Phylogenetic Distribution, Ultrastructure, and Function of Bacterial Flagellar Sheaths
Abstract
:1. Introduction
2. Phylogenetic Distribution of Flagellar Sheaths
3. Composition of Flagellar Sheaths
4. Rotation of the Sheathed Flagellum
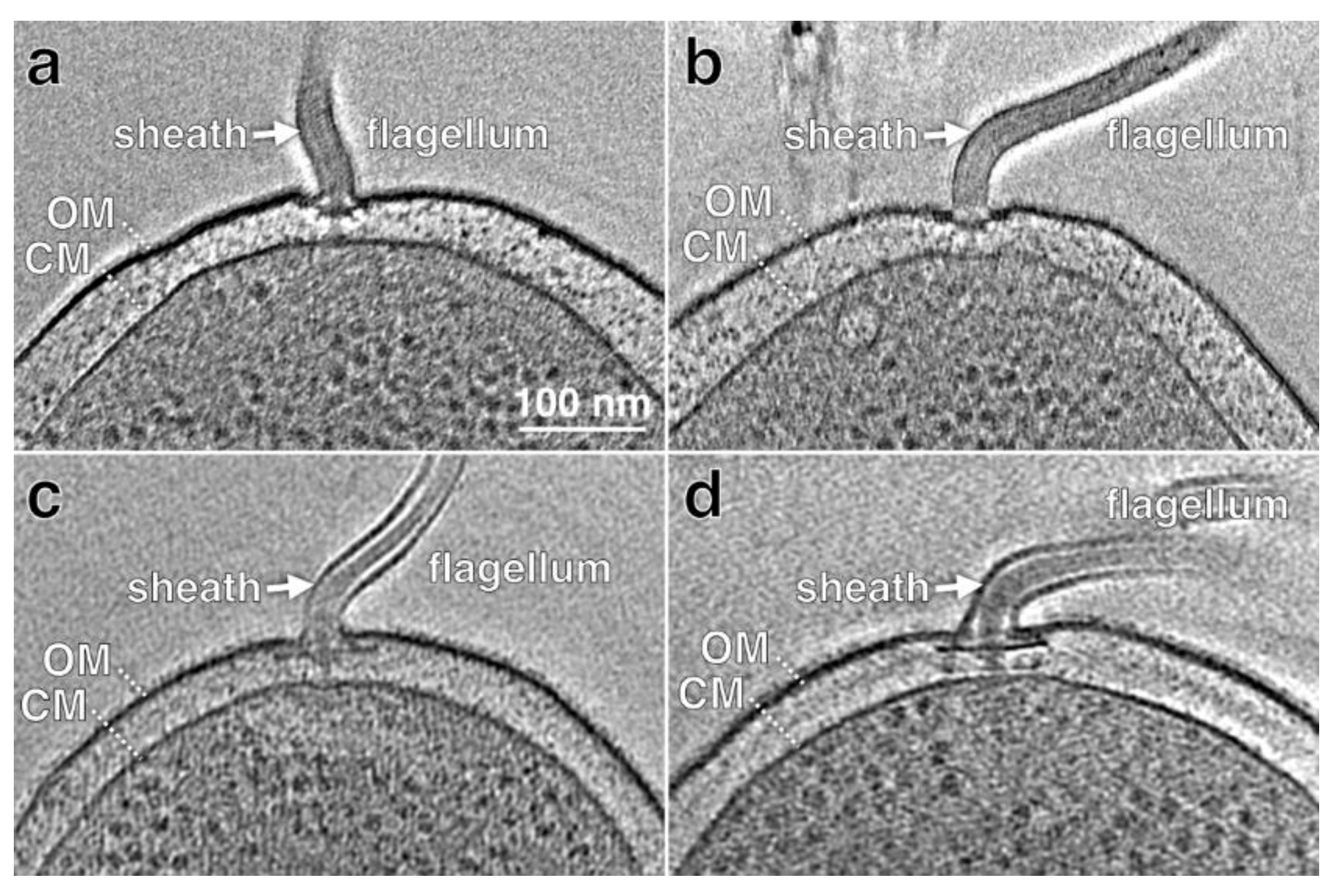
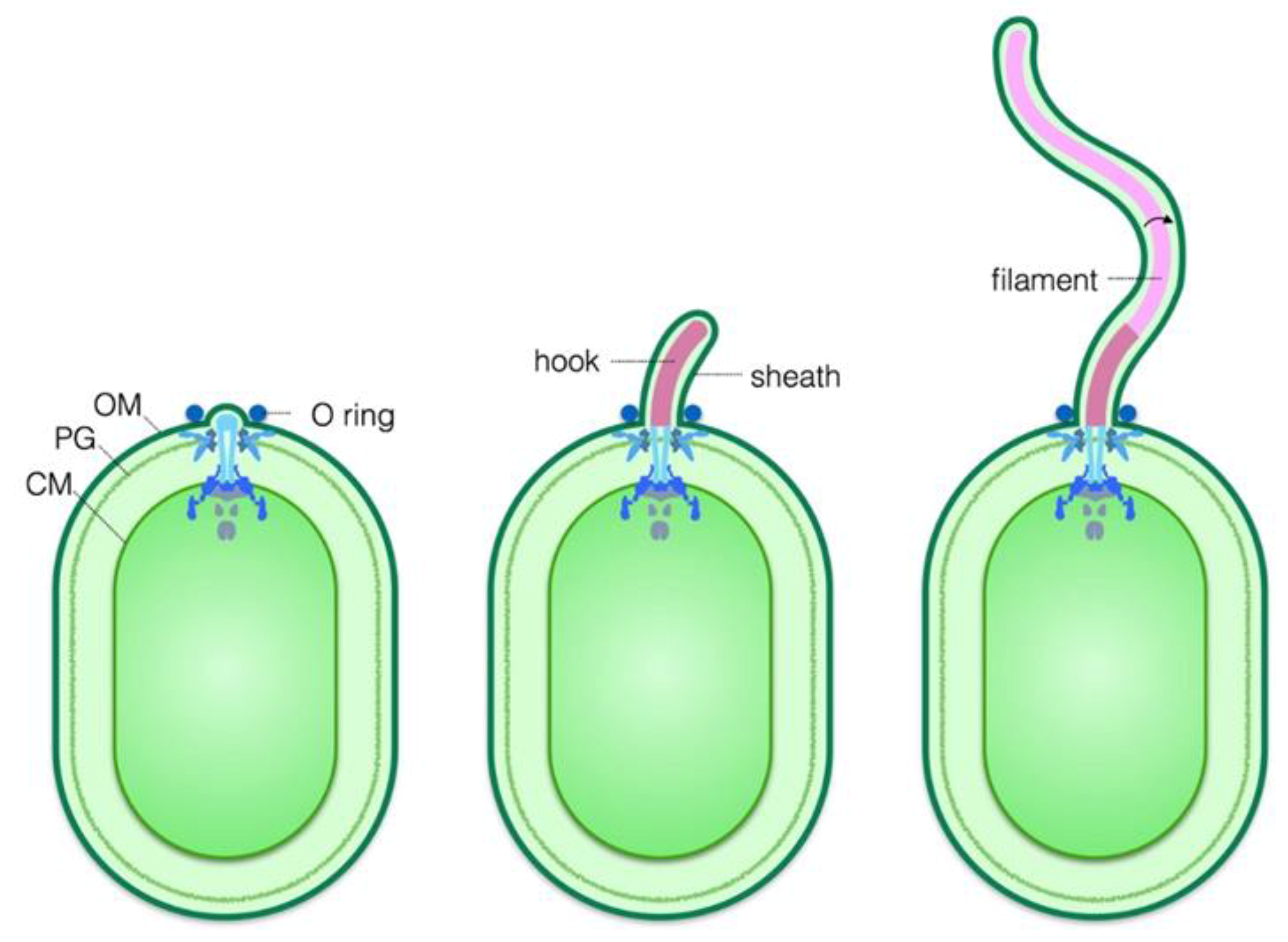
5. Biogenesis of the Flagellar Sheath
6. Proposed Functions for Flagellar Sheaths
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lefevre, C.T.; Santini, C.L.; Bernadac, A.; Zhang, W.J.; Li, Y.; Wu, L.F. Calcium ion-mediated assembly and function of glycosylated flagellar sheath of marine magnetotactic bacterium. Mol. Microbiol 2010, 78, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolgemuth, C.W. Flagellar motility of the pathogenic spirochetes. Semin Cell Dev. Biol. 2015, 46, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoblad, R.D.; Emala, C.W.; Doetsch, R.N. Invited review: Bacterial flagellar sheaths: Structures in search of a function. Cell Motil. 1983, 3, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, J.A. The planctomycetes: Emerging models for microbial ecology, evolution and cell biology. Microbiology 1995, 141, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesner, H. Pirella marina sp. nov., a budding, peptidoglycan-less bacterium from brackish water. Syst. Appl. Microbiol. 1986, 8, 177–180. [Google Scholar] [CrossRef]
- Burygin, G.L.; Shirokov, A.A.; Shelud’ko, A.V.; Katsy, E.I.; Shchygolev, S.Y.; Matora, L.Y. Detection of a sheath on Azospirillum brasilense polar flagellum. Microbiology 2007, 76, 728–734. [Google Scholar] [CrossRef]
- Ferooz, J.; Letesson, J.J. Morphological analysis of the sheathed flagellum of Brucella melitensis. BMC Res. Notes 2010, 3, 333. [Google Scholar] [CrossRef] [Green Version]
- Ragatz, L.; Jiang, Z.Y.; Bauer, C.E.; Gest, H. Macroscopic phototactic behavior of the purple photosynthetic bacterium Rhodospirillum centenum. Arch. Microbiol. 1995, 163, 1–6. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Starr, M.P. Unidirectional polar growth of cells of Seliberia stellata and aquatic seliberia-like bacteria revealed by immunoferritin labeling. Arch. Microbiol. 1984, 138, 89–95. [Google Scholar] [CrossRef]
- Fuerst, J.A.; Hayward, A.C. The sheathed flagellum of Pseudomonas stizolobii. J. Gen. Microbiol. 1969, 58, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Busse, H.-J.; Auling, G. Achromobacter. In Bergy’s Manual of Systematics of Archaea and Bacteria; Whitman, W., Rainey, F., Kampfer, P., Trujillo, M., Chun, J., DeVos, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Yabuuchi, E.; Yano, I.; Goto, S.; Tanimura, E.; Ito, T.; Ohyama, A. Description of Achromobacter xylosoxidans Yabuuchi and Ohyama 1971. Int. J. Syst. Bacteriol. 1974, 24, 470–477. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Truper, H.G. Ectothiorhodospira abdelmalekii sp. nov., a new halophilic and alkaliphilic phototrophic bacterium. Zbl Bakt Hyg I Abt Orig C 1981, 2, 228–234. [Google Scholar] [CrossRef]
- Enger, O.; Nygaard, H.; Solberg, M.; Schei, G.; Nielsen, J.; Dundas, I. Characterization of Alteromonas denitrificans sp. nov. Int. J. Syst. Bacteriol. 1987, 37, 416–421. [Google Scholar] [CrossRef]
- Hansen, A.J.; Ingebritsen, A.; Weeks, O.B. Flagellation of Flavabacterium piscicida. J. Bacteriol. 1963, 86, 602–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmstrom, C.; James, S.; Neilan, B.A.; White, D.C.; Kjelleberg, S. Pseudoalteromonas tunicata sp. nov., a bacterium that produces antifouling agents. Int. J. Syst. Bacteriol. 1998, 48, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novick, J.J.; Tyler, M.E. Isolation and characterization of Alteromonas luteoviolacea strains with sheathed flagella. Int. J. Syst. Bacteriol. 1985, 35, 111–113. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.J., III; Janda, J.M.; Brenner, F.W.; Cameron, D.N.; Birkhead, K.M. Genus I. Vibrio Pacini 1854, 411AL. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.R., Garrity, G., Eds.; Springer: New York, NY, USA, 2005; Volume 2, Part B; pp. 494–546. [Google Scholar]
- Gonzalez, Y.; Venegas, D.; Mendoza-Hernandez, G.; Camarena, L.; Dreyfus, G. Na+- and H+-dependent motility in the coral pathogen Vibrio shilonii. Fems. Microbiol. Lett. 2010, 312, 142–150. [Google Scholar] [CrossRef] [Green Version]
- McCarter, L.L. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001, 65, 445–462, table of contents. [Google Scholar] [CrossRef] [Green Version]
- McCarter, L.L. Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol 2004, 7, 18–29. [Google Scholar] [CrossRef]
- Tarrand, J.J.; Krieg, N.R.; Dobereiner, J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can. J. Microbiol. 1978, 24, 967–980. [Google Scholar] [CrossRef]
- Burnham, J.C.; Hashimoto, T.; Conti, S.F. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J. Bacteriol. 1968, 96, 1366–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidler, R.J.; Starr, M.P. Structure of the flagellum of Bdellovibrio bacteriovorus. J. Bacteriol. 1968, 95, 1952–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, H.; Baer, M. Genus II. Bacteriovorax Baer, Ravel, Chun, Hill and Williams 2000, 222VP. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.R., Garrity, G., Eds.; Springer: New York, NY, USA, 2005; Volume 2, Part C; pp. 1053–1057. [Google Scholar]
- Berthenet, E.; Benejat, L.; Menard, A.; Varon, C.; Lacomme, S.; Gontier, E.; Raymond, J.; Boussaba, O.; Toulza, O.; Ducournau, A.; et al. Whole-genome sequencing and bioinformatics as pertinent tools to support Helicobacteracae taxonomy, based on three strains suspected to belong to novel Helicobacter species. Front. Microbiol. 2019, 10, 2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.D.; Baumann, P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J. Bacteriol. 1971, 107, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geis, G.; Leying, H.; Suerbaum, S.; Mai, U.; Opferkuch, W. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J. Clin. Microbiol. 1989, 27, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Thomashow, L.S.; Rittenberg, S.C. Isolation and composition of sheathed flagella from Bdellovibrio bacteriovorus 109J. J. Bacteriol. 1985, 163, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Beeby, M.; Ribardo, D.A.; Brennan, C.A.; Ruby, E.G.; Jensen, G.J.; Hendrixson, D.R. Diverse high-torque bacterial flagellar motors assemble wider stator rings using a conserved protein scaffold. Proc. Natl. Acad. Sci. USA 2016, 113, E1917–E1926. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Beeby, M.; Murphy, G.E.; Leadbetter, J.R.; Hendrixson, D.R.; Briegel, A.; Li, Z.; Shi, J.; Tocheva, E.I.; Muller, A.; et al. Structural diversity of bacterial flagellar motors. EMBO J. 2011, 30, 2972–2981. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Lin, W.T.; Zhu, S.; Franco, A.T.; Liu, J. Imaging the motility and chemotaxis machineries in Helicobacter pylori by cryo-electron tomography. J. Bacteriol. 2017, 199, e00695-16. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Nishikino, T.; Hu, B.; Kojima, S.; Homma, M.; Liu, J. Molecular architecture of the sheathed polar flagellum in Vibrio alginolyticus. Proc. Natl. Acad. Sci. USA 2017, 114, 10966–10971. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Nishikino, T.; Takekawa, N.; Terashima, H.; Kojima, S.; Imada, K.; Homma, M.; Liu, J. In situ structure of the Vibrio polar flagellum reveals distinct outer membrane complex and its specific interaction with the stator. J. Bacteriol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hranitzky, K.W.; Mulholland, A.; Larson, A.D.; Eubanks, E.R.; Hart, L.T. Characterization of a flagellar sheath protein of Vibrio cholerae. Infect. Immun. 1980, 27, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.C.; Schrank, G.D.; Freeman, B.A. Purification of flagellar cores of Vibrio cholerae. J. Bacteriol. 1977, 129, 1121–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuerst, J.A.; Perry, J.W. Demonstration of lipopolysaccharide on sheathed flagella of Vibrio cholerae O:1 by protein A-gold immunoelectron microscopy. J. Bacteriol. 1988, 170, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Norqvist, A.; Wolf-Watz, H. Characterization of a novel chromosomal virulence locus involved in expression of a major surface flagellar sheath antigen of the fish pathogen Vibrio anguillarum. Infect. Immun. 1993, 61, 2434–2444. [Google Scholar] [CrossRef] [Green Version]
- Geis, G.; Suerbaum, S.; Forsthoff, B.; Leying, H.; Opferkuch, W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 1993, 38, 371–377. [Google Scholar] [CrossRef]
- Bernal, P.; Munoz-Rojas, J.; Hurtado, A.; Ramos, J.L.; Segura, A. A Pseudomonas putida cardiolipin synthesis mutant exhibits increased sensitivity to drugs related to transport functionality. Environ. Microbiol. 2007, 9, 1135–1145. [Google Scholar] [CrossRef]
- Kawai, F.; Shoda, M.; Harashima, R.; Sadaie, Y.; Hara, H.; Matsumoto, K. Cardiolipin domains in Bacillus subtilis Marburg membranes. J. Bacteriol. 2004, 186, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- Mileykovskaya, E.; Dowhan, W. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 2000, 182, 1172–1175. [Google Scholar] [CrossRef] [Green Version]
- Romantsov, T.; Helbig, S.; Culham, D.E.; Gill, C.; Stalker, L.; Wood, J.M. Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol. Microbiol. 2007, 64, 1455–1465. [Google Scholar] [CrossRef]
- Chu, J. Understanding the Role of Cardiolipin in Helicobacter Pylori Flagellar Synthesis. Ph.D. Thesis, University of Georgia, Athens, Georgia, 2019. [Google Scholar]
- Chu, J.K.; Zhu, S.; Herrera, C.M.; Henderson, J.C.; Liu, J.; Trent, M.S.; Hoover, T.R. Loss of a cardiolipin synthase in Helicobacter pylori G27 blocks flagellum assembly. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Haque, M.; Yoshida, T.; Yokota, K.; Yasuda, T.; Oguma, K. Unique cholesteryl glucosides in Helicobacter pylori: Composition and structural analysis. J. Bacteriol. 1995, 177, 5327–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Hu, R.; Chandan, V.; Kuolee, R.; Liu, X.; Chen, W.; Liu, B.; Altman, E.; Li, J. Simultaneous analysis of cardiolipin and lipid A from Helicobacter pylori by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Mol. Biosyst 2012, 8, 720–725. [Google Scholar] [CrossRef]
- Doig, P.; Trust, T.J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect. Immun. 1994, 62, 4526–4533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuno, M.; Sato, K.; Kawagishi, I.; Homma, M. Characterization of a flagellar sheath component, PF60, and its structural gene in marine Vibrio. J. Biochem. 2000, 127, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Luke, C.J.; Kubiak, E.; Cockayne, A.; Elliott, T.S.; Penn, C.W. Identification of flagellar and associated polypeptides of Helicobacter (formerly Campylobacter) pylori. Fems. Microbiol. Lett. 1990, 59, 225–230. [Google Scholar] [CrossRef]
- Luke, C.J.; Penn, C.W. Identification of a 29 kDa flagellar sheath protein in Helicobacter pylori using a murine monoclonal antibody. Microbiology 1995, 141, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Bari, W.; Lee, K.M.; Yoon, S.S. Structural and functional importance of outer membrane proteins in Vibrio cholerae flagellum. J. Microbiol. 2012, 50, 631–637. [Google Scholar] [CrossRef]
- Evans, D.G.; Evans, D.J., Jr.; Moulds, J.J.; Graham, D.Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: A putative colonization factor antigen. Infect. Immun. 1988, 56, 2896–2906. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, P.W.; Janzon, L.; Doig, P.; Huang, J.; Kostrzynska, M.; Trust, T.J. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pylori CCUG 17874 is a lipoprotein. J. Bacteriol. 1995, 177, 6049–6057. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.C.; Logan, R.P.H.; Foynes, S.; Cackayne, A.; Wren, B.W.; Penn, C.W. A flagellar sheath protein of Helicobacter pylori is identical to HpaA, a putative N-acetylneuraminyllactose-binding hemagglutinin, but is not an adhesin for AGS cells. J. Bacteriol. 1997, 179, 5643–5647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundstrom, A.M.; Blom, K.; Sundaeus, V.; Bolin, I. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb Pathog 2001, 31, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Radin, J.N.; Gaddy, J.A.; Gonzalez-Rivera, C.; Loh, J.T.; Algood, H.M.; Cover, T.L. Flagellar localization of a Helicobacter pylori autotransporter protein. MBio 2013, 4, e00613-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehus, E.; Gressmann, H.; Ye, F.; Schlapbach, R.; Dehio, M.; Dehio, C.; Stack, A.; Meyer, T.F.; Suerbaum, S.; Josenhans, C. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the flagellar system of Helicobacter pylori. Mol. Microbiol. 2004, 52, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Voss, B.J.; Gaddy, J.A.; McDonald, W.H.; Cover, T.L. Analysis of surface-exposed outer membrane proteins in Helicobacter pylori. J. Bacteriol. 2014, 196, 2455–2471. [Google Scholar] [CrossRef] [Green Version]
- Ottemann, K.M.; Lowenthal, A.C. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 2002, 70, 1984–1990. [Google Scholar] [CrossRef] [Green Version]
- Meuskens, I.; Saragliadis, A.; Leo, J.C.; Linke, D. Type V secretion systems: An overview of passenger domain functions. Front. Microbiol. 2019, 10, 1163. [Google Scholar] [CrossRef]
- Fuerst, J.A. Bacterial sheathed flagella and the rotary motor model for the mechanism of bacterial motility. J. Biol. 1980, 84, 761–774. [Google Scholar] [CrossRef]
- Aschtgen, M.S.; Lynch, J.B.; Koch, E.; Schwartzman, J.; McFall-Ngai, M.; Ruby, E. Rotation of Vibrio fischeri flagella produces outer membrane vesicles that induce host development. J. Bacteriol. 2016, 198, 2156–2165. [Google Scholar] [CrossRef] [Green Version]
- Brennan, C.A.; Hunt, J.R.; Kremer, N.; Krasity, B.C.; Apicella, M.A.; McFall-Ngai, M.J.; Ruby, E.G. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. Elife 2014, 3, e01579. [Google Scholar] [CrossRef]
- Millikan, D.S.; Ruby, E.G. Vibrio fischeri flagellin A is essential for normal motility and for symbiotic competence during initial squid light organ colonization. J. Bacteriol. 2004, 186, 4315–4325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhove, A.S.; Duperthuy, M.; Charriere, G.M.; Le Roux, F.; Goudenege, D.; Gourbal, B.; Kieffer-Jaquinod, S.; Coute, Y.; Wai, S.N.; Destoumieux-Garzon, D. Outer membrane vesicles are vehicles for the delivery of Vibrio tasmaniensis virulence factors to oyster immune cells. Environ. Microbiol. 2015, 17, 1152–1165. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, N. Outer-membrane phospholipase A: Known structure, unknown biological function. Mol. Microbiol. 2000, 35, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinverni, J.C.; Silhavy, T.J. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc. Natl. Acad. Sci. USA 2009, 106, 8009–8014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.C.; Mukhopadhyay, R.; Wingreen, N.S. A curvature-mediated mechanism for localization of lipids to bacterial poles. PLoS Comput. Biol. 2006, 2, e151. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci USA 2011, 108, 6264–6269. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, M. Polymorphic phase behavior of cardiolipin derivatives studied by coarse-grained molecular dynamics. J. Phys. Chem. B 2007, 111, 7194–7200. [Google Scholar] [CrossRef]
- Schlame, M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid. Res. 2008, 49, 1607–1620. [Google Scholar] [CrossRef] [Green Version]
- Schlame, M.; Rua, D.; Greenberg, M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid. Res. 2000, 39, 257–288. [Google Scholar] [CrossRef]
- Nichols-Smith, S.; Teh, S.Y.; Kuhl, T.L. Thermodynamic and mechanical properties of model mitochondrial membranes. Biochim Biophys. Acta 2004, 1663, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Planas-Iglesias, J.; Dwarakanath, H.; Mohammadyani, D.; Yanamala, N.; Kagan, V.E.; Klein-Seetharaman, J. Cardiolipin interactions with proteins. Biophys. J. 2015, 109, 1282–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buskirk, S.W.; Lafontaine, E.R. Moraxella catarrhalis expresses a cardiolipin synthase that impacts adherence to human epithelial cells. J. Bacteriol. 2014, 196, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romantsov, T.; Gonzalez, K.; Sahtout, N.; Culham, D.E.; Coumoundouros, C.; Garner, J.; Kerr, C.H.; Chang, L.; Turner, R.J.; Wood, J.M. Cardiolipin synthase A colocalizes with cardiolipin and osmosensing transporter ProP at the poles of Escherichia coli cells. Mol. Microbiol. 2018, 107, 623–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, R.M.; Yum, L.; Agaisse, H.; Payne, S.M. Cardiolipin synthesis and outer membrane localization are required for Shigella flexneri virulence. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treuner-Lange, A.; Sogaard-Andersen, L. Regulation of cell polarity in bacteria. J. Cell Biol. 2014, 206, 7–17. [Google Scholar] [CrossRef]
- Richardson, K.; Nixon, L.; Mostow, P.; Kaper, J.B.; Michalski, J. Transposon-induced non-motile mutants of Vibrio cholerae. J. Gen. Microbiol. 1990, 136, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Chaban, B.; Coleman, I.; Beeby, M. Evolution of higher torque in Campylobacter-type bacterial flagellar motors. Sci. Rep. 2018, 8, 97. [Google Scholar] [CrossRef]
- Terashima, H.; Koike, M.; Kojima, S.; Homma, M. The flagellar basal body-associated protein FlgT is essential for a novel ring structure in the sodium-driven Vibrio motor. J. Bacteriol. 2010, 192, 5609–5615. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Nishikino, T.; Kojima, S.; Homma, M.; Liu, J. The Vibrio H-ring facilitates the outer membrane penetration of the polar sheathed flagellum. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.G.; Karjalainen, T.K.; Evans, D.J., Jr.; Graham, D.Y.; Lee, C.H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J. Bacteriol. 1993, 175, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsohn, E.; Nystrom, J.; Bolin, I.; Nilsson, C.L.; Svennerholm, A.M. HpaA is essential for Helicobacter pylori colonization in mice. Infect. Immun. 2006, 74, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.S.; Mekalanos, J.J. Decreased potency of the Vibrio cholerae sheathed flagellum to trigger host innate immunity. Infect. Immun. 2008, 76, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Stack, A.; Katzowitsch, E.; Aizawa, S.I.; Suerbaum, S.; Josenhans, C. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 2003, 5, 1345–1356. [Google Scholar] [CrossRef]
- Logan, S.M. Flagellar glycosylation-a new component of the motility repertoire? Microbiology 2006, 152, 1249–1262. [Google Scholar] [CrossRef] [Green Version]
- Merino, S.; Tomas, J.M. Gram-negative flagella glycosylation. Int. J. Mol. Sci. 2014, 15, 2840–2857. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.K.; Neely, A.N.; Blair, B.; Lory, S.; Ramphal, R. Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect. Immun. 2005, 73, 4395–4398. [Google Scholar] [CrossRef] [Green Version]
- Ewing, C.P.; Andreishcheva, E.; Guerry, P. Functional characterization of flagellin glycosylation in Campylobacter jejuni 81-176. J. Bacteriol. 2009, 191, 7086–7093. [Google Scholar] [CrossRef] [Green Version]
- Guerry, P.; Ewing, C.P.; Schirm, M.; Lorenzo, M.; Kelly, J.; Pattarini, D.; Majam, G.; Thibault, P.; Logan, S. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 2006, 60, 299–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanuszkiewicz, A.; Pittock, P.; Humphries, F.; Moll, H.; Rosales, A.R.; Molinaro, A.; Moynagh, P.N.; Lajoie, G.A.; Valvano, M.A. Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses. J. Biol. Chem. 2014, 289, 19231–19244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, S.L.; Jagannathan, A.; Soo, E.C.; Hui, J.P.; Aubry, A.J.; Ahmed, I.; Karlyshev, A.; Kelly, J.F.; Jones, M.A.; Stevens, M.P.; et al. Campylobacter jejuni glycosylation island important in cell charge, legionaminic acid biosynthesis, and colonization of chickens. Infect. Immun. 2009, 77, 2544–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichinose, Y.; Taguchi, F.; Yamamoto, M.; Ohnishi-Kameyama, M.; Atsumi, T.; Iwaki, M.; Manabe, H.; Kumagai, M.; Nguyen, Q.T.; Nguyen, C.L.; et al. Flagellin glycosylation is ubiquitous in a broad range of phytopathogenic bacteria. J. Gen. Plant. Path 2013, 79, 359–365. [Google Scholar] [CrossRef]
- Schirm, M.; Soo, E.C.; Aubry, A.J.; Austin, J.; Thibault, P.; Logan, S.M. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 2003, 48, 1579–1592. [Google Scholar] [CrossRef]
- Logan, S.M.; Hui, J.P.; Vinogradov, E.; Aubry, A.J.; Melanson, J.E.; Kelly, J.F.; Nothaft, H.; Soo, E.C. Identification of novel carbohydrate modifications on Campylobacter jejuni 11168 flagellin using metabolomics-based approaches. FEBS J. 2009, 276, 1014–1023. [Google Scholar] [CrossRef]
- Schirm, M.; Schoenhofen, I.C.; Logan, S.M.; Waldron, K.C.; Thibault, P. Identification of unusual bacterial glycosylation by tandem mass spectrometry analyses of intact proteins. Anal. Chem. 2005, 77, 7774–7782. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, L.; Zhao, Z.; Peng, D.; Zhou, X. Polar flagella rotation in Vibrio parahaemolyticus confers resistance to bacteriophage infection. Sci. Rep. 2016, 6, 26147. [Google Scholar] [CrossRef]
- Belas, R. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol. 2014, 22, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ferreira, R.C.; Viollier, P.H.; Ely, B.; Poindexter, J.S.; Georgieva, M.; Jensen, G.J.; Wright, E.R. Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 2011, 108, 9963–9968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldvinsson, S.B.; Sorensen, M.C.; Vegge, C.S.; Clokie, M.R.; Brondsted, L. Campylobacter jejuni motility is required for infection of the flagellotropic bacteriophage F341. Appl. Environ. Microbiol. 2014, 80, 7096–7106. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, J.; Liu, J.; Hoover, T.R. Phylogenetic Distribution, Ultrastructure, and Function of Bacterial Flagellar Sheaths. Biomolecules 2020, 10, 363. https://doi.org/10.3390/biom10030363
Chu J, Liu J, Hoover TR. Phylogenetic Distribution, Ultrastructure, and Function of Bacterial Flagellar Sheaths. Biomolecules. 2020; 10(3):363. https://doi.org/10.3390/biom10030363
Chicago/Turabian StyleChu, Joshua, Jun Liu, and Timothy R. Hoover. 2020. "Phylogenetic Distribution, Ultrastructure, and Function of Bacterial Flagellar Sheaths" Biomolecules 10, no. 3: 363. https://doi.org/10.3390/biom10030363



