Structure-Based Virtual Screening of Pseudomonas aeruginosa LpxA Inhibitors using Pharmacophore-Based Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Binding Pocket and Volumetric Analysis
2.2. Ligand Preparation
2.3. Pharmacophore Modeling and Virtual Screening
2.4. Molecular Docking
2.5. Born Interaction Energies and Binding Affinities
2.6. Protein–Peptide Docking
2.7. Bioavailability
3. Results and Discussion
3.1. PaLpxA-Substrate Analysis
3.2. LpxA Pocket Analysis among Orthologs
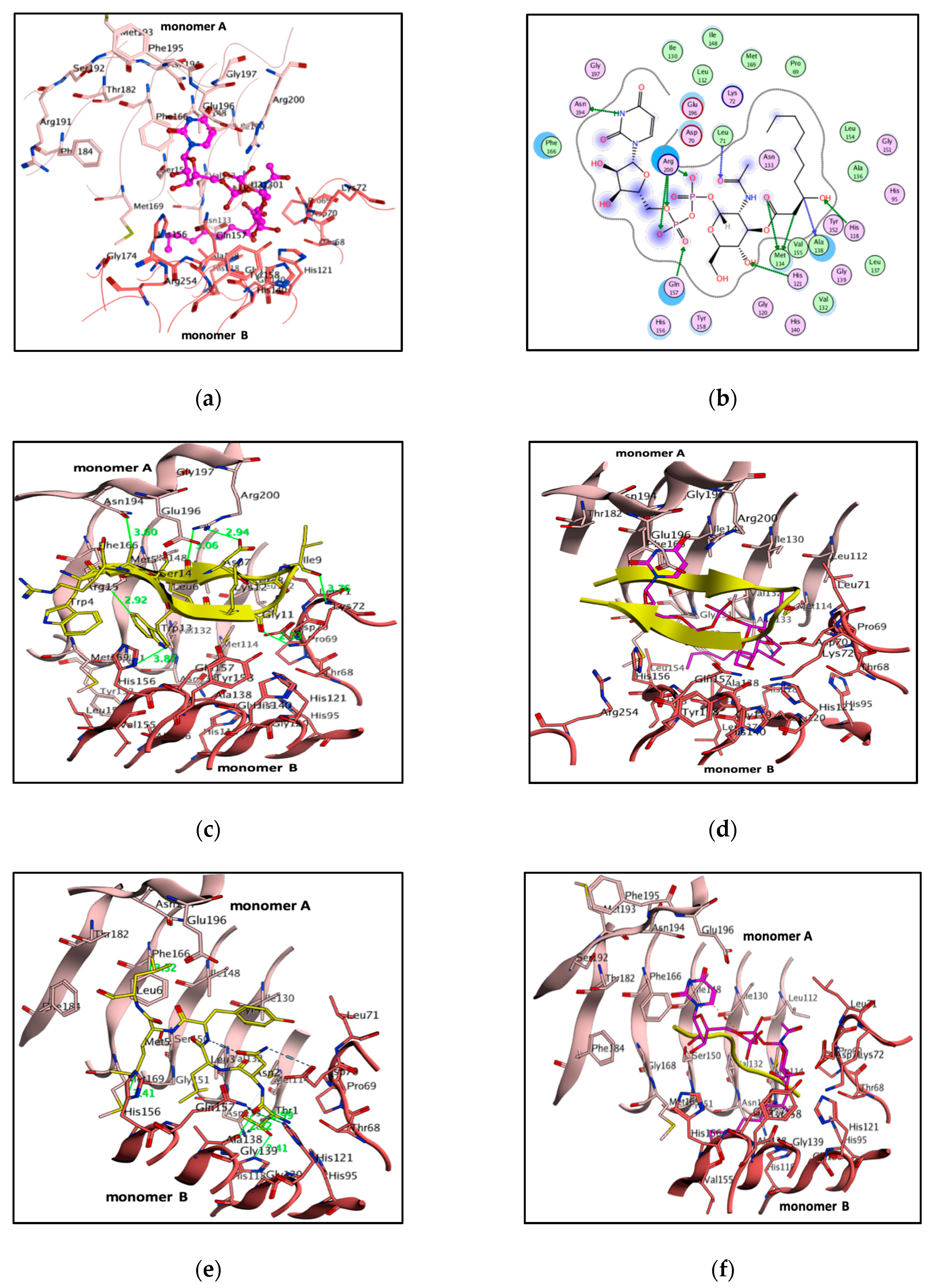
3.3. PaLpxA-Peptide Docking
3.4. Pharmacophore-Based Virtual Screening
3.5. Molecular Docking
3.6. Born Interaction Energies and Binding Affinities
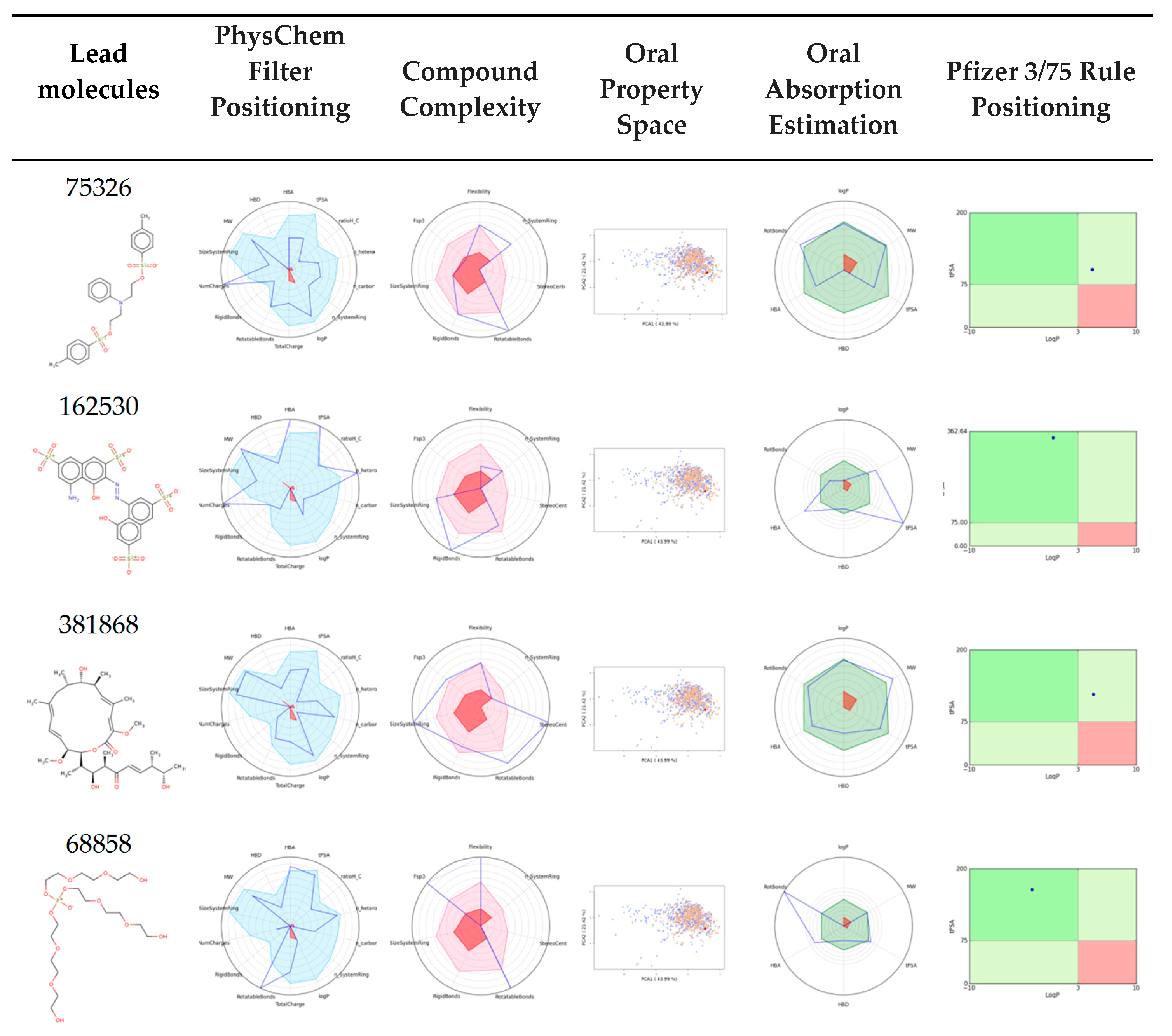
3.7. Bioavailability and ADME Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giamarellou, H.; Poulakou, G. Multidrug resistant gram negative infections: What are the treatment options? Drugs 2009, 69, 1879–1901. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.T. Where will new antibiotics come from? Nat. Rev. Microbiol. 2003, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Projan, S.J. New (and not so new) antibacterial targets from where and when will the novel drugs come? Curr. Opin. Pharmacol. 2002, 2, 513–522. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hypo responsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Raetz, C.R.H. Biochemistry of endotoxins. Annu. Rev. Biochem. 1990, 59, 129–170. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- King, J.D.; Kocincova, D.; Westman, E.L.; Lam, J.S. Review: Lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Innate Immun. 2009, 15, 261–312. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid. Res. 2010, 49, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Roderick, S.L.A. left-handed parallel beta helix in the structure of UDP-N-acetylglucosamine acyltransferase. Science 1995, 270, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, T.J.; Raetz, C.R. The active site of Escherichia coli UDP-N-acetylglucosamine acyltransferase. Chemical modification and site-directed mutagenesis. J. Biol. Chem. 1999, 274, 27047–27055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.W.; Zhang, X.; Behzadi, C.; Andrews, L.D.; Cohen, F.; Chen, Y. Structures of Pseudomonas aeruginosa LpxA Reveal the Basis for Its Substrate Selectivity. Biochemistry 2015, 54, 5937–5948. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Buetow, L.; Hunter, W.N. Nucleotide substrate recognition by UDP-N-acetylglucosamine acyltransferase (LpxA) in the first step of lipid A biosynthesis. J. Mol. Biol. 2007, 369, 305–312. [Google Scholar] [CrossRef]
- Williams, A.H.; Immormino, R.M.; Gewirth, D.T.; Raetz, C.R. Structure of UDP-N acetylglucosamine acyltransferase with a bound antibacterial pentadecapeptide. Proc. Natl. Acad. Sci. USA 2006, 103, 10877–10882. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.H.; Raetz, C.R. Structural basis for the acyl chain selectivity and mechanism of UDP-N-acetylglucosamine acyltransferase. Proc. Natl. Acad. Sci. USA 2007, 104, 13543–13550. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.J.; Heslip, K.A.; Meagher, J.L.; Stuckey, J.A.; Dotson, G.D. Structural basis for the recognition of peptide RJPXD33 by acyltransferases in lipid A biosynthesis. J. Biol. Chem. 2014, 289, 15527–15535. [Google Scholar] [CrossRef] [Green Version]
- Dangkulwanich, M.; Raetz, C.R.H.; Williams, A.H. Structure guided design of an antibacterial peptide that targets UDP-N-acetyl glucosamine acyltransferase. Sci. Rep. 2019, 9, 3947. [Google Scholar] [CrossRef]
- Pratap, S.; Kesari, P.; Yadav, R.; Dev, A.; Narwal, M.; Kumar, P. Acyl chain preference and inhibitor identification of Moraxella catarrhalis LpxA: Insight through crystal structure and computational studies. Int. J. Biol. Macromol. 2017, 96, 759–765. [Google Scholar] [CrossRef]
- Badger, J.; Chie-Leon, B.; Logan, C.; Sridhar, V.; Sankaran, B.; Zwart, P.H.; Nienaber, V. Structure determination of LpxA from the lipopolysaccharide-synthesis pathway of Acinetobacter baumannii. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.I.; Suh, S.W. Crystal structure of UDP-N-acetylglucosamine acyltransferase from Helicobacter pylori. Proteins 2003, 53, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Robins, L.I.; Williams, A.H.; Raetz, C.R. Structural basis for the sugar nucleotide and acyl-chain selectivity of Leptospira interrogans LpxA. Biochemistry 2009, 48, 6191–6201. [Google Scholar] [CrossRef] [PubMed]
- Baugh, L.; Gallagher, L.A.; Patrapuvich, R.; Clifton, M.C.; Gardberg, A.S.; Edwards, T.E.; Armour, B.; Begley, D.W.; Dieterich, S.H.; Dranow, D.M.; et al. Combining functional and structural genomics to sample the essential Burkholderia structome. PLoS ONE 2013, 8, e53851. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), C.C.G.I. 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2013. Available online: https://www.chemcomp.com/MOE-Molecular_Operating_Environment.htm (accessed on 4 January 2017).
- Edelsbrunner, H.; Facello, M.; Fu, P.; Liang, J. Measuring proteins and voids in protiens. In Proceedings of the 28th Annual Hawaii International Conference on System Sciences, Wailea, HI, USA, 3–6 January 1995. [Google Scholar]
- Soga, S.; Shirai, H.; Kobori, M.; Hirayama, N. Use of amino acid composition to predict ligand-binding sites. J. Chem. Inf. Model. 2007, 47, 400–406. [Google Scholar] [CrossRef]
- Gerber, P.R.; Muller, K. MAB, a generally applicable molecular force field for structure modelling in medicinal chemistry. J. Comput. Aided Mol. Des. 1995, 9, 251–268. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef]
- Babu, T.M.C.; Rammohan, A.; Baki, V.B.; Devi, S.; Gunasekar, D.; Rajendra, W. Development of novel HER2 inhibitors against gastric cancer derived from flavonoid source of Syzygium alternifolium through molecular dynamics and pharmacophore-based screening. Drug Des. Devel. Ther. 2016, 10, 3611–3632. [Google Scholar]
- Vijaya Bhaskar, B.; Ram Mohan, A.; Munichandra babu, T.; Siva Rajesh, S.; Srinivasan, T.; Guna Sekar, D.; Rajendra, W. Antibacterial efficacy of fractions and compounds from Indigofera barberi: Identification of DNA gyrase B inhibitors through pharmacophore based virtual screening. Process Biochem. 2016, 51, 2208–2221. [Google Scholar] [CrossRef]
- Labute, P. The generalized born/volume integral (GB/VI) implicit solvent model: Estimation of the free energy of hydration using London dispersion instead of atomic surface area. J. Comput. Chem. 2008, 29, 1693–1698. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.Y. HDOCK: A web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 3, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wen, Z.; Wang, X.; Huang, S.Y. Addressing recent docking challenges: A hybrid strategy to integrate template-based and free protein-protein docking. Proteins 2017, 85, 497–512. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Lagorce, D.; Sperandio, O.; Baell, J.B.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs3: A web server for compound property calculation and chemical library design. Nucleic Acids Res. 2015, 43, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Pauli, I.; dos Santos, R.N.; Rostirolla, D.C.; Martinelli, L.K.; Ducati, R.G.; Timmers, L.F.; Basso, L.A.; Santos, D.S.; Guido, R.V.; Andricopulo, A.D.; et al. Discovery of new inhibitors of mycobacterium tuberculosis inha enzyme using virtual screening and a 3D-pharmacophore-based approach. J. Chem. Inf. Model. 2013, 53, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
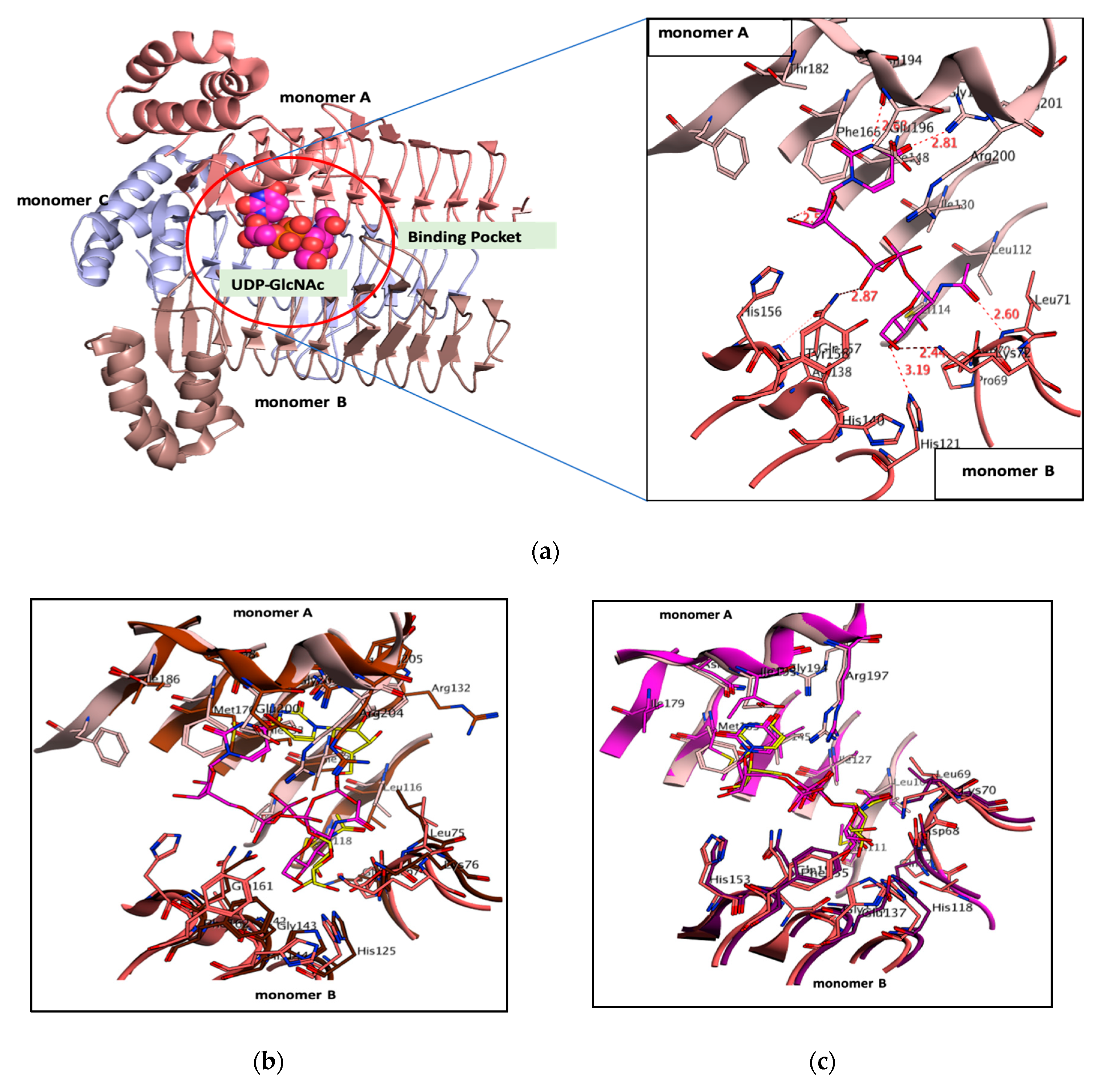
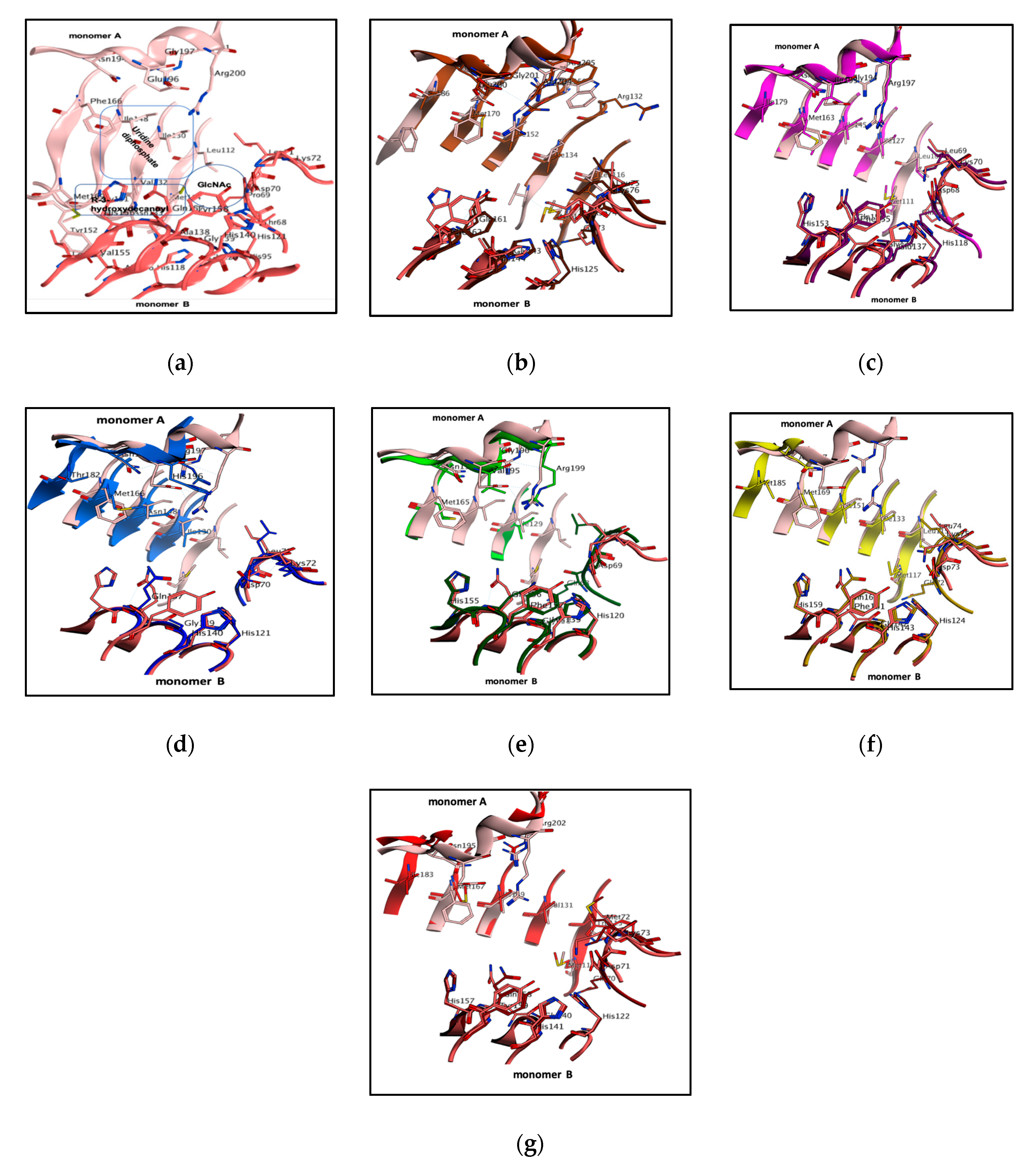
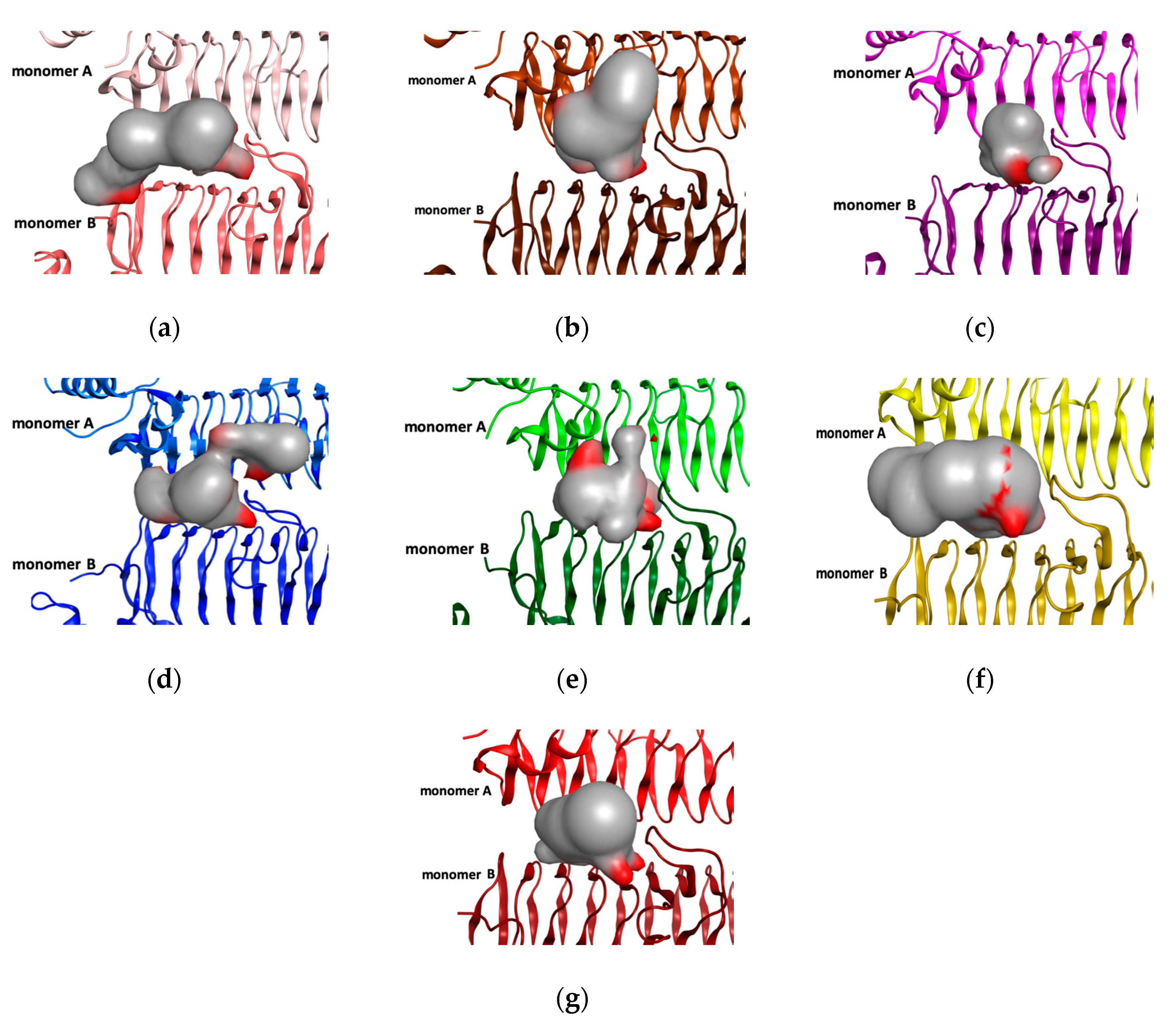


| Organism | PDB ID | Monomer A | Monomer B | RMSD (Å) | Pocket Size (Å) |
|---|---|---|---|---|---|
| UDP Pocket | GlcNAc Pocket | ||||
| P. aeruginosa | 5DEM | Arg200, Arg201, Gly197 Glu196, Asn194, Phe166, Ile148, Ile130, Leu112, Met114 | Asp70, Leu71, Lys72, Ala138, Gly139, His121, His140, His156, Gln157, Tyr158 | 213 | |
| E. coli | 2JF3 | Arg204, Arg205, Glu201 Asn198, Met170, Ile152 Ile134, Leu116, Met118 | Gln73, Asp74, Leu75, Lys76, His125, Gly143, His144, Gln161, Phe162 | 0.6 | 156 |
| B. fragilis | 4R37 | Arg197, Arg198, Gly194, Ile193, Asn191, Ile199, Met163, Ile145, Ile127 Met111 | Gln167, Asp68, Leu69, Lys70, His118, Gly136, Glu137, His153, Gln154, Phe155 | 0.8 | 123 |
| H. pylori | IJ2Z | Arg197, His196, Asn194 Thr182, Met166, Asn148, Met130 | Asp70, Leu71, Lys72, His121, Gly139, His140, Gln157, Phe158 | 0.6 | 208 |
| L. interrogans | 3HSQ | Arg199, Val195, Gly196, Asn193, Met163, Ile129 | Gln168, Asp69, Leu70,, Gly71, His120, His130, Gly138, His155, Gln156, Phe157 | 0.8 | 205 |
| A. baumannii | 4E6Q | Asn197, Met185, Met169, Ile151, Ile133, Leu115, Met117 | Gln172, Asp73, Leu74, Lys75, His124, Gly142, His143, His159, Gln160, Phe161 | 0.5 | 170 |
| B. thailandensis | 4EQY | Arg202, Gly198, Asn195, Ile183, Met167, Ile149, Val131, Met115 | Gln70, Asp71, Met72, His122, Gly140, His141, His157, Gln158, Tyr159 | 0.6 | 145 |
| Peptide Inhibitor | Binding Interactions Peptide | Protein | Distance (Å) | Binding Energy (Kcal/mol) |
|---|---|---|---|---|
| Peptide920 | Trp13-----------Phe166 Met5-----------Asn194 Leu6-----------Arg200 Asp7-----------Arg200 | monomer A | 2.9 3.6 3.0 2.9 3.7 2.3 3.8 | −263 |
| Ileu9-------------Lys72 Gly11-------------Lys72 Trp13------------His156 | monomer B | |||
| RJPXD33 | Leu6------------Phe166 Met5-----------Met169 | monomer A | 2.9 2.4 3.5 3.5 3.4 3.3 | −177 |
| Thr1------------His121 Thr1------------His118 Thr1------------Gln157 Asn2-------------Asp70 | monomer B |
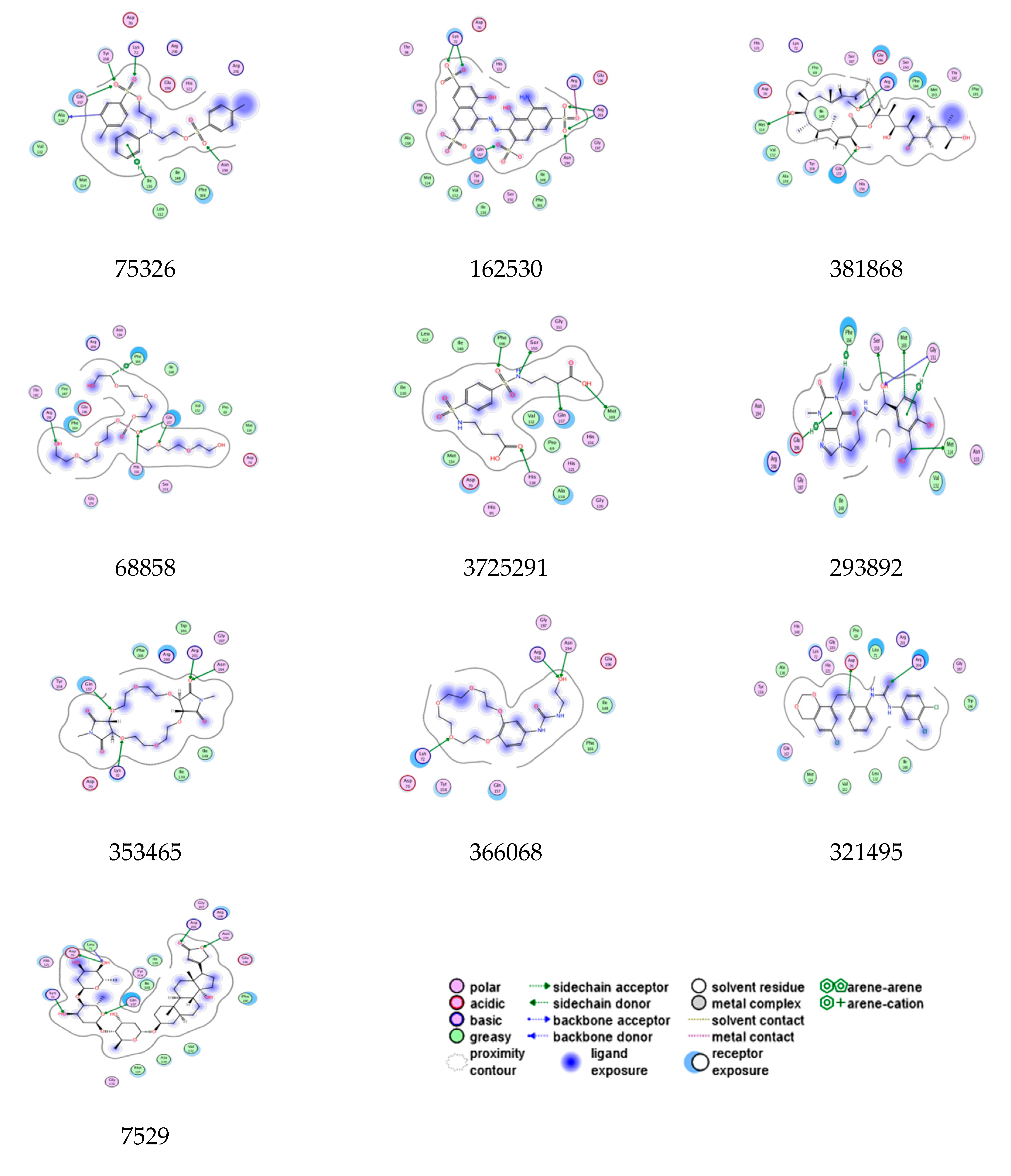
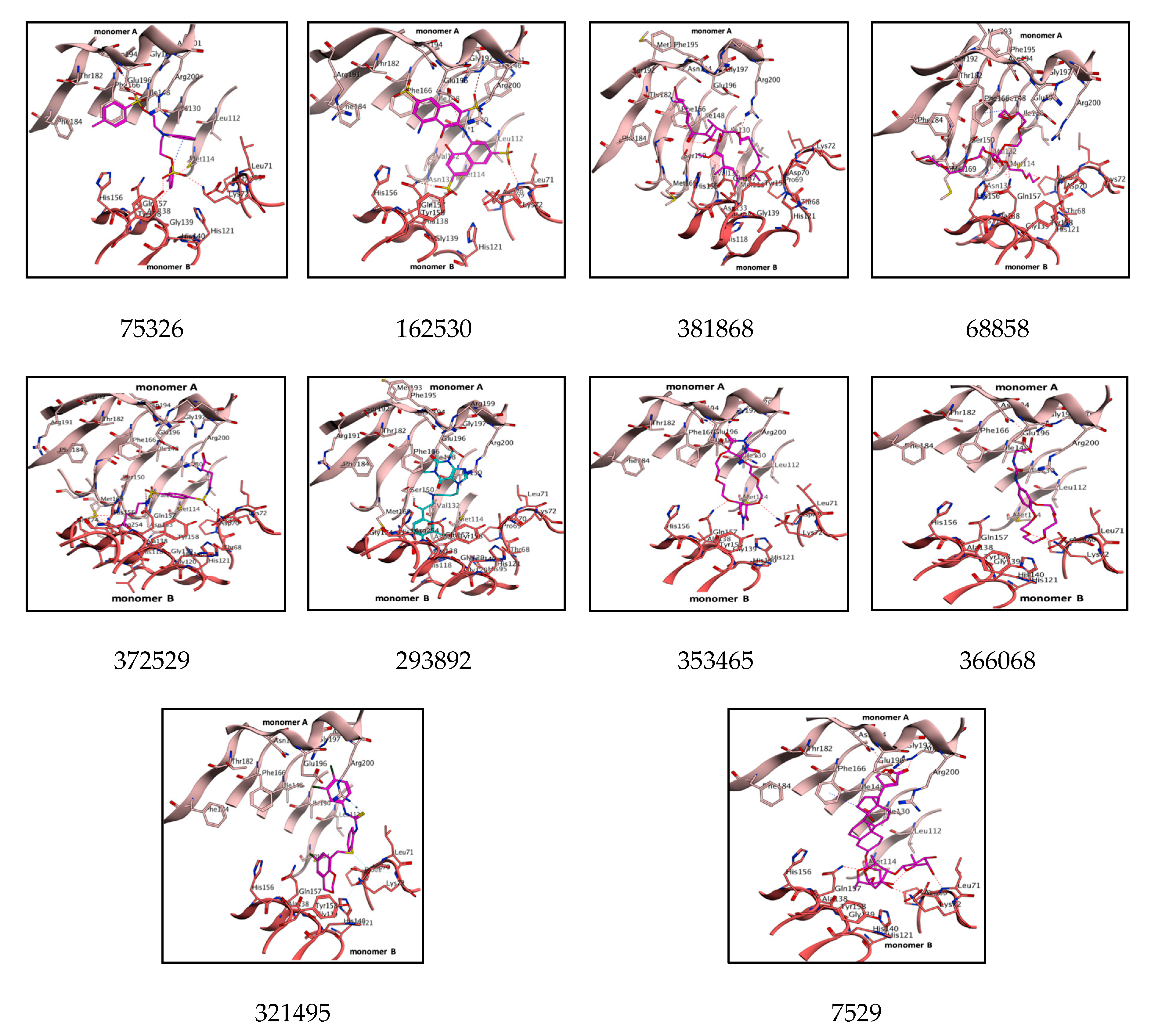
| Lead Molecules | Binding Interaction | Atoms Involved in Angle | Angle (∠) | Distance (Å) | Docking Score (S) | Binding Affinity (pKi) | |
|---|---|---|---|---|---|---|---|
| Protein | Ligand | ||||||
| 75326 | Asn194----------O Ile130---------arene Ala138---------arene Lys72---------S=O Tyr158-------- S=O | OD2-OD1-H38 HE2-NE2-O27 HE2-NE2-O25 NE2-HE2-CN1 NE2-HE2-O26 | 26.5 49.2 40.4 24.4 40.6 | 3.4 3.2 3.3 3.6 3.9 | −10.5 | −6.7 | |
| 162530 | Asn194----------O=S Arg200---------O=S Arg201---------O-S Arg201---------O=S Lys72---------O=S Lys72---------O-S | NZ-HZ-O28 NE2-HE-O29 N1-N-C O27-N-C HE2-NE2-O25 NE2-HE2-CN1 | 64.6 53.6 22.9 95.0 49.2 79.8 | 3.0 3.7 3.1 3.1 4.3 3.3 | −10.1 | −6.9 | |
| 381868 | Arg200---------O Gln157---------O Met114--------OH | NZ-HZ-O31 NE2-HE-O32 N-N-C | 30.5 35.4 21.8 | 3.1 3.0 4.0 | −7.4 | −7.9 | |
| 68858 | Phe166---------C Gln157---------O-C Gln157---------O-P His156---------O-P Arg191---------OH | NE2-HE-O27 ND1-CE1-H42 N-N-O N-O-C ND1-CE1-H42 | 69.2 84.1 29.6 19.9 80.3 | 2.9 3.9 3.2 3.5 | −7.4 | −7.1 | |
| 372529 | Phe166---------O=S Ser150---------HN Gln157---------C His118---------O Met169---------OH | NZ-HZ-O27 NE2-HE-N3 NE-HE-N3 NZ-HZ-O31 NE2-CD2-O28 | 33.8 35.3 90.8 60.7 84.1 | 3.1 3.3 3.9 3.1 4.0 | −7.6 | −7.6 | |
| 293892 | Phe166---------HN Ser150---------OH Glu196---------arene Gly151---------arene Gly151---------OH Met169---------arene Gly151---------C | CG-OD2-H46 NZ-HZ-O31 O-O-N O-N-C O-N-C OH-HH-O27 O-N-C | 54.5 4.6 60.7 45.8 97.7 76.9 35.3 | 4.6 4.3 4.5 3.0 3.1 3.3 3.9 | −7.3 | −7.3 | |
| 353465 | Asn194----------O=C Arg201---------O=C Lys72-----------O Gln157---------O | F-N-C F-N-C C-C-O N-O-C | 22.2 86.8 76.9 76.7 | 3.5 3.3 4.0 4.9 | −8.9 | −6.7 | |
| 366068 | Asn194----------OH Arg201---------OH Lys72---------O | HE2-NE2-O25 NE2-HE2-CN1 NE-HE-N3 | 45.8 97.7 76.9 | 3.3 3.9 3.1 | −8.6 | −6.4 | |
| 321495 | Arg200---------S Asp72---------S | NE2-HE-O29 C-C-O | 60.7 45.8 | 3.7 3.1 | −8.5 | −6.6 | |
| 7529 | Asn194----------O Arg201---------O=C Lys72---------O Leu71---------OH Gln157---------O | NZ-HZ-O28 N1-N-C NE2-HE2-CN1 NZ-HZ-O31 OH-HH-O27 | 4.6 60.7 45.8 84.1 29.6 | 4.5 3.0 3.1 3.3 3.1 | −8.5 | −8.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhaskar, B.V.; Babu, T.M.C.; Rammohan, A.; Zheng, G.Y.; Zyryanov, G.V.; Gu, W. Structure-Based Virtual Screening of Pseudomonas aeruginosa LpxA Inhibitors using Pharmacophore-Based Approach. Biomolecules 2020, 10, 266. https://doi.org/10.3390/biom10020266
Bhaskar BV, Babu TMC, Rammohan A, Zheng GY, Zyryanov GV, Gu W. Structure-Based Virtual Screening of Pseudomonas aeruginosa LpxA Inhibitors using Pharmacophore-Based Approach. Biomolecules. 2020; 10(2):266. https://doi.org/10.3390/biom10020266
Chicago/Turabian StyleBhaskar, Baki Vijaya, Tirumalasetty Muni Chandra Babu, Aluru Rammohan, Gui Yu Zheng, Grigory V. Zyryanov, and Wei Gu. 2020. "Structure-Based Virtual Screening of Pseudomonas aeruginosa LpxA Inhibitors using Pharmacophore-Based Approach" Biomolecules 10, no. 2: 266. https://doi.org/10.3390/biom10020266





