Non-Targeted Metabolomics Analysis of Golden Retriever Muscular Dystrophy-Affected Muscles Reveals Alterations in Arginine and Proline Metabolism, and Elevations in Glutamic and Oleic Acid In Vivo
Abstract
:1. Introduction
2. Results
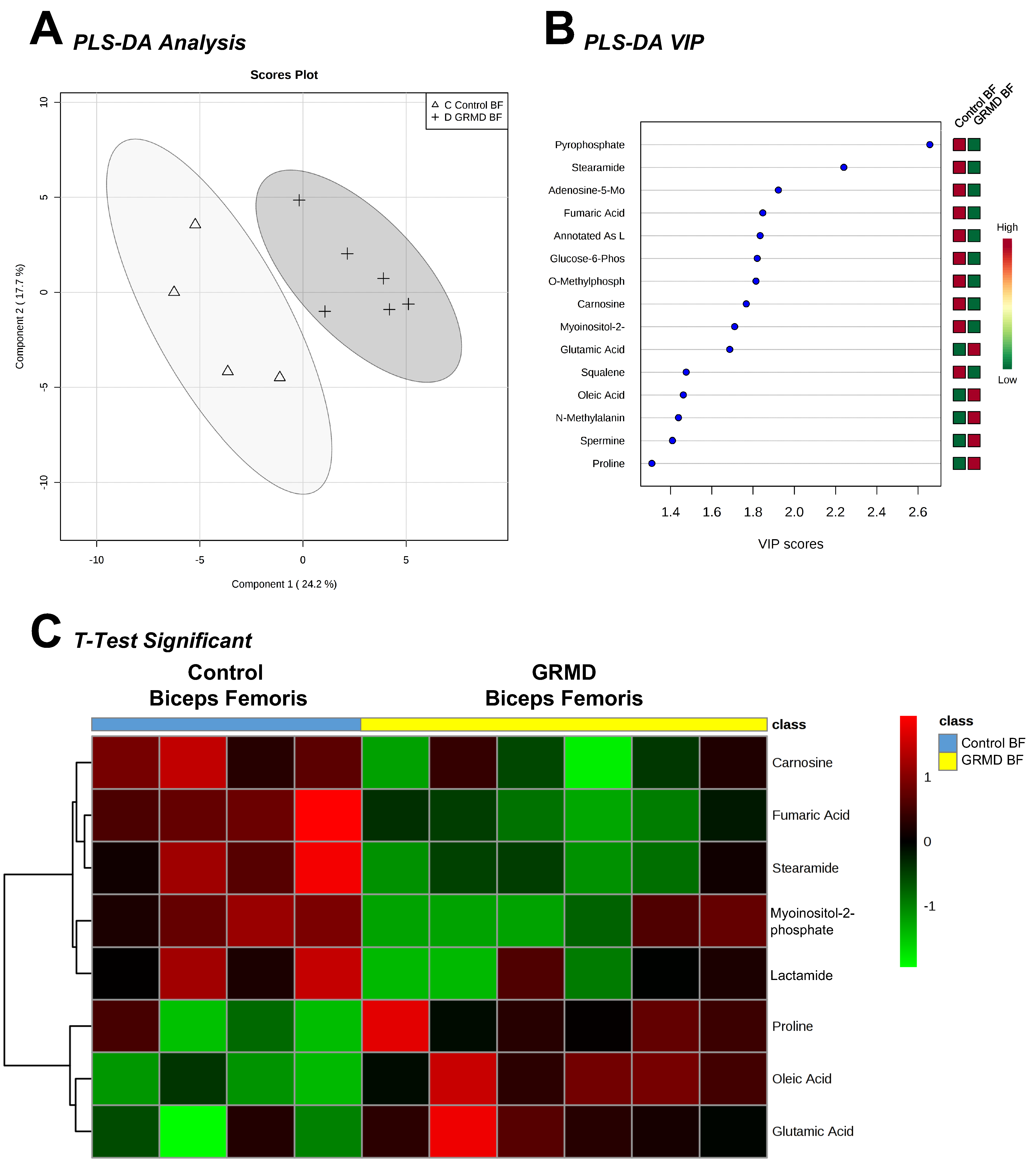
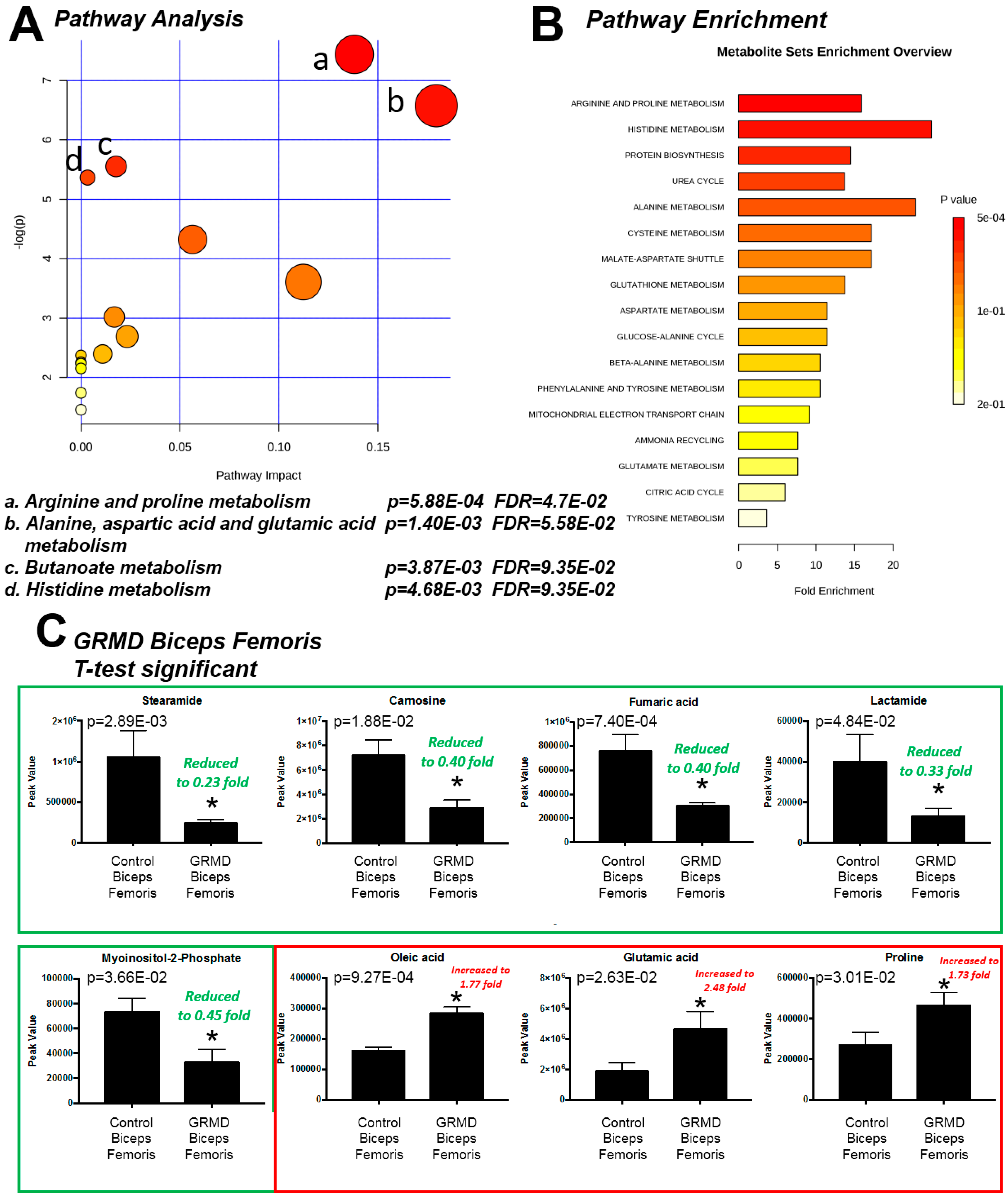
2.1. Determination of Metabolomics Changes in the GRMD Biceps Femoris vs. Control (t-Test)
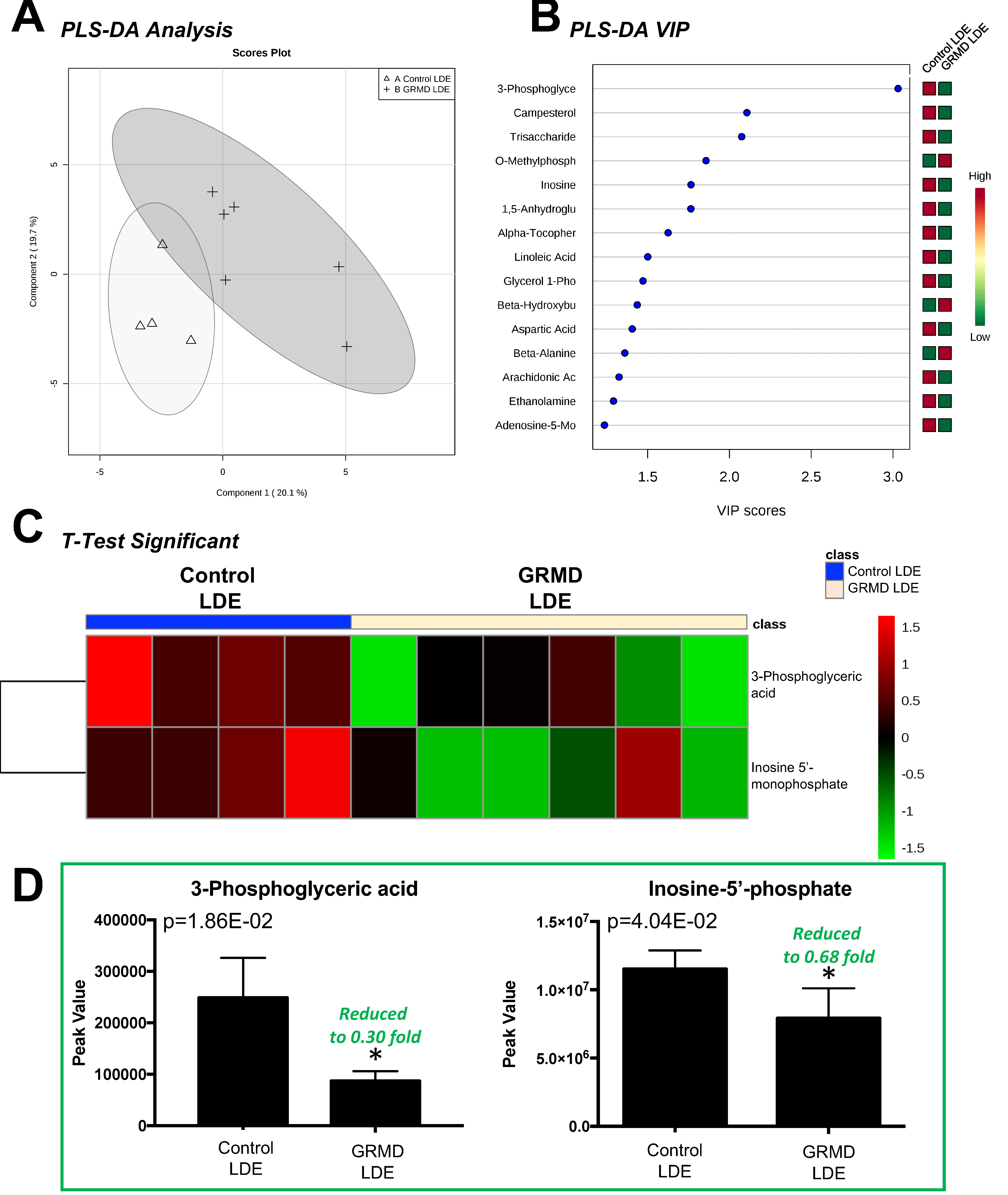
2.2. GRMD vs. Control Long Digital Extensor Muscle vs. Control (t-Test)
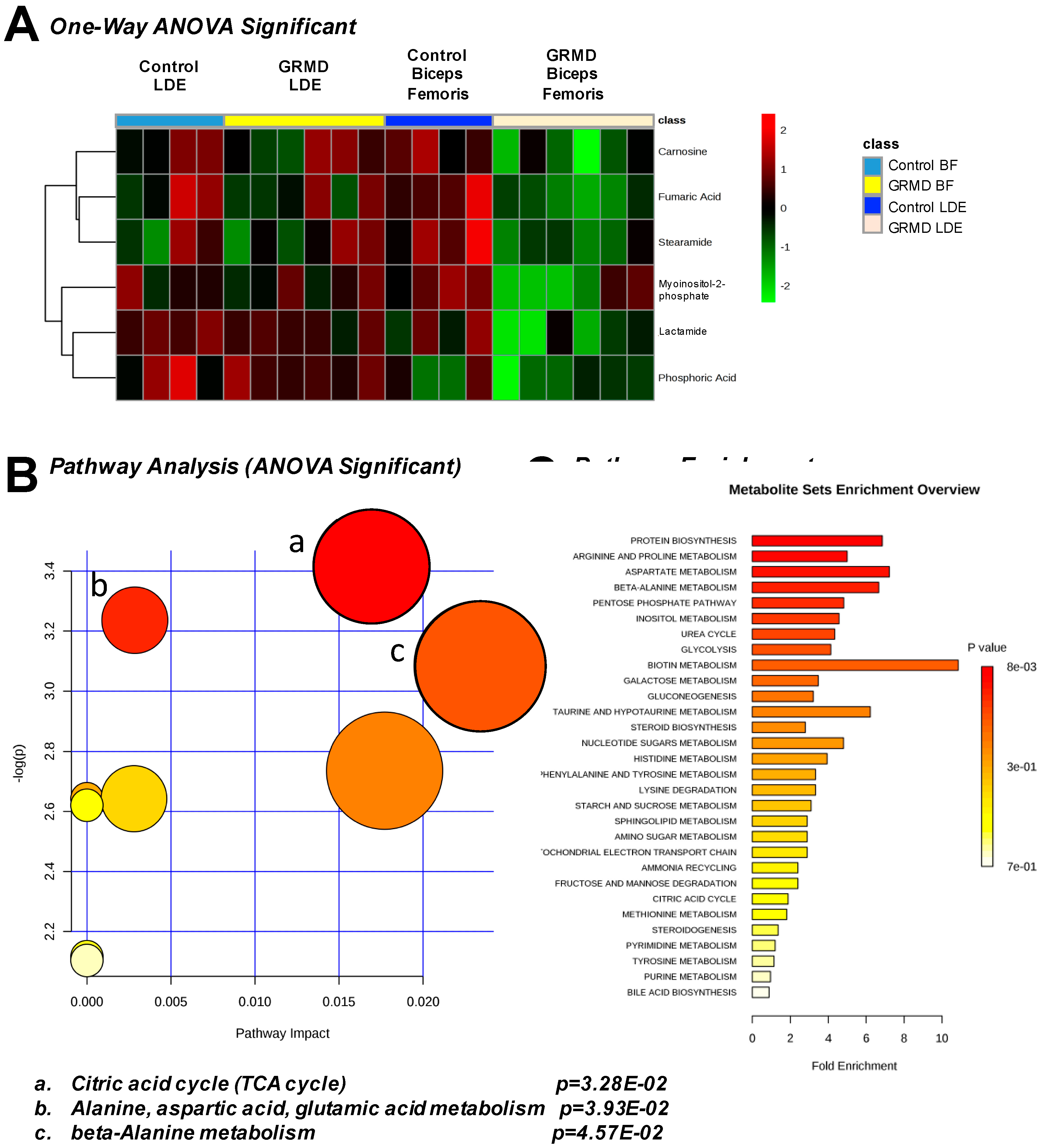
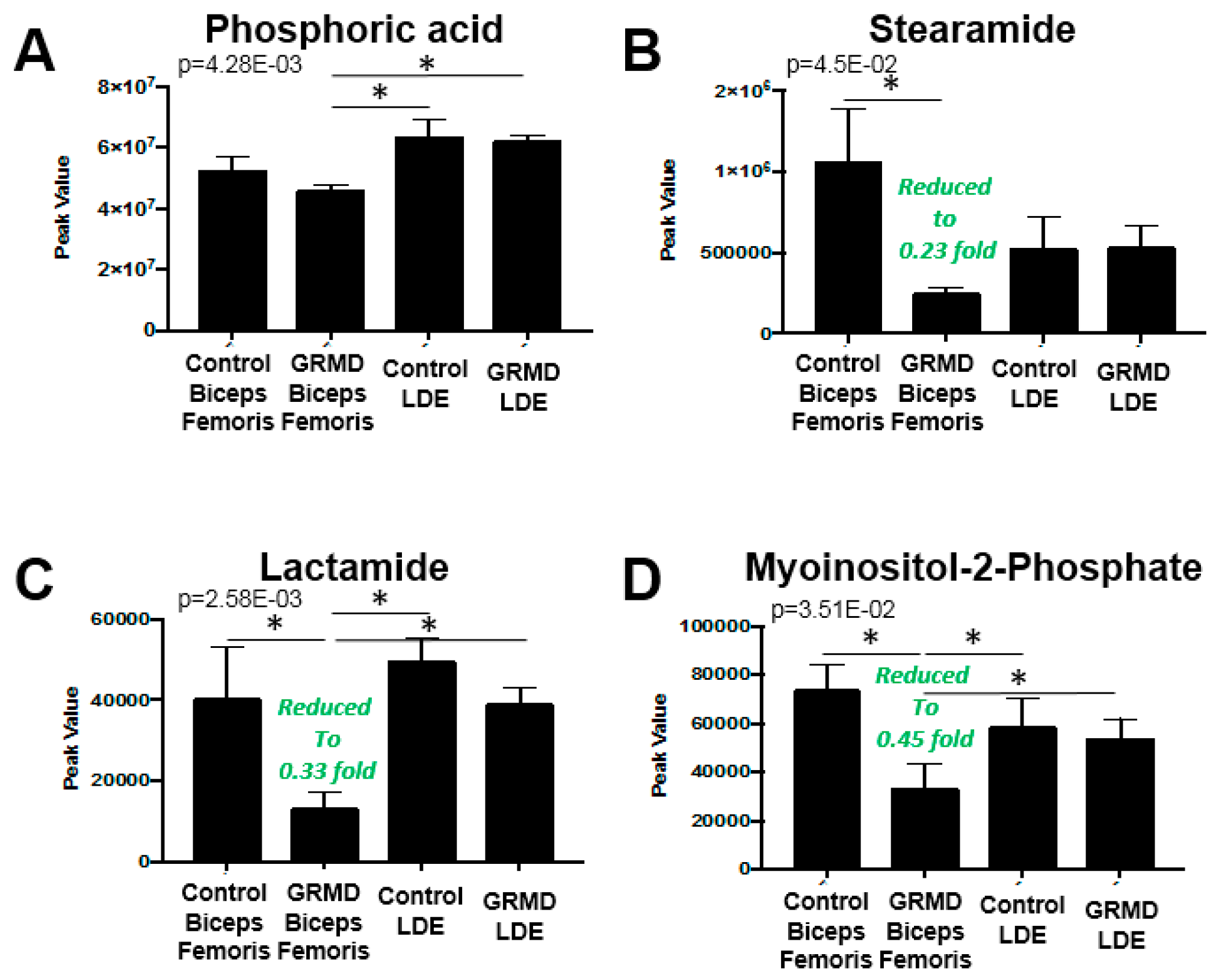
2.3. GRMD LDE vs. GRMD BF vs. Control LDE vs. Control BF (ANOVA)
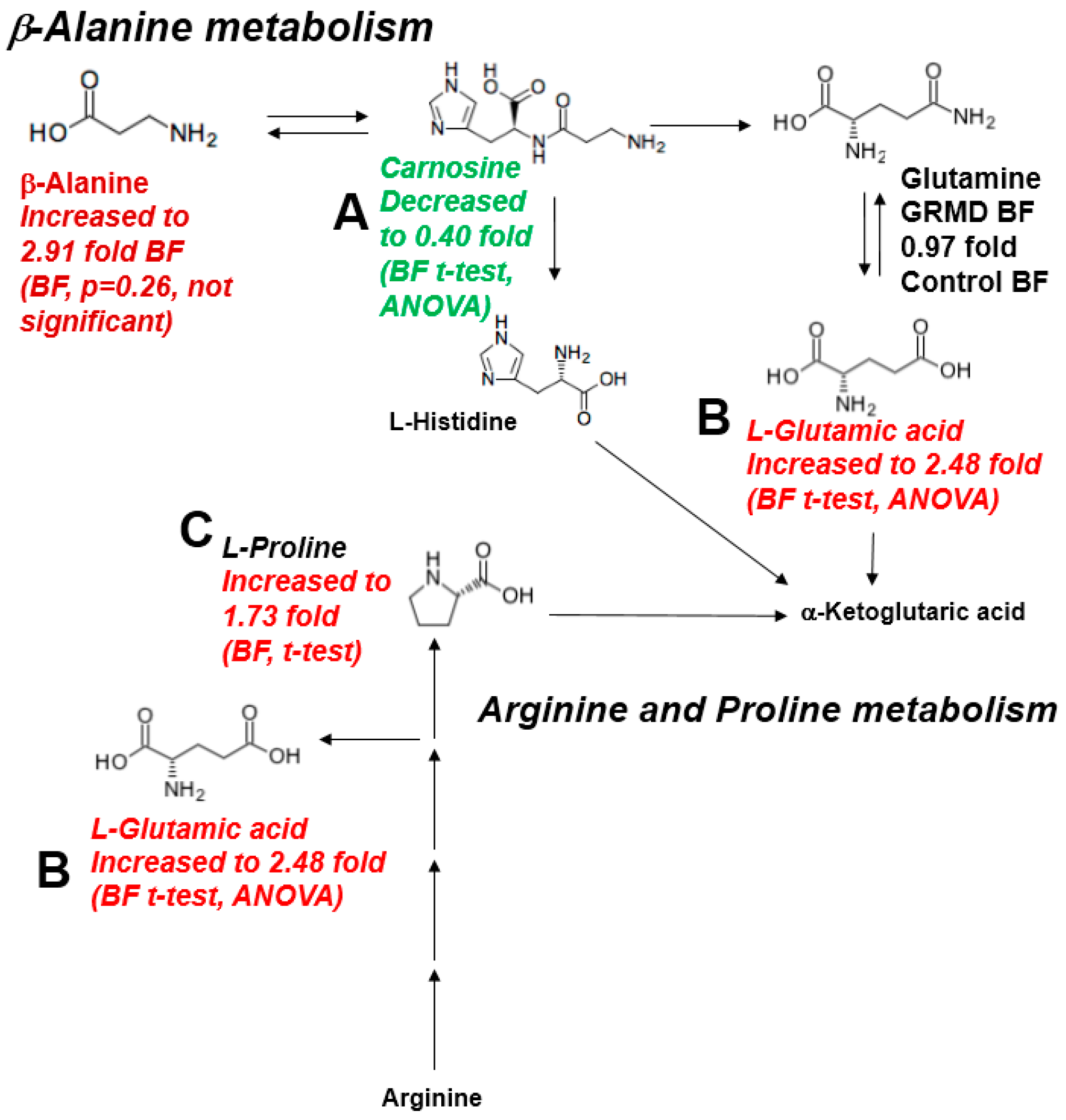
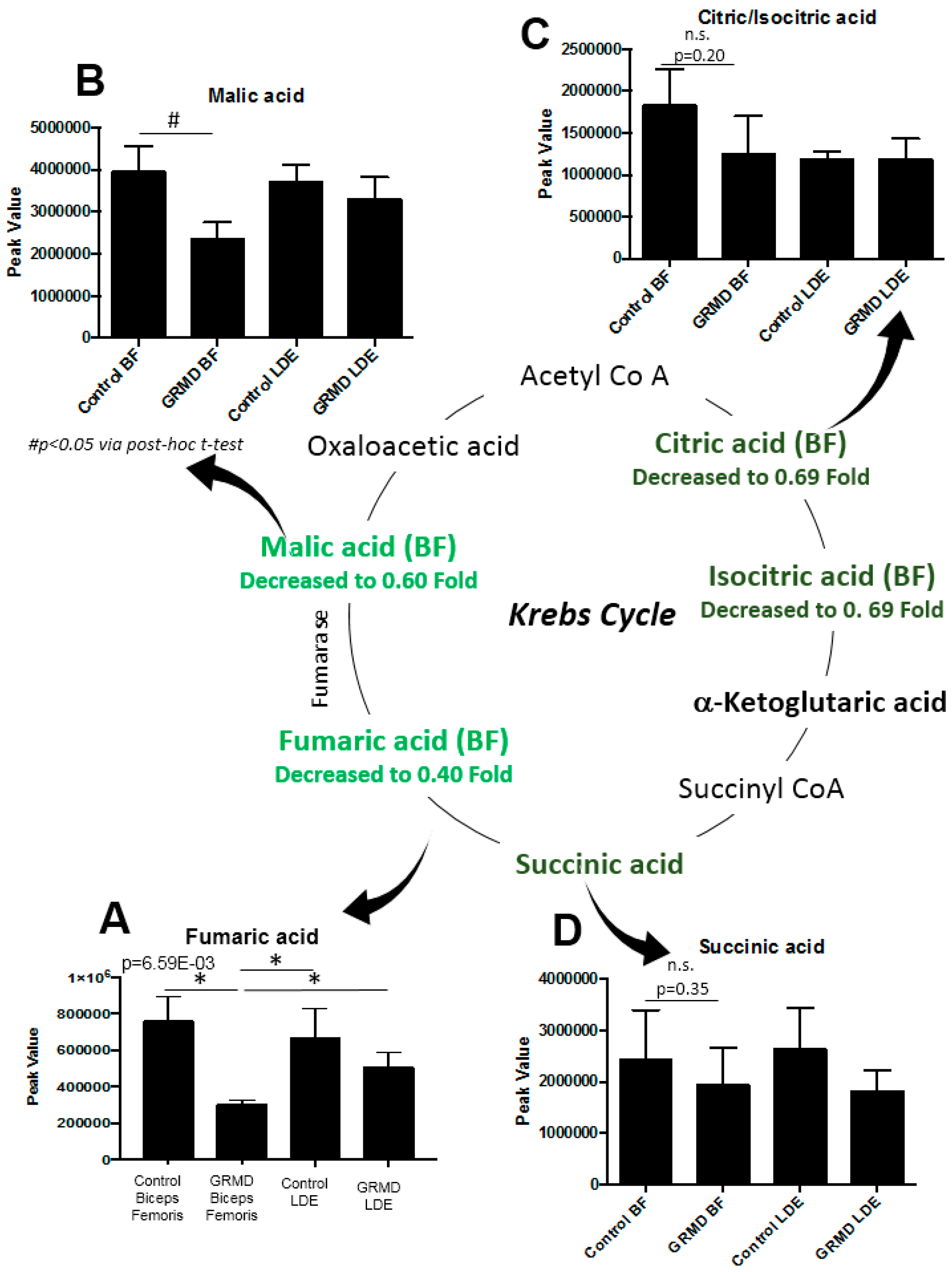
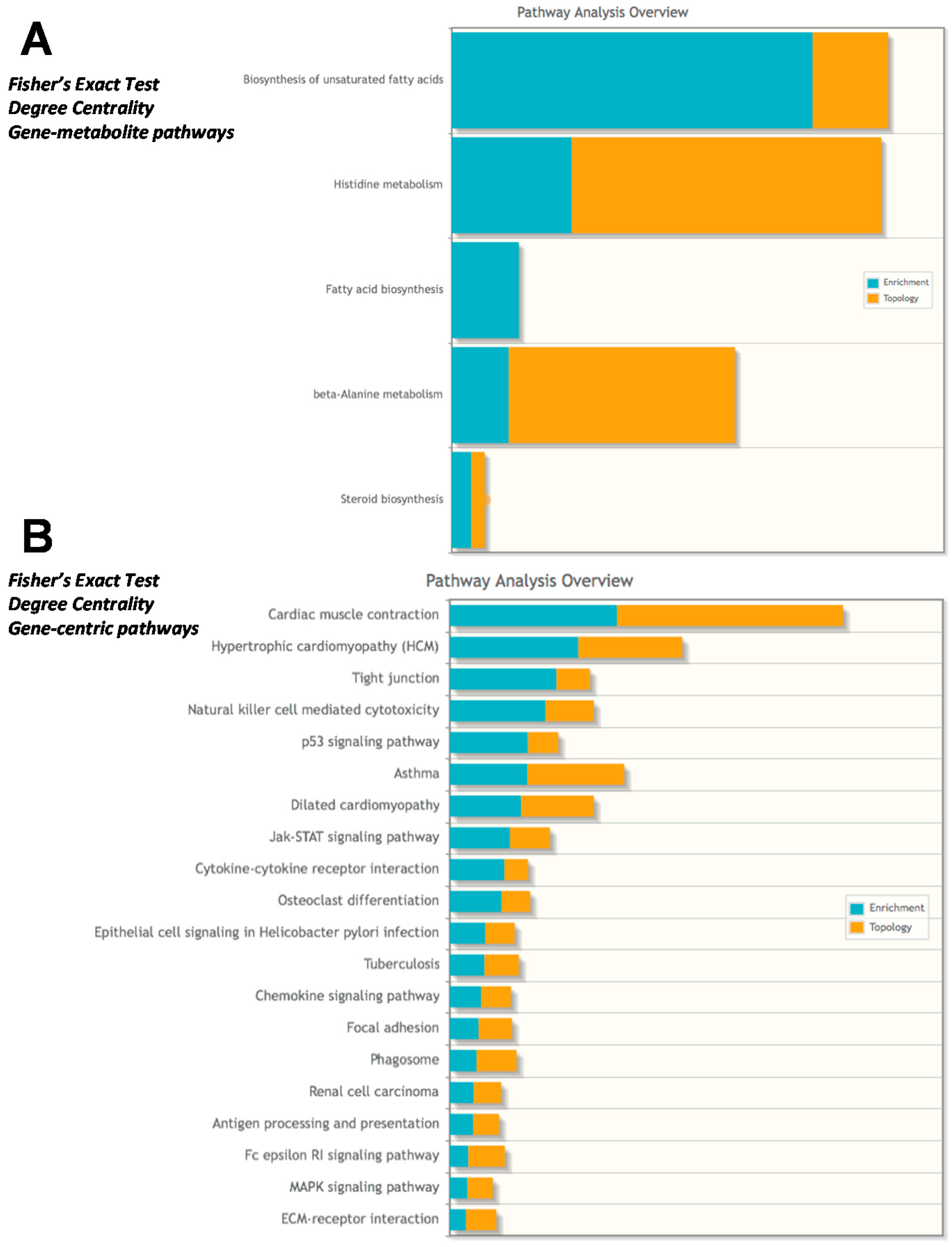
2.4. Integrated Metabolomics Analysis
3. Discussion
Summary
4. Materials and Methods
4.1. Golden Retriever Muscular Dystrophy Dog Model
4.2. Metabolomics Determination by GC–MS Instrumentation
4.3. Metabolomic Statistical Analysis
4.4. Integrated Microarray and Metabolomics Statistical Analysis
4.5. Other Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BF | biceps femoris |
| DMD | Duchenne muscular dystrophy |
| FDR | false discovery rate |
| GRMD | golden retriever muscular dystrophy |
| LDE | long digital extensor |
References
- Malhotra, S.B.; Hart, K.A.; Klamut, H.J.; Thomas, N.S.; Bodrug, S.E.; Burghes, A.H.; Bobrow, M.; Harper, P.S.; Thompson, M.W.; Ray, P.N.; et al. Frame-shift deletions in patients with duchenne and becker muscular dystrophy. Science 1988, 242, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Anderson, M.S.; Kunkel, L.M. The molecular and biochemical basis of duchenne muscular dystrophy. Trends Biochem. Sci. 1992, 17, 289–292. [Google Scholar] [CrossRef]
- Willmann, R.; Possekel, S.; Dubach-Powell, J.; Meier, T.; Ruegg, M.A. Mammalian animal models for duchenne muscular dystrophy. Neuromuscul. Disord. 2009, 19, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Morgan, J.E. Duchenne’s muscular dystrophy: Animal models used to investigate pathogenesis and develop therapeutic strategies. Int. J Exp. Pathol. 2003, 84, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chamberlain, J.S.; Tapscott, S.J.; Storb, R. Gene therapy in large animal models of muscular dystrophy. ILAR J. 2009, 50, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kornegay, J.N.; Bogan, D.J.; Bogan, J.R.; Childers, M.K.; Cundiff, D.D.; Petroski, G.F.; Schueler, R.O. Contraction force generated by tarsal joint flexion and extension in dogs with golden retriever muscular dystrophy. J. Neurol. Sci. 1999, 166, 115–121. [Google Scholar] [CrossRef]
- Kornegay, J.N.; Cundiff, D.D.; Bogan, D.J.; Bogan, J.R.; Okamura, C.S. The cranial sartorius muscle undergoes true hypertrophy in dogs with golden retriever muscular dystrophy. Neuromuscul. Disord. 2003, 13, 493–500. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Yadav, R.; Mukherjee, S.; Pal, L.; Sinha, N. Abnormal lipid metabolism in skeletal muscle tissue of patients with muscular dystrophy: In vitro, high-resolution nmr spectroscopy based observation in early phase of the disease. Magn. Reson. Imaging 2017, 38, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Galindo, C.L.; Soslow, J.H.; Brinkmeyer-Langford, C.L.; Gupte, M.; Smith, H.M.; Sengsayadeth, S.; Sawyer, D.B.; Benson, D.W.; Kornegay, J.N.; Markham, L.W. Translating golden retriever muscular dystrophy microarray findings to novel biomarkers for cardiac/skeletal muscle function in duchenne muscular dystrophy. Pediatr. Res. 2016, 79, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Fontes-Oliveira, C.C.; Steinz, M.; Schneiderat, P.; Mulder, H.; Durbeej, M. Bioenergetic impairment in congenital muscular dystrophy type 1a and leigh syndrome muscle cells. Sci. Rep. 2017, 7, 45272. [Google Scholar] [CrossRef] [PubMed]
- van den Oord, E.J. Controlling false discoveries in genetic studies. Am. J. Med. Genet. 2008, 147B, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.P.; Hoffman, E.P.; Mittal, P.; Brown, K.J.; Schatzberg, S.J.; Ghimbovschi, S.; Wang, Z.; Kornegay, J.N. Sparing of the dystrophin-deficient cranial sartorius muscle is associated with classical and novel hypertrophy pathways in grmd dogs. Am. J. Pathol. 2013, 183, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Msea: A web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Metpa: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. Metaboanalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Masuda, S.; Tamura, T.; Kai, K.; Ojima, K.; Fukase, A.; Motoyoshi, K.; Kamakura, K.; Miyagoe-Suzuki, Y.; Takeda, S. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: A role for osteopontin. Am. J. Pathol. 2003, 163, 203–215. [Google Scholar] [CrossRef]
- Markham, L.W.; Brinkmeyer-Langford, C.L.; Soslow, J.H.; Gupte, M.; Sawyer, D.B.; Kornegay, J.N.; Galindo, C.L. Grmd cardiac and skeletal muscle metabolism gene profiles are distinct. BMC Med. Genom. 2017, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.; Wouters, E.F.; Deutz, N.E.; Menheere, P.P.; Schols, A.M. Factors contributing to alterations in skeletal muscle and plasma amino acid profiles in patients with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2000, 72, 1480–1487. [Google Scholar] [PubMed]
- Pouw, E.M.; Schols, A.M.; Deutz, N.E.; Wouters, E.F. Plasma and muscle amino acid levels in relation to resting energy expenditure and inflammation in stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Fazelzadeh, P.; Hangelbroek, R.W.; Tieland, M.; de Groot, L.C.; Verdijk, L.B.; van Loon, L.J.; Smilde, A.K.; Alves, R.D.; Vervoort, J.; Muller, M.; et al. The muscle metabolome differs between healthy and frail older adults. J. Proteom. Res. 2016, 15, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins-Bach, A.B.; Bloise, A.C.; Vainzof, M.; Rahnamaye Rabbani, S. Metabolic profile of dystrophic mdx mouse muscles analyzed with in vitro magnetic resonance spectroscopy (MRS). Magn. Reson. Imaging 2012, 30, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Atri, S.; Sharma, M.C.; Sarkar, C.; Jagannathan, N.R. Skeletal muscle metabolism in duchenne muscular dystrophy (dmd): An in-vitro proton nmr spectroscopy study. Magn. Reson. Imaging 2003, 21, 145–153. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S.; Nagarajan, K. Glutamic acid as anticancer agent: An overview. Saudi Pharm. J. 2013, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.S.; Rennie, M.J.; Watt, P.W. Characteristics of acidic, basic and neutral amino acid transport in the perfused rat hindlimb. J. Physiol. 1989, 408, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Rutten, E.P.; Engelen, M.P.; Schols, A.M.; Deutz, N.E. Skeletal muscle glutamate metabolism in health and disease: State of the art. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Laferte, R.O.; Rosenkrantz, H.; Berlinguet, L. Transamination in muscular dystrophy and the effect of exogenous glutamate: A study of vitamine e deficient rabbits, and mice with hereditary dystrophy. Can. J. Biochem. Physiol. 1963, 41, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C.; Evans, N.P.; Grange, R.W.; Tuazon, M.A. Compared with that of mufa, a high dietary intake of n-3 pufa does not reduce the degree of pathology in mdx mice. Br. J. Nutr. 2014, 111, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Papapanagiotou, A.; Kalofoutis, C.; Zisaki, K.; Michalaki, V.; Tziraki, A.; Kalofoutis, A. Altered long chain fatty acids composition in duchenne muscular dystrophy erythrocytes. In Vivo 2004, 18, 799–802. [Google Scholar] [PubMed]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Arafat, E.S.; Trimble, J.W.; Andersen, R.N.; Dass, C.; Desiderio, D.M. Identification of fatty acid amides in human plasma. Life Sci. 1989, 45, 1679–1687. [Google Scholar] [CrossRef]
- Nichols, K.K.; Ham, B.M.; Nichols, J.J.; Ziegler, C.; Green-Church, K.B. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest. Ophthalmol. Vis. Sci. 2007, 48, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ezzili, C.; Otrubova, K.; Boger, D.L. Fatty acid amide signaling molecules. Bioorg. Med. Chem. Lett. 2010, 20, 5959–5968. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Takehara, M.; Ushimaru, M. Inhibitory action of linoleamide and oleamide toward sarco/endoplasmic reticulum ca2+-atpase. Biochim. Biophys. Acta 2017, 1861, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Davey, C.L. The effects of carnosine and anserine on glycolytic reactions in skeletal muscle. Arch. Biochem. Biophys. 1960, 89, 296–302. [Google Scholar] [CrossRef]
- Quinn, P.J.; Boldyrev, A.A.; Formazuyk, V.E. Carnosine: Its properties, functions and potential therapeutic applications. Mol. Asp. Med. 1992, 13, 379–444. [Google Scholar] [CrossRef]
- Parker, C.J., Jr.; Ring, E. A comparative study of the effect of carnosine on myofibrillar-atpase activity of vertebrate and invertebrate muscles. Comp. Biochem. Physiol. 1970, 37, 413–419. [Google Scholar] [CrossRef]
- Rayment, I. The structural basis of the myosin atpase activity. J. Biol. Chem. 1996, 271, 15850–15853. [Google Scholar] [CrossRef] [PubMed]
- Suidasari, S.; Stautemas, J.; Uragami, S.; Yanaka, N.; Derave, W.; Kato, N. Carnosine content in skeletal muscle is dependent on vitamin b6 status in rats. Front. Nutr. 2015, 2, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suidasari, S.; Hasegawa, T.; Yanaka, N.; Kato, N. Dietary supplemental vitamin b6 increases carnosine and anserine concentrations in the heart of rats. Springerplus 2015, 4, 280. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Oyadomari, S.; Terasaki, Y.; Takeya, M.; Suga, M.; Mori, M.; Gotoh, T. Induction of arginase I and II in bleomycin-induced fibrosis of mouse lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 285, L313–L321. [Google Scholar] [CrossRef] [PubMed]
- Kornegay, J.N.; Bogan, D.J.; Bogan, J.R.; Dow, J.L.; Wang, J.; Fan, Z.; Liu, N.; Warsing, L.C.; Grange, R.W.; Ahn, M.; et al. Dystrophin-deficient dogs with reduced myostatin have unequal muscle growth and greater joint contractures. Skelet. Muscle 2016, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.M.; DeFrancesco, T.C.; Boyle, M.C.; Malarkey, D.E.; Ritchey, J.W.; Atkins, C.E.; Cullen, J.M.; Kornegay, J.N.; Keene, B.W. Cardiac structure and function in female carriers of a canine model of duchenne muscular dystrophy. Res. Vet. Sci. 2013, 94, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Dong, S.; Wei, B.; Liu, P.; Zhang, Y.Y.; Su, S.B. Metabolomic mechanisms of gypenoside against liver fibrosis in rats: An integrative analysis of proteomics and metabolomics data. PLoS One 2017, 12, e0173598. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Banoei, M.M.; Nienow, C.K.; Abassi, T.; Hakim, A.; Schraufnagel, D.; Winston, B.W.; Sweiss, N.; Baughman, R.; Garcia, J.G.; et al. Plasma metabolomic profile in fibrosing pulmonary sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2016, 33, 29–38. [Google Scholar] [PubMed]
- Huffman, K.M.; Jessee, R.; Andonian, B.; Davis, B.N.; Narowski, R.; Huebner, J.L.; Kraus, V.B.; McCracken, J.; Gilmore, B.F.; Tune, K.N.; et al. Molecular alterations in skeletal muscle in rheumatoid arthritis are related to disease activity, physical inactivity, and disability. Arthr. Res. Ther. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Valkovic, L.; Chmelik, M.; Ukropcova, B.; Heckmann, T.; Bogner, W.; Frollo, I.; Tschan, H.; Krebs, M.; Bachl, N.; Ukropec, J.; et al. Skeletal muscle alkaline pi pool is decreased in overweight-to-obese sedentary subjects and relates to mitochondrial capacity and phosphodiester content. Sci. Rep. 2016, 6, 20087. [Google Scholar] [CrossRef] [PubMed]
- Howarth, K.R.; LeBlanc, P.J.; Heigenhauser, G.J.; Gibala, M.J. Effect of endurance training on muscle TCA cycle metabolism during exercise in humans. J. Appl. Physiol. 2004, 97, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Valentine, B.A.; Cooper, B.J.; de Lahunta, A.; O'Quinn, R.; Blue, J.T. Canine x-linked muscular dystrophy: An animal model of duchenne muscular dystrophy: Clinical studies. J. Neurol. Sci. 1988, 88, 69–81. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Winand, N.J.; Secore, S.L.; Singer, J.T.; Fletcher, S.; Wilton, S.; Bogan, D.J.; Metcalf-Bogan, J.R.; Bartlett, W.T.; Howell, J.M.; et al. Mutation segregation and rapid carrier detection of x-linked muscular dystrophy in dogs. Am J Vet Res 1996, 57, 650–654. [Google Scholar] [PubMed]
- Brinkmeyer-Langford, C.; Balog-Alvarez, C.; Cai, J.J.; Davis, B.W.; Kornegay, J.N. Genome-wide association study to identify potential genetic modifiers in a canine model for duchenne muscular dystrophy. BMC Genom. 2016, 17, 665. [Google Scholar] [CrossRef] [PubMed]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Technical advance: Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant. J. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting msi-compliant studies. Plant. J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. Fiehnlib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Mallard, W.G.; Reed, J. Automated mass spectral deconvolution and identification system: Amdis user guide. Available online: http://chemdata.nist.gov/mass-spc/amdis/docs/amdis.pdf (accessed on 25 July 2017).
- Halket, J.M.; Przyborowska, A.; Stein, S.E.; Mallard, W.G.; Down, S.; Chalmers, R.A. Deconvolution gas chromatography/mass spectrometry of urinary organic acids-potential for pattern recognition and automated identification of metabolic disorders. Rapid Commun. Mass Spectrom. 1999, 13, 279–284. [Google Scholar] [CrossRef]
- Stein, S.E. An integrated method for spectrum extraction and compound identification from GC/MS data. J. Am. Soc. Mass Spectrom. 1999, 10, 770–781. [Google Scholar] [CrossRef]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmuller, E.; Dormann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. [email protected]: The golm metabolome database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, M.P.; Moxley, J.F.; Tong, L.V.; Walther, J.L.; Jensen, K.L.; Stephanopoulos, G.N. Systematic identification of conserved metabolites in gc/ms data for metabolomics and biomarker discovery. Anal. Chem. 2007, 79, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14 10 11–14 10 91. [Google Scholar] [CrossRef]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. Metaboanalyst 2.0--a comprehensive server for metabolomic data analysis. Nucleic Acids Res 2012, 40, W127–W133. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Metabolomic data processing, analysis, and interpretation using metaboanalyst. Current Protocols Bioinformatics 2011, 6. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21633943 (accessed on 25 July 2017).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, M.; Kornegay, J.N.; Honcoop, A.; Parry, T.L.; Balog-Alvarez, C.J.; O’Neal, S.K.; Bain, J.R.; Muehlbauer, M.J.; Newgard, C.B.; Patterson, C.; et al. Non-Targeted Metabolomics Analysis of Golden Retriever Muscular Dystrophy-Affected Muscles Reveals Alterations in Arginine and Proline Metabolism, and Elevations in Glutamic and Oleic Acid In Vivo. Metabolites 2017, 7, 38. https://doi.org/10.3390/metabo7030038
Abdullah M, Kornegay JN, Honcoop A, Parry TL, Balog-Alvarez CJ, O’Neal SK, Bain JR, Muehlbauer MJ, Newgard CB, Patterson C, et al. Non-Targeted Metabolomics Analysis of Golden Retriever Muscular Dystrophy-Affected Muscles Reveals Alterations in Arginine and Proline Metabolism, and Elevations in Glutamic and Oleic Acid In Vivo. Metabolites. 2017; 7(3):38. https://doi.org/10.3390/metabo7030038
Chicago/Turabian StyleAbdullah, Muhammad, Joe N. Kornegay, Aubree Honcoop, Traci L. Parry, Cynthia J. Balog-Alvarez, Sara K. O’Neal, James R. Bain, Michael J. Muehlbauer, Christopher B. Newgard, Cam Patterson, and et al. 2017. "Non-Targeted Metabolomics Analysis of Golden Retriever Muscular Dystrophy-Affected Muscles Reveals Alterations in Arginine and Proline Metabolism, and Elevations in Glutamic and Oleic Acid In Vivo" Metabolites 7, no. 3: 38. https://doi.org/10.3390/metabo7030038





