Application of 1H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet
Abstract
1. Introduction
2. Results
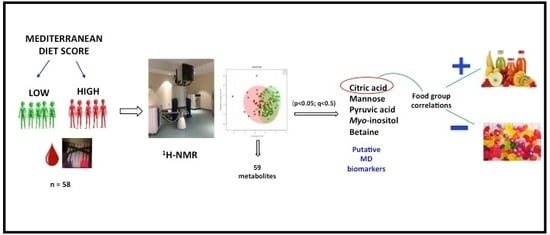
2.1. Univariate Analysis
2.2. Multivariate Analysis
2.3. Correlation of Metabolite Concentrations with MDS, and Consumption of Food Components
2.4. Identification of Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. MD Scoring
4.3. 1H-NMR Analysis of Blood
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vázquez-Fresno, R.; Llorach, R.; Urpi-Sarda, M.; Lupianez-Barbero, A.; Estruch, R.; Corella, D.; Fitó, M.; Arós, F.; Ruiz-Canela, M.; Salas-Salvadó, J.; et al. Metabolomic pattern analysis after Mediterranean diet intervention in a nondiabetic population: A 1- and 3-year follow-up in the PREDIMED study. J. Proteome Res. 2015, 14, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, N.; Sala-Vila, A.; Cofán, M.; Pérez-Heras, A.; Fitó, M.; Ruiz-Gutiérrez, V.; Martínez-González, M.; Corella, D.; Arós, F.; Estruch, R.; et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013, 230, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Mistretta, A.; Marventano, S.; Purrello, A.; Vitaglione, P.; Calabrese, G.; Drago, F.; Galvano, F. Beneficial effects of the Mediterranean diet on metabolic syndrome. Curr. Pharm. Des. 2014, 20, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Topor-Mądry, R.; Stefler, D.; Szafraniec, K.; Bobak, M.; Pająk, A. A Mediterranean-type diet is associated with better metabolic profile in urban Polish adults: Results from the HAPIEE study. Metabolism 2015, 64, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Ahluwalia, N.; Lassale, C.; Hercberg, S.; Fezeu, L.; Lairon, D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: A 6-year prospective study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 677–683. [Google Scholar] [CrossRef]
- Babio, N.; Bulló, M.; Basora, J.; Martínez-González, M.; Fernández-Ballart, J.; Márquez-Sandoval, F.; Molina, C.; Salas-Salvadó, J. Adherence to the Mediterranean diet and risk of metabolic syndrome and its components. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 563–570. [Google Scholar] [CrossRef]
- Esko, T.; Hirschhorn, J.N.; Feldman, H.A.; Hsu, Y.H.; Deik, A.A.; Clish, C.B.; Ebbeling, C.B.; Ludwig, D.S. Metabolomic profiles as reliable biomarkers of dietary composition. Am. J. Clin. Nutr. 2017, 105, 547–554. [Google Scholar] [CrossRef]
- Woodside, J.V.; Draper, J.; Lloyd, A.; McKinley, M.C. Use of biomarkers to assess fruit and vegetable intake. Proc. Nutr. Soc. 2017, 76, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Aguilera, E.; Urpi-Sarda, M.; Llorach, R.; Vázquez-Fresno, R.; Garcia-Aloy, M.; Carmona, F.; Sanchez, A.; Madrid-Gambin, F.; Estruch, R.; Corella, D.; et al. Microbial metabolites are associated with a high adherence to a Mediterranean dietary pattern using a 1H-NMR-based untargeted metabolomics approach. J. Nutr. Biochem. 2017, 48, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.J.; Woodside, J.V.; Young, I.S.; McKinley, M.C.; Perkins-Porras, L.; McKeown, P.P. Adoption and maintenance of a Mediterranean diet in patients with coronary heart disease from a Northern European population: A pilot randomised trial of different methods of delivering Mediterranean diet advice. J. Hum. Nutr. Diet. 2010, 23, 30–37. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Gibbons, H.; Brennan, L. Metabolomics in the identification of biomarkers of dietary intake. Comput. Struct. Biotechnol. J. 2013, 4, e201301004. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L. Progress in nuclear magnetic resonance spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 83, 42–49. [Google Scholar] [CrossRef] [PubMed]
- González-Guardia, L.; Yubero-Serrano, E.; Delgado-Lista, J.; Perez-Martínez, P.; Garcia-Rios, A.; Marin, C.; Camargo, A.; Delgado-Casado, N.; Roche, H.; Perez-Jimenez, F.; et al. Effects of the Mediterranean diet supplemented with Coenzyme Q10 on metabolomic profiles in elderly men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 70, 78–84. [Google Scholar] [CrossRef]
- Garcia-Aloy, M.; Llorach, R.; Urpi-Sarda, M.; Tulipani, S.; Estruch, R.; Martínez-González, M.; Corella, D.; Fitó, M.; Ros, E.; Salas-Salvadó, J.; et al. Novel multimetabolite prediction of walnut consumption by a urinary biomarker model in a free-living population: the PREDIMED study. J. Proteome Res. 2014, 13, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Martínez, J.; de la Iglesia, R.; Lopez-Legarrea, P.; Poutanen, K.; Hanhineva, K.; Zulet, M. Effects of short- and long-term Mediterranean-based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: The Metabolic Syndrome Reduction in Navarra (RESMENA) randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 711–728. [Google Scholar] [CrossRef]
- Playdon, M.; Moore, S.; Derkach, A.; Reedy, J.; Subar, A.; Sampson, J.; Albanaes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Papadaki, A.; Johnson, L.; Toumpakari, Z.; England, C.; Rai, M.; Toms, S.; Penfold, C.; Zazpe, I.; Martínez-González, M.A.; Feder, G. Validation of the English Version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in People at High Cardiovascular Risk in the UK. Nutrients 2018, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- George, E.; Kucianski, T.; Mayr, H.; Moschonis, G.; Tierney, A.; Itsiopoulos, C. A Mediterranean Diet Model in Australia: Strategies for Translating the Traditional Mediterranean Diet into a Multicultural Setting. Nutrients 2018, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, S.; Cai, S.; Dong, J.; Li, X.; Chen, Z. Identification of biochemical changes in lactovegetarian urine using 1H NMR spectroscopy and pattern recognition. Anal. Bioanal. Chem. 2010, 396, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, M.; Martinez, P.J.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Prado, J.C.; Segura, J.; de la Cuesta, F.; Barderas, M.G.; Vivanco, F.; Ruilope, L.M.; et al. Citric Acid Metabolism in Resistant Hypertension Underlying Mechanisms and Metabolic Prediction of Treatment Response. Hypertension 2017, 70, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Ameta, K.; Gupta, A.; Kumar, S.; Sethi, R.; Kumar, D.; Mahdi, A.A. Essential hypertension: A filtered serum based metabolomics study. Sci. Rep. 2017, 7, 2153. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Ji, E.; Christison, T.; Lopez, L.; Huang, Y. Targeted Metabolomic Analysis of Head and Neck Cancer Cells Using High Performance Ion Chromatography Coupled with a Q Exactive HF Mass Spectrometer. Anal. Chem. 2015, 87, 6371–6379. [Google Scholar] [CrossRef]
- Ahmed, S.; Santosh, W.; Kumar, S.; Thanka Christlet, H.T. Metabolic profiling of Parkinson’s disease: Evidence of biomarker from gene expression analysis and rapid neural network detection. J. Biomed. Sci. 2009, 16, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kori, M.; Aydın, B.; Unal, S.; Arga, K.; Kazan, D. Metabolic Biomarkers and Neurodegeneration: A Pathway Enrichment Analysis of Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. OMICS 2016, 20, 645–661. [Google Scholar] [CrossRef]
- Zhang, S.; Nagana Gowda, G.A.; Asiago, V.; Shanaiah, N.; Barbas, C.; Raftery, D. Correlative and quantitative 1H NMR-based metabolomics reveals specific metabolic pathway disturbances in diabetic rats. Anal. Biochem. 2008, 383, 76–84. [Google Scholar] [CrossRef]
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322. [Google Scholar] [CrossRef]
- Menni, C.; Fauman, E.; Erte, I.; Perry, J.R.; Kastenmuller, G.; Shin, S.Y.; Petersen, A.K.; Hyde, C.; Psatha, M.; Ward, K.J.; et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 2013, 62, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Clements, R.; Darnell, B. Myo-inositol content of common foods: Development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Palomba, S.; Laganà, A.; Orio, F. Current insights into inositol isoforms, Mediterranean and Ketogenic diets for polycystic ovary syndrome: From bench to bedside. Curr. Pharm. Des. 2016, 22, 5554–5557. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.; Mar, M.; Howe, J.; Holden, J. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.F.; Hollis, J.H.; Migaud, M.; Browne, R.A. Analysis of Betaine and Choline Contents of Aleurone, Bran, and Flour Fractions of Wheat (Triticum aestivum L.) Using 1H Nuclear Magnetic Resonance (NMR) Spectroscopy. J. Agric. Food Chem. 2009, 57, 1948–1951. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Panagiotakos, D.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2008, 87, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Lichtenstein, A.H.; Zheng, Z.; Appel, L.J.; Coresh, J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am. J. Clin. Nutr. 2018, 108, 243–255. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.; Ruiz-Canela, M.; Bulló, M.; Toledo, E.; Wang, D.D.; Corella, D.; Gómez-Gracia, E.; Fiol, M.; Estruch, R.; et al. Plasma Metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (Prevention with Mediterranean Diet) study. J. Am. Heart Assoc. 2017, 6, e006524. [Google Scholar] [CrossRef]
- Roe, A.J.; Zhang, S.; Bhadelia, R.A.; Johnson, E.J.; Lichtenstein, A.H.; Rogers, G.T.; Rosenberg, I.H.; Smith, C.E.; Zeisel, S.H.; Scott, T.M. Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am. J. Clin. Nutr. 2017, 105, 1283–1290. [Google Scholar] [CrossRef]
- Martínez-González, M.; Fernández-Jarne, E.; Serrano-Martínez, M.; Wright, M.; Gomez-Gracia, E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur. J. Clin. Nutr. 2004, 58, 1550–1552. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Moore, S.E.; Appleton, K.M.; Cupples, M.E.; Erwin, C.M.; Hunter, S.J.; Kee, F.; McCance, D.; Patterson, C.C.; Young, I.S.; et al. Trial to Encourage Adoption and Maintenance of a Mediterranean Diet (TEAM-MED): Protocol for a Randomised Feasibility Trial of a Peer Support Intervention for Dietary Behaviour Change in Adults at High Cardiovascular Disease Risk. Int. J. Environ. Res. Public Health 2018, 15, 1130. [Google Scholar] [CrossRef] [PubMed]
- Mercier, P.; Lewis, M.; Chang, D.; Baker, D.; Wishart, D. Towards automatic metabolomic profiling of high-resolution one-dimensional proton NMR spectra. J. Biomol. NMR 2011, 49, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, S.; Liu, P.; Bjordahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C.; et al. Accurate, fully-automated NMR spectral profiling for metabolomics. PLoS ONE 2015, 10, e0124219. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.F.; Holscher, C.; Green, B.D. Metabolic signatures of human Alzheimer’s disease (AD): 1H NMR analysis of the polar metabolome of post-mortem brain tissue. Metabolomics 2013, 10, 744–753. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]


| Name | Mean [µM] Low MDS (n = 64) | SD. Low MDS | Mean [µM] High MDS (n = 73) | SD. High MDS | p-Value | q-Value (FDR) | AUC (ROC) | ↑/↓ | % Change | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | citric acid* | 76.62 | 30.50 | 92.36 | 32.58 | 5.99 × 10−4* | 0.03 | 0.67 | ↑ | 20.5 |
| 2 | pyruvic acid* | 63.50 | 34.80 | 50.19 | 34.36 | 0.005* | 0.16 | 0.64 | ↓ | −20.9 |
| 3 | betaine* | 40.37 | 17.83 | 46.64 | 18.42 | 0.01* | 0.28 | 0.62 | ↑ | 15.5 |
| 4 | mannose* | 55.08 | 44.14 | 39.25 | 36.58 | 0.02* | 0.32 | 0.61 | ↓ | −28.7 |
| 5 | acetic acid | 13.06 | 7.54 | 14.94 | 6.55 | 0.03* | 0.34 | 0.61 | ↑ | 14.39 |
| 6 | myo-inositol* | 104.67 | 74.41 | 79.22 | 47.25 | 0.03* | 0.34 | 0.60 | ↓ | −24.3 |
| 7 | tyrosine | 4.58 | 2.74 | 5.48 | 2.86 | 0.06 | 0.52 | 0.59 | ↑ | 19.7 |
| 8 | glycerol | 1521.19 | 817.49 | 1304.62 | 875.72 | 0.08 | 0.54 | 0.59 | ↓ | −14.23 |
| 9 | dimethylglycine | 3.01 | 1.75 | 2.37 | 1.56 | 0.09 | 0.54 | 0.58 | ↓ | −21.3 |
| 10 | malonate | 8.35 | 7.03 | 8.99 | 4.35 | 0.09 | 0.54 | 0.58 | ↑ | 7.66 |
| 11 | pyroglutamic acid | 87.23 | 28.57 | 95.35 | 29.65 | 0.10 | 0.54 | 0.58 | ↑ | 9.3 |
| Name | AUC (ROC) | ↑/↓ | % Change | |
|---|---|---|---|---|
| 1 | citric acid/pyruvic acid | 0.74 | ↑ | 53.3 |
| 2 | citric acid/l-lactic acid | 0.73 | ↑ | 29.2 |
| 3 | citric acid/aspartate | 0.72 | ↑ | 38.3 |
| 4 | citric acid/l-phenylalanine | 0.71 | ↑ | 27.1 |
| 5 | betaine/pyruvic acid | 0.70 | ↑ | 47.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macias, S.; Kirma, J.; Yilmaz, A.; Moore, S.E.; McKinley, M.C.; McKeown, P.P.; Woodside, J.V.; Graham, S.F.; Green, B.D. Application of 1H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet. Metabolites 2019, 9, 201. https://doi.org/10.3390/metabo9100201
Macias S, Kirma J, Yilmaz A, Moore SE, McKinley MC, McKeown PP, Woodside JV, Graham SF, Green BD. Application of 1H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet. Metabolites. 2019; 9(10):201. https://doi.org/10.3390/metabo9100201
Chicago/Turabian StyleMacias, Shirin, Joseph Kirma, Ali Yilmaz, Sarah E. Moore, Michelle C. McKinley, Pascal P. McKeown, Jayne V. Woodside, Stewart F. Graham, and Brian D. Green. 2019. "Application of 1H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet" Metabolites 9, no. 10: 201. https://doi.org/10.3390/metabo9100201
APA StyleMacias, S., Kirma, J., Yilmaz, A., Moore, S. E., McKinley, M. C., McKeown, P. P., Woodside, J. V., Graham, S. F., & Green, B. D. (2019). Application of 1H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet. Metabolites, 9(10), 201. https://doi.org/10.3390/metabo9100201