Involvement of Spermidine in the Reduced Lifespan of Caenorhabditis elegans During Vitamin B12 Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organisms
2.2. Preparation of a Homogenate of Worms
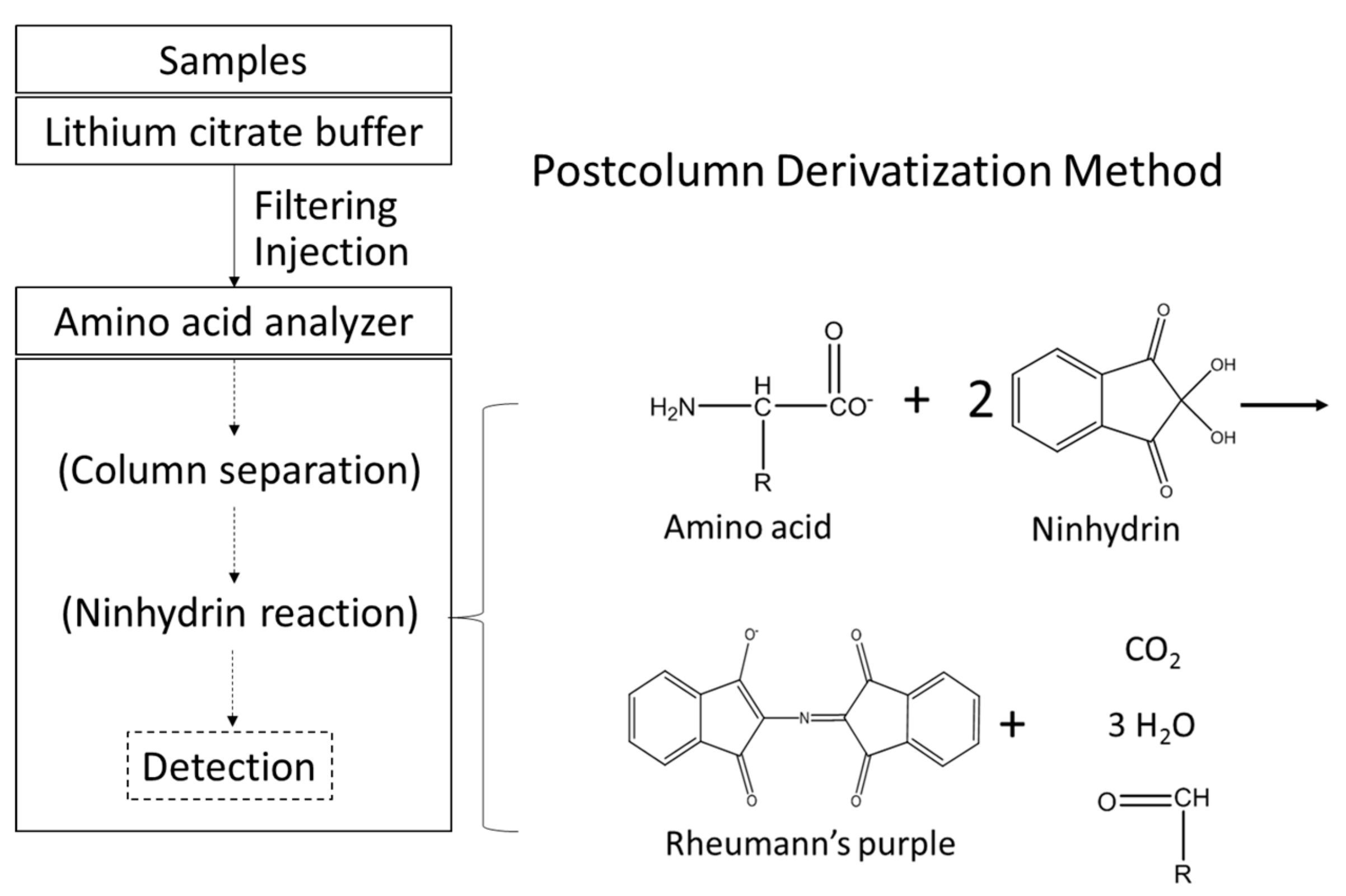
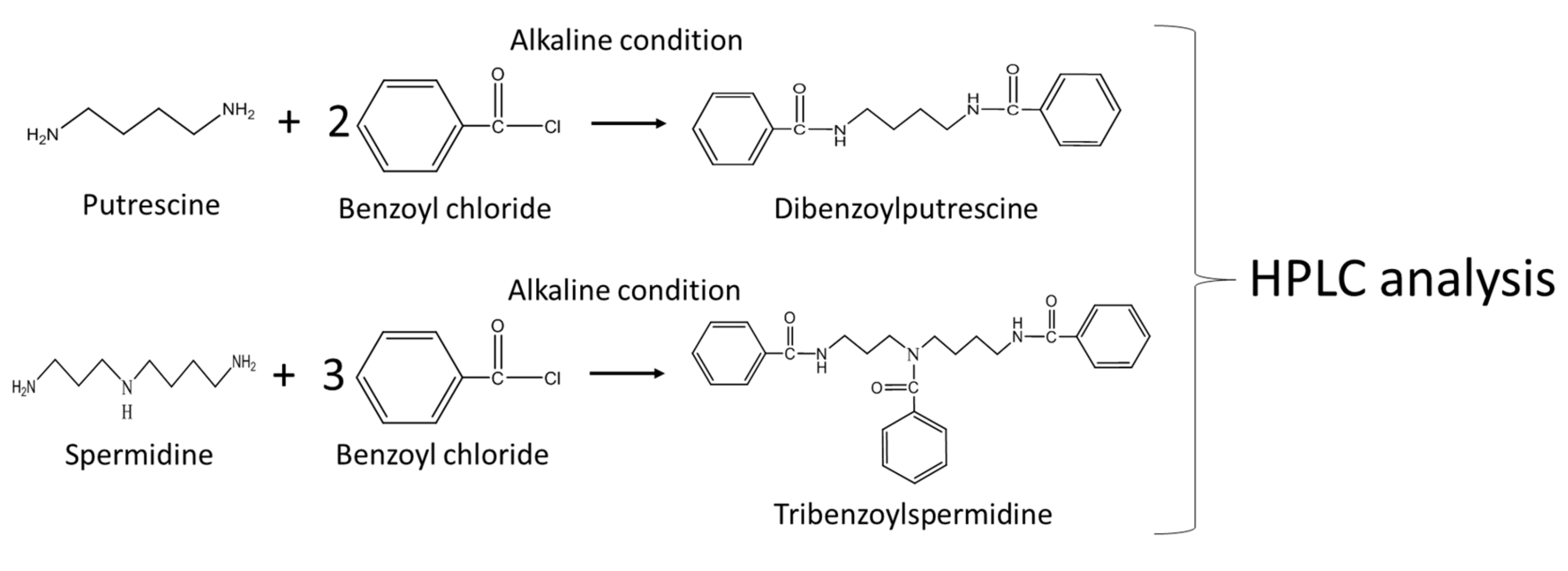
2.3. Determination of Free Amino Acids in Worm Bodies
2.4. Enzyme Assays
2.5. Other Assays
2.6. Quantitative PCR Analysis
2.7. Lifespan Analysis
2.8. Protein Quantitation
2.9. Statistical Analysis
3. Results
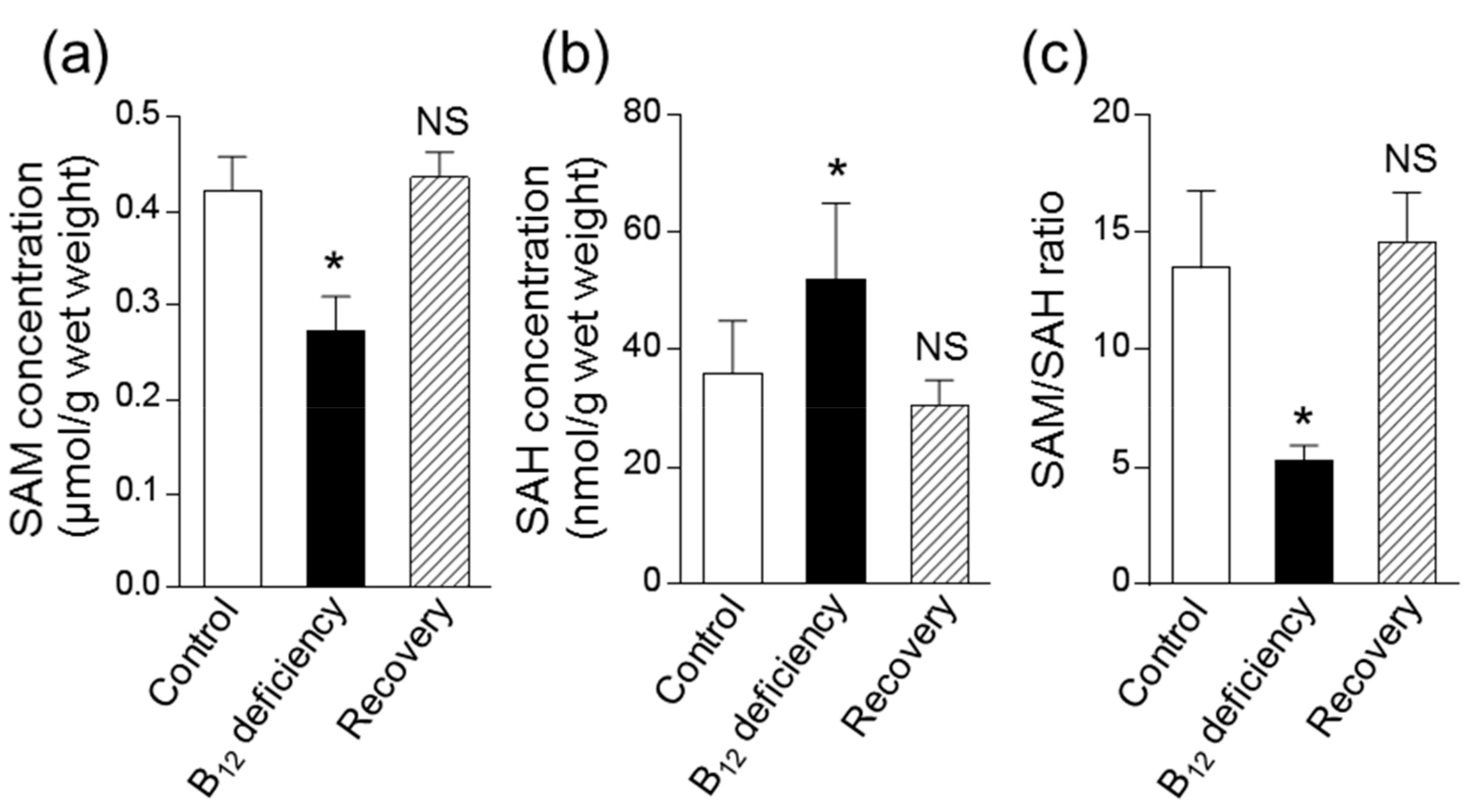
3.1. Effect of B12 Deficiency on Free Amino Acids in C. elegans
3.2. Effects of B12 Deficiency on Arginase Activity and the Levels of mRNAs Encoding Putative Arginase in C. elegans
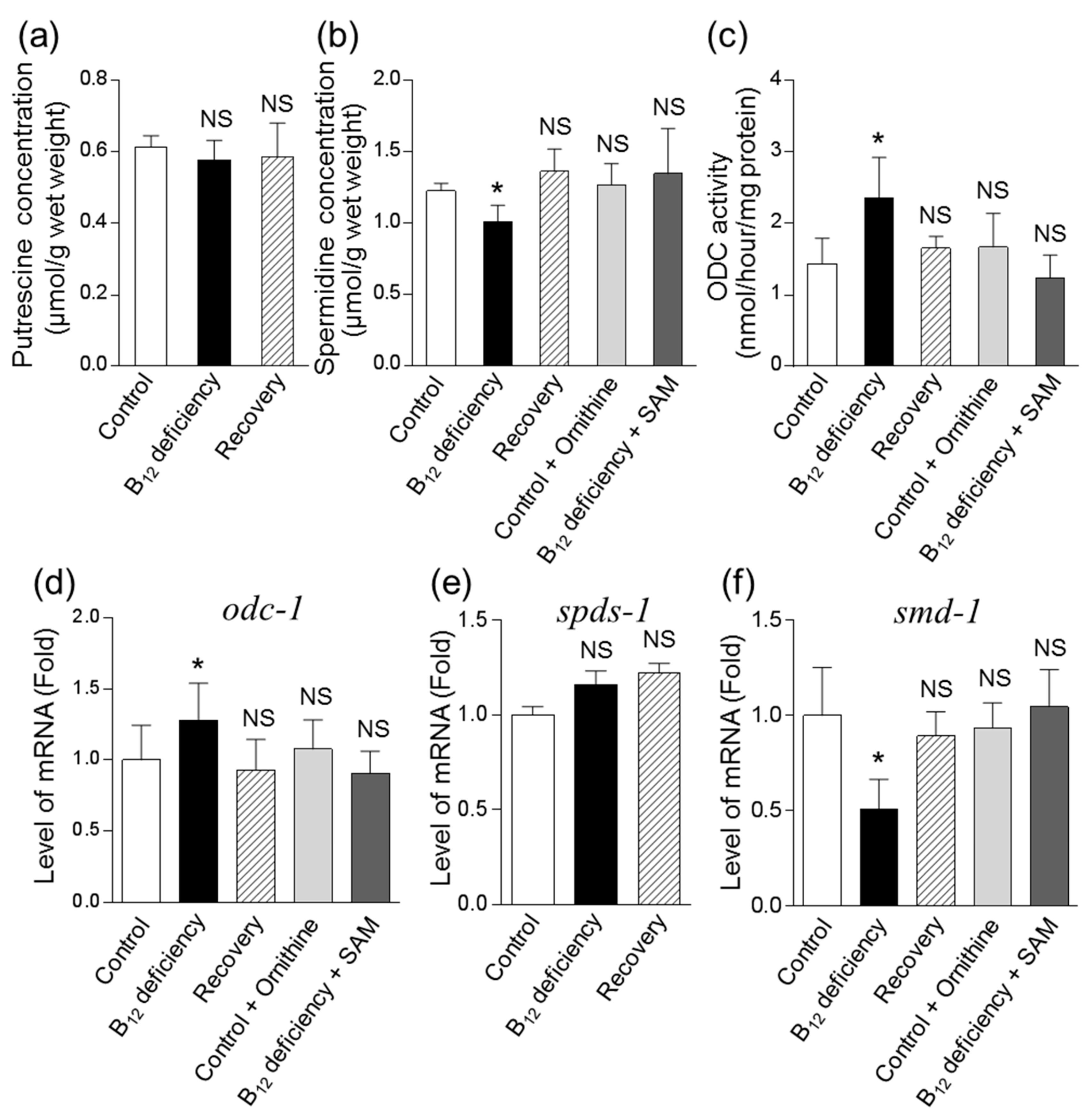
3.3. Effect of B12 Deficiency on Polyamine Levels of C. elegans
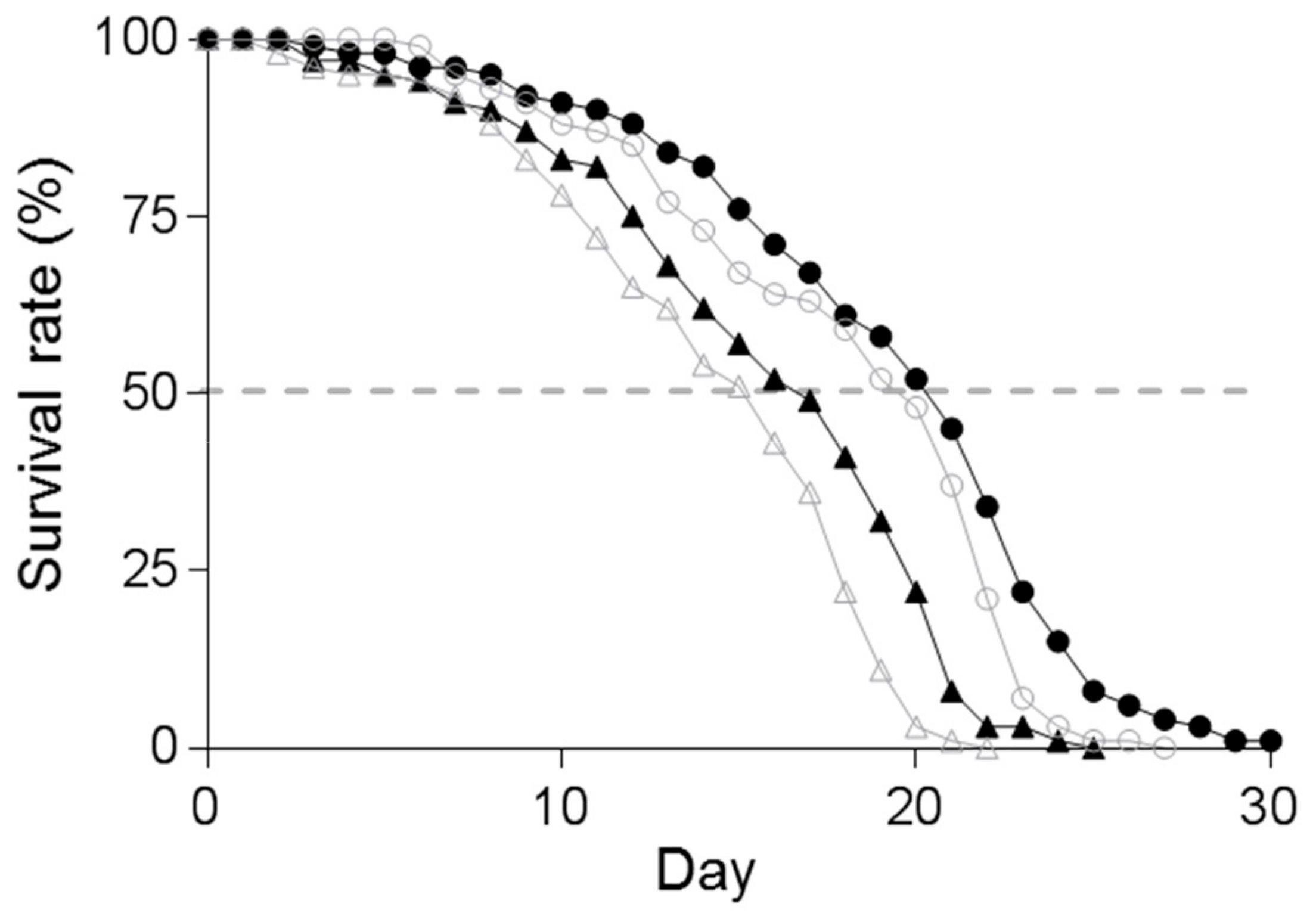
3.4. Effect of Supplementation of Spd on the Lifespan of C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Watanabe, F.; Bito, T. Vitamin B12 sources and microbial interaction. Exp. Biol. Med. 2017, 243, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B. Genetic defects of folate and cobalamin metabolism. Eur. J. Pediatr. 1998, 157, S60–S66. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. Radical peregrinations catalyzed by coenzyme B12-dependent enzymes. Biochemistry 2001, 40, 6191–6198. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.G.; Cannavan, A.; Molloy, A.; O’harte, F. Methylmalonyl-CoA mutase (EC 5.4.99.2) and methionine synthetase (EC 2.1.1.13) in the tissues of cobalt-vitamin B12 deficient sheep. Br. J. Nutr. 1990, 64, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, S.; Watanabe, F.; Saido, H.; Miyatake, K.; Nakano, Y. Methylmalonic acid inhibits respiration in rat liver mitochondria. J. Nutr. 1995, 125, 2846–2850. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. S-Adenosylmethionine. Int. J. Biochem. Cell Biol. 2000, 32, 391–395. [Google Scholar] [CrossRef]
- Bito, T.; Matsunaga, Y.; Yabuta, Y.; Kawano, T.; Watanabe, F. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio 2013, 3, 112–117. [Google Scholar] [CrossRef]
- Ebara, S.; Toyoshime, S.; Matsumura, T.; Adachi, S.; Takenaka, S.; Yamaji, R.; Watanabe, F.; Miyatake, K.; Inui, H.; Nakano, Y. Cobalamin deficiency results in severe metabolic disorder of serine and threonine in rats. Biochim. Biophys. Acta 2001, 1568, 111–117. [Google Scholar] [CrossRef]
- Li, H.; Ren, C.; Shi, J.; Hang, X.; Zhang, F.; Gao, Y.; Wu, Y.; Xu, L.; Chen, C.; Zhang, C. A proteomic view of Caenorhabditis elegans caused by short-term hypoxic stress. Proteome Sci. 2010, 8, 49–62. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Kvannes, J.; Flatmark, T. Rapid and sensitive assay of ornithine decarboxylase activity by high-performance liquid chromatography of the o-phthalaldehyde derivative of putrescine. J. Chromatogr. B Biomed. Sci. Appl. 1987, 419, 291–295. [Google Scholar] [CrossRef]
- Wang, W.; Kramer, P.M.; Yang, S.; Pereira, M.A.; Tao, L. Reversed-phase high-performance liquid chromatography procedure for the simultaneous determination of S-adenosyl-L-methionine and S-adenosyl-L-homocysteine in mouse liver and the effect of methionine on their contractions. J. Chromatogr. B Biomed. Sci. Appl. 2001, 762, 59–65. [Google Scholar] [CrossRef]
- Yamamoto, A.; Shim, I.S.; Fujiwara, S.; Yonezawa, T.; Utsui, K. Effect of difference in nitrogen media on salt-stress response and contents of nitrogen compounds in rice seeding. Soil Sci. Plant Nutr. 2004, 50, 85–93. [Google Scholar] [CrossRef]
- Apfeld, J.; Kenyon, C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 1998, 95, 199–210. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Daniel, D.S.; Iva, G. OrthoList: A compendium of C. elegans genes with human orthologs. PLoS ONE 2011, 6, e20085. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef]
- Bito, T.; Misaki, T.; Yabuta, Y.; Ishikawa, T.; Kawano, T.; Watanabe, F. Vitamin B12 deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans. Redox Biol. 2017, 11, 21–29. [Google Scholar] [CrossRef]
- Chandra, S.; Romero, M.J.; Shatanawi, A.; Alkilany, A.M.; Caldwell, R.B.; Caldwell, R.W. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br. J. Pharmacol. 2012, 165, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.A.; Mishur, R.J.; Bokov, A.F.; Hakala, K.W.; Weintraub, S.T.; Rea, S.L. Profiling the anaerobic response of C. elegans using GC-MS. PLoS ONE 2012, 7, e46140. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.J. Elimination of nitrogenous compounds by Panagrellus redivivus, goodey, 1945 (Nematoda: Cephalobidae). Comp. Biochem. Physiol. Part B Comp. Biochem. 1975, 52, 247–253. [Google Scholar] [CrossRef]
- Ash, D.E. Structure and function of Arginases. J. Nutr. 2004, 134, 2760S–2764S. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Doi, S.; Mizutani, S.; Azuma, H. Roles of accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, and attenuated nitric oxide synthase activity in endothelial cells for pulmonary hypertention in rats. Am. J. Physiol. Lung Cell Physiol. 2007, 292, L1480–L1487. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Kumar, K.A.; Basak, T.; Bhardwaj, G.; Yadav, D.K.; Lalitha, A.; Chandak, G.R.; Raghunath, M.; Chandak, G.R. PPAR signaling pathway is a key modulator of liver proteome in pups born to vitamin B12 deficient rats. J. Proteomics. 2013, 91, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, L.; Aouffen, M.; Mateescu, M.A.; Nadeau, R.; Wang, R. Novel cardiac protective effects of urea: From shark to rat. Br. J. Pharmacol. 1999, 128, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Shinohara, E.M.; Morita, O.E.; Pagliusi, R.A.; Blaia-d’ Avila, V.L.; Allen, R.H.; Stabler, S.P. Elevated serum S-adenosylhomocysteine in cobalamin-deficient megaloblastic anemia. Metabolism 2007, 56, 339–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taban-Shomal, O.; Kilter, H.; Wagner, A.; Schorr, H.; Umanskaya, N.; Hübner, U.; Böhm, M.; Herrmann, W.; Herrmann, M. The cardiac effects of prolonged vitamin B12 and folate deficiency in rats. Cardiovasc. Toxicol. 2009, 9, 95–102. [Google Scholar] [CrossRef]
- Kenyon, S.H.; Nicolaou, A.; Ast, T.; Gibbons, W.A. Stimulation in vitro of vitamin B12-dependent methionine synthase by polyamines. Biochem. J. 1996, 316, 661–665. [Google Scholar] [CrossRef]
- Persson, L. Polyamine homoeostasis. Essays Biochem. 2009, 46, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.L.; Sufrin, J.R.; Porter, C.W. Relative effects of S-adenosylmethionine depletion on nucleic acid methylation and polyamine biosynthesis. Biochem. J. 1987, 247, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Scalabrino, G.; Monzio-Compagnoni, B.; Ferioli, M.E.; Lorenzini, E.C.; Chiodini, E.; Candiani, R. Subacute combined degeneration and induction of ornithine decarboxylase in spinal cords of totally gastrectomized rats. Lab. Investig. 1990, 62, 297–304. [Google Scholar] [PubMed]
- Pegg, A.E.; Michael, A.J. Spermine synthase. Cell Mol. Life Sci. 2010, 67, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining mysteries of molecular biology: The role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Tahira, F.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front Chem. 2018, 6, 10. [Google Scholar] [CrossRef]



| C. elegans (mmol/g wet weight) | |||
|---|---|---|---|
| Control | B12 Deficiency | Recovery | |
| Amino acids | |||
| Aspatic acid | 1.29 ± 0.22 | 0.58 ± 0.22 | 1.37 ± 0.29 |
| Threonine | 0.55 ± 0.04 | 0.80 ± 0.10 * | 0.49 ± 0.08 |
| Serine | 0.76 ± 0.07 | 0.93 ± 0.17 | 0.82 ± 0.08 |
| Asparagine | 0.57 ± 0.07 | 0.41 ± 0.07 | 0.61 ± 0.05 |
| Glutamic acid | 3.27 ± 0.38 | 3.41 ± 0.28 | 3.32 ± 0.45 |
| Glutamine | 1.32 ± 0.22 | 1.28 ± 0.29 | 1.27 ± 0.15 |
| Glycine | 0.73 ± 0.06 | 0.78 ± 0.05 | 0.63 ± 0.11 |
| Alanine | 5.80 ± 0.66 | 6.05 ± 0.22 | 5.53 ± 0.39 |
| Valine | 0.58 ± 0.06 | 1.03 ± 0.13 * | 0.52 ± 0.09 |
| Methionine | 0.19 ± 0.03 | 0.12 ± 0.03 * | 0.22 ± 0.04 |
| Isoleucine | 0.37 ± 0.05 | 0.61 ± 0.08 * | 0.36 ± 0.05 |
| Leucine | 0.70 ± 0.09 | 1.07 ± 0.12 * | 0.63 ± 0.10 |
| Tyrosine | 0.22 ± 0.02 | 0.30 ± 0.02 | 0.24 ± 0.04 |
| Phenylalanine | 0.35 ± 0.05 | 0.47 ± 0.10 | 0.40 ± 0.06 |
| Ornithine | 0.21 ± 0.07 | 0.43 ± 0.05 * | 0.29 ± 0.10 |
| Lysine | 0.43 ± 0.04 | 0.71 ± 0.01 * | 0.40 ± 0.04 |
| Histidine | 0.34 ± 0.04 | 0.55 ± 0.12 | 0.39 ± 0.05 |
| Arginine | 0.88 ± 0.10 | 1.14 ± 0.16 | 0.81 ± 0.05 |
| Hydroxyproline | 0.07 ± 0.06 | 0.03 ± 0.01 | 0.05 ± 0.03 |
| Others | |||
| Cystathionine | 0.57 ± 0.06 | 3.57 ± 0.31 * | 0.78 ± 0.13 |
| Aminoadipic acid | 0.79 ± 0.09 | 1.44 ± 0.12 * | 0.92 ± 0.15 |
| Urea | 1.66 ± 0.52 | 2.44 ± 0.90 | 1.84 ± 0.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bito, T.; Okamoto, N.; Otsuka, K.; Yabuta, Y.; Arima, J.; Kawano, T.; Watanabe, F. Involvement of Spermidine in the Reduced Lifespan of Caenorhabditis elegans During Vitamin B12 Deficiency. Metabolites 2019, 9, 192. https://doi.org/10.3390/metabo9090192
Bito T, Okamoto N, Otsuka K, Yabuta Y, Arima J, Kawano T, Watanabe F. Involvement of Spermidine in the Reduced Lifespan of Caenorhabditis elegans During Vitamin B12 Deficiency. Metabolites. 2019; 9(9):192. https://doi.org/10.3390/metabo9090192
Chicago/Turabian StyleBito, Tomohiro, Naho Okamoto, Kenji Otsuka, Yukinori Yabuta, Jiro Arima, Tsuyoshi Kawano, and Fumio Watanabe. 2019. "Involvement of Spermidine in the Reduced Lifespan of Caenorhabditis elegans During Vitamin B12 Deficiency" Metabolites 9, no. 9: 192. https://doi.org/10.3390/metabo9090192





