of antifreeze glycoprotein activity. J. Biol. Chem. 1986, 261, 6390–6397. [Google Scholar] [PubMed]
- Scotter, A.J.; Marshall, C.B.; Graham, L.A.; Gilbert, J.A.; Garnham, C.P.; Davies, P.L. The basis for hyperactivity of antifreeze proteins. Cryobiology 2006, 53, 229–239. [Google Scholar] [CrossRef]
- Kuiper, M.J.; Lankin, C.; Gauthier, S.Y.; Walker, V.K.; Davies, P.L. Purification of antifreeze proteins by adsorption to ice. Biochem. Biophys. Res. Commun. 2003, 300, 645–648. [Google Scholar] [CrossRef]
- Adar, C.; Sirotinskaya, V.; Bar Dolev, M.; Friehmann, T.; Braslavsky, I. Falling water ice affinity purification of ice-binding proteins. Sci. Rep. 2018, 8, 11046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, C.A.; Cheng, C.C.; DeVries, A.L. Adsorption of α-helical antifreeze peptides on specific ice crystal surface planes. Biophys. J. 1999, 59, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, E.; Zachariassen, K.E. The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology 2005, 51, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Nishi, N.; Miyamoto, T.; Waku, T.; Tanaka, N.; Hagiwara, Y. Ice growth inhibition in antifreeze polypeptide solution by short-time solution preheating. PLoS ONE 2016, 11, e0154782. [Google Scholar] [CrossRef]
- Knight, C.A.; Driggers, E.; DeVries, A.L. Adsorption to ice of fish antifreeze glycopeptides 7 and 8. Biophys. J. 1993, 64, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Poget, S.F.; Legge, G.B.; Proctor, M.R.; Butler, P.J.G.; Bycroft, M.; Williams, R.L. The structure of a tunicate C-type lectin from Polyandrocarpa misakiensis with D-Galactose. J. Mol. Biol. 1999, 290, 867–879. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.R.; Nagar., B.; Young, N.M.; Hirama, T.; Rini, J.M. X-ray crystal structure of a galactose-specific C-type lectin possessing a novel decameric quaternary structure. Biochemistry 2004, 43, 3783–3792. [Google Scholar] [CrossRef]
- Zelensky, A.D.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef]
- Arai, T.; Nishimiya, Y.; Ohyama, Y.; Kondo, H.; Tsuda, S. Calcium-binding generates the semi-clathrate waters on a type II antifreeze protein to adsorb onto an ice crystal surface. Biomolecules 2019, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. Ccp4 Newsl. Protein Chem. 2002, 40, 82–92. [Google Scholar]
- Mantz, Y.A.; Geiger, F.M.; Molina, L.T.; Molina, M.J.; Trout, B.L. First-principles molecular-dynamics study of surface disordering of the (0001) face of hexagonal ice. J. Chem. Phys. 2000, 113, 10733–10743. [Google Scholar] [CrossRef]
- Hayward, J.A.; Haymet, A.D.J. The ice/water interface: Molecular dynamics simulations of the basal, prism, {20-21}, and {2-1-10} Interfaces of Ice Ih. J. Chem. Phys. 2001, 114, 3713–3726. [Google Scholar] [CrossRef]
- Duboué-Dijon, E.; Laage, E. Characterization of the local structure in liquid water by various order parameters. J. Phys. Chem. B 2015, 119, 8406–8418. [Google Scholar] [CrossRef]
- Ball, P. Water–An enduring mystery. Nature 2008, 452, 291–292. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuda, S.; Yamauchi, A.; Khan, N.M.-M.U.; Arai, T.; Mahatabuddin, S.; Miura, A.; Kondo, H. Fish-Derived Antifreeze Proteins and Antifreeze Glycoprotein Exhibit a Different Ice-Binding Property with Increasing Concentration. Biomolecules 2020, 10, 423. https://doi.org/10.3390/biom10030423
Tsuda S, Yamauchi A, Khan NM-MU, Arai T, Mahatabuddin S, Miura A, Kondo H. Fish-Derived Antifreeze Proteins and Antifreeze Glycoprotein Exhibit a Different Ice-Binding Property with Increasing Concentration. Biomolecules. 2020; 10(3):423. https://doi.org/10.3390/biom10030423
Chicago/Turabian StyleTsuda, Sakae, Akari Yamauchi, N. M.-Mofiz Uddin Khan, Tatsuya Arai, Sheikh Mahatabuddin, Ai Miura, and Hidemasa Kondo. 2020. "Fish-Derived Antifreeze Proteins and Antifreeze Glycoprotein Exhibit a Different Ice-Binding Property with Increasing Concentration" Biomolecules 10, no. 3: 423. https://doi.org/10.3390/biom10030423