Detection of Volatile Metabolites of Garlic in Human Breast Milk
Abstract
:1. Introduction
2. Results
2.1. Determination of Odor Qualities of Reference Compounds
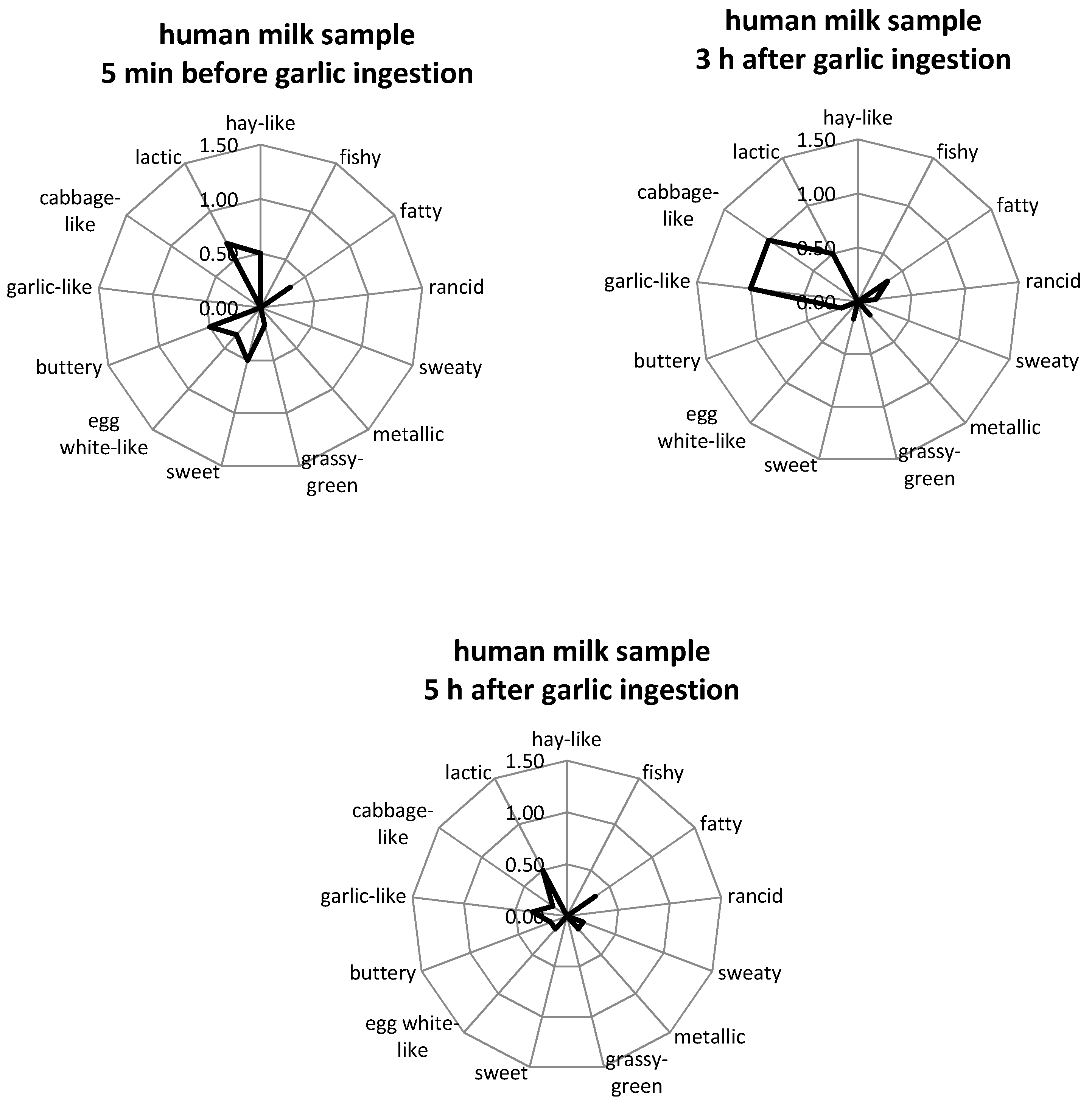
2.2. Aroma Profile Analysis
2.3. Comparative Aroma Extract Dilution Analysis (cAEDA) of the Milk before and after Garlic Consumption
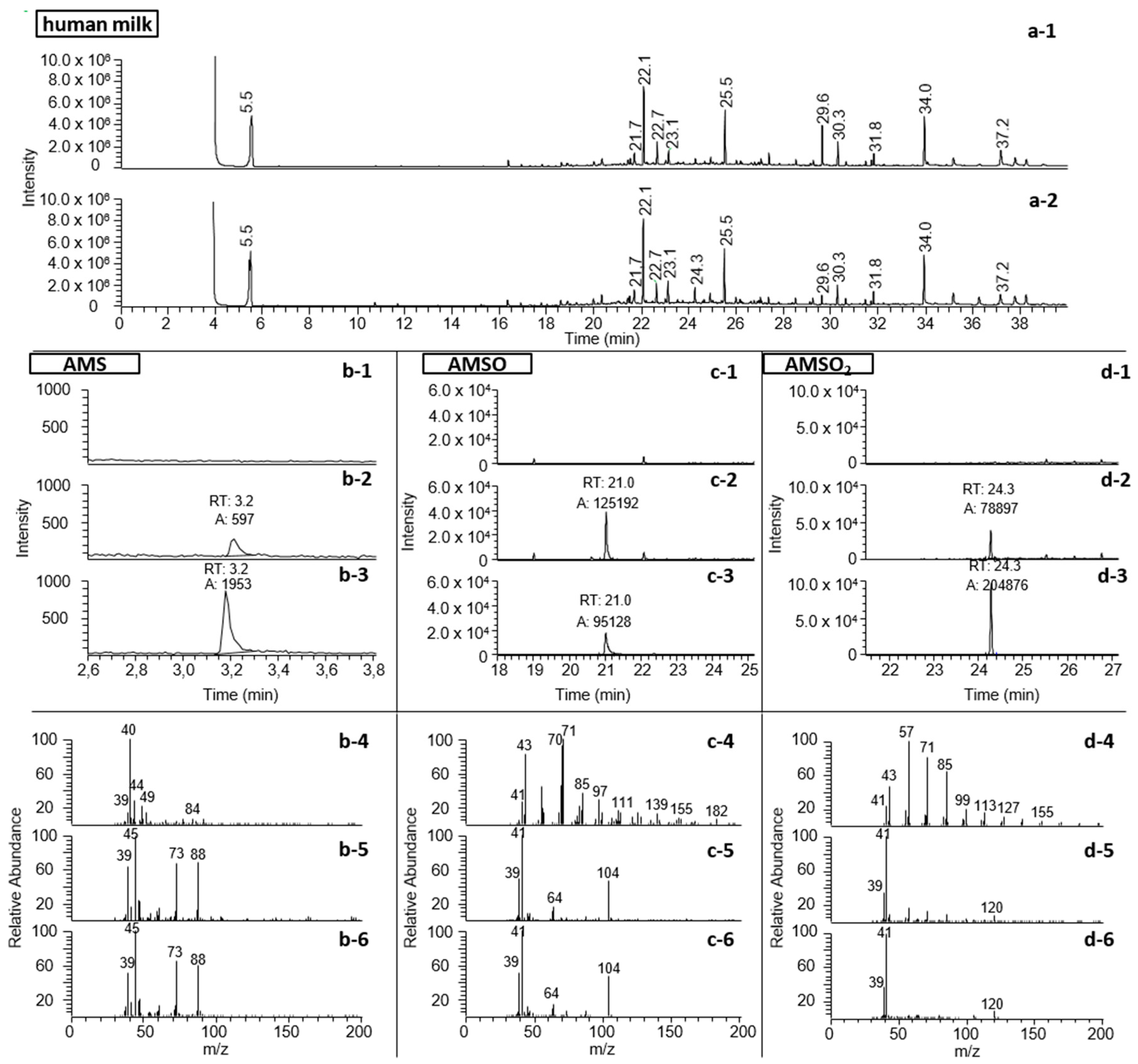
2.4. Identification of Garlic-Derived Metabolites in Human Milk
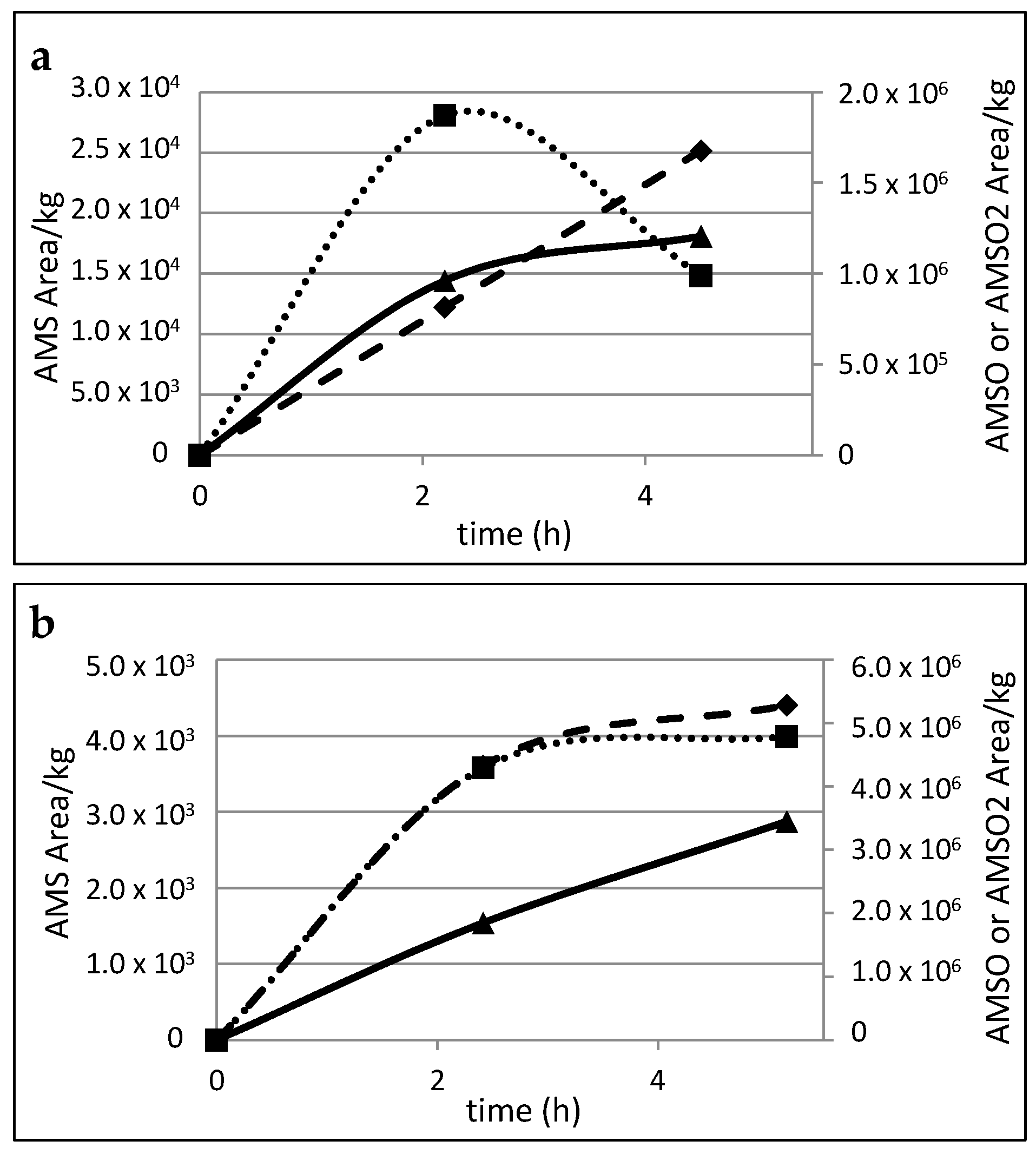
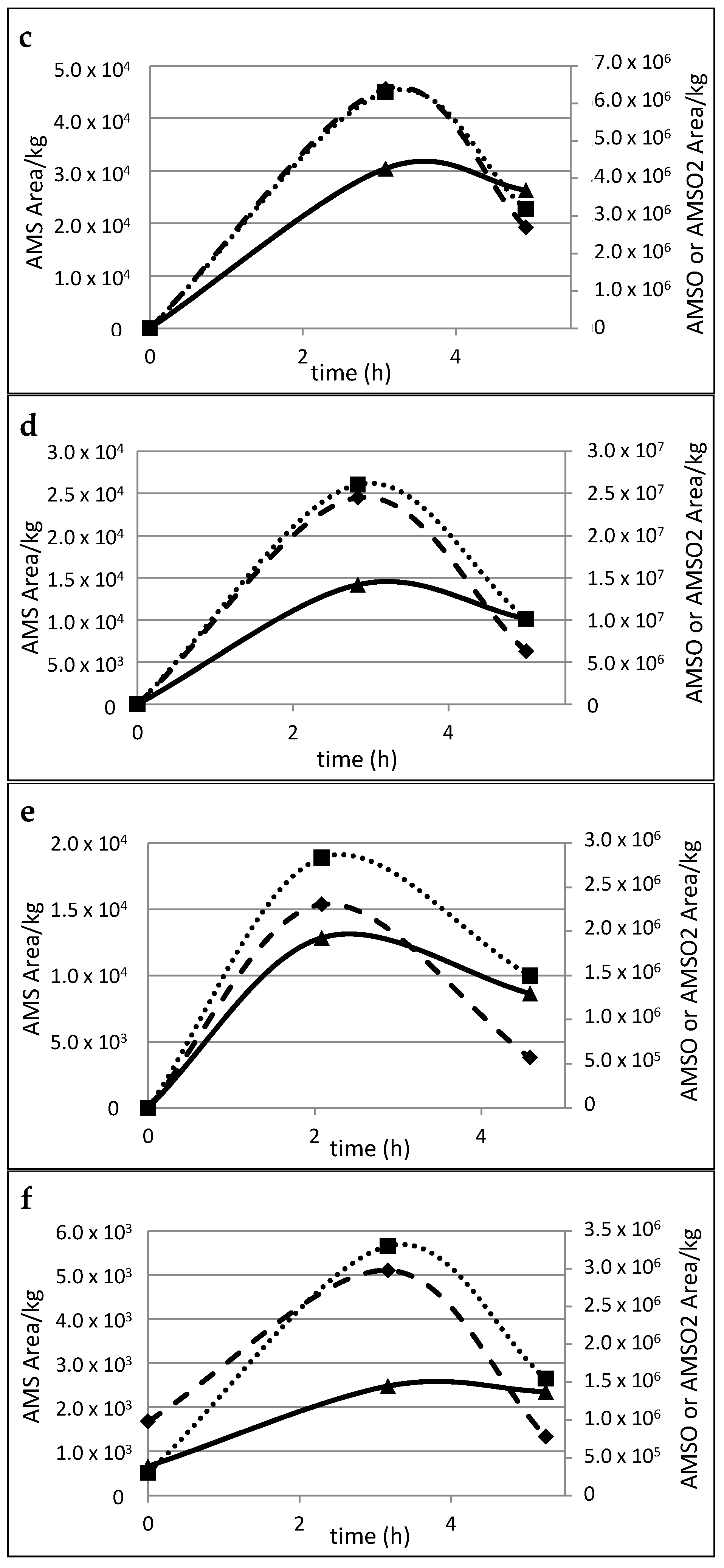
2.5. Time Dependency of Appearance of the Garlic-Derived Metabolites in the Human Milk after Consumption of Garlic
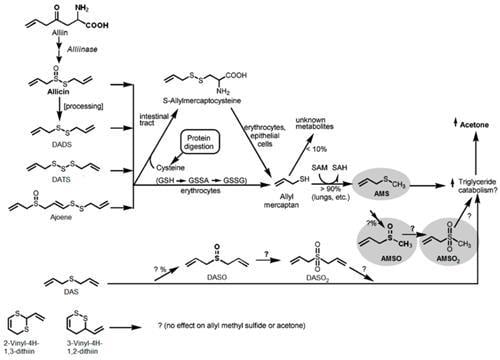
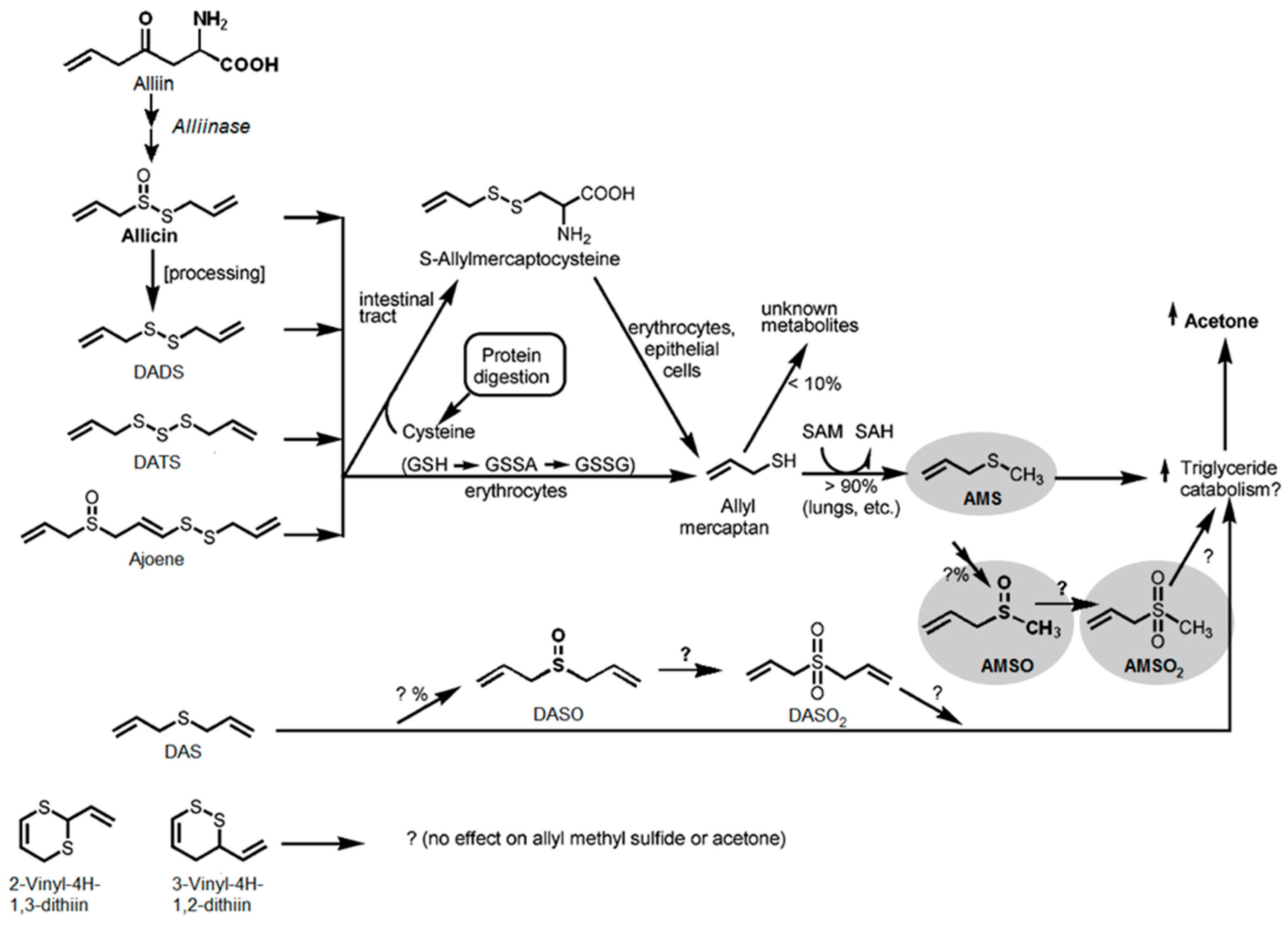
3. Discussion
3.1. Aroma Profile Analysis
3.2. Identification of Garlic-Derived Metabolites in Human Milk
3.3. Metabolism of Garlic
4. Materials and Methods
4.1. Chemicals/Materials
4.2. Syntheses of Reference Substances
4.3. Human Milk Samples
4.4. Study Design
4.5. Aroma Profile Analysis
4.6. Solvent-Assisted Flavor Evaporation (SAFE) of Volatiles from Human Milk
4.7. High-Resolution Gas Chromatography-Olfactometry (HRGC-O)
4.8. Determination of Odor Qualities of Reference Compounds
4.9. Comparative Aroma Extract Dilution Analysis (cAEDA)
4.10. High-Resolution Gas Chromatography−Mass Spectrometry (HRGC-MS)
4.11. Two-Dimensional High-Resolution Gas Chromatography−Mass Spectrometry/Olfactometry (HRGC-GC-MS/O) (Heart-Cut)
4.12. Identification of Metabolites and Calculation of Metabolite Profiles
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMDS | Allyl methyl disulfide |
| AMS | Allyl methyl sulfide |
| AMSO | Allyl methyl sulfoxide |
| AMSO2 | Allyl methyl sulfone |
| APA | Aroma profile analysis |
| cAEDA | Comparative aroma extract dilution analysis |
| DADS | Diallyl disulfide |
| DAS | Diallyl sulfide |
| DASO | Diallyl sulfoxide |
| DASO2 | Diallyl sulfone |
| DATS | Diallyl trisulfide |
| DMDS | Dimethyl disulfide |
| DMTS | Dimethyl trisulfide |
| FD-factor | Flavor dilution factor |
| FID | Flame ionization detector |
| HRGC-GC-MS/O | Two-dimensional high-resolution gas chromatography−mass spectrometry/olfactometry |
| HRGC-MS | High-resolution gas chromatography−mass spectrometry |
| RI | Linear retention indices |
| SAFE | Solvent-assisted flavor evaporation |
References
- Peterson, R.; Cheah, W.Y.; Grinyer, J.; Packer, N. Glycoconjugates in human milk: Protecting infants from disease. Glycobiology 2013, 23, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Jagnow, C.P.; Beauchamp, G.K. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001, 107, e88. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Beauchamp, G.K. Maternal diet alters the sensory qualities of human milk and the nurslings’s behavior. Pediatrics 1991, 88, 737–744. [Google Scholar] [PubMed]
- Mennella, J.A.; Beauchamp, G.K. The transfer of alcohol to human milk. N. Engl. J. Med. 1991, 325, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Beauchamp, G.K. The human infants’ response to vanilla flavors in mother’s milk and formula. Infant Behav. Dev. 1996, 19, 13–19. [Google Scholar] [CrossRef]
- Mennella, J.A.; Beauchamp, G.K. Beer, breast feeding, and folklore. Dev. Psychobiol. 1993, 26, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Denzer, M.Y.; Kirsch, F.; Buettner, A. Are odorant constituents of herbal tea transferred into human milk? J. Agric. Food Chem. 2015, 63, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sandgruber, S.; Much, D.; Amann-Gassner, U.; Hauner, H.; Buettner, A. Sensory and molecular characterisation of human milk odour profiles after maternal fish oil supplementation during pregnancy and breastfeeding. Food Chem. 2011, 128, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, J.; Doucet, S.; Buettner, A. The influence of storage conditions on flavour changes in human milk. Food Qual. Preference 2010, 21, 998–1007. [Google Scholar] [CrossRef]
- Spitzer, J.; Buettner, A. Characterization of aroma changes in human milk during storage at −19 °C. Food Chem. 2010, 120, 240–246. [Google Scholar] [CrossRef]
- Spitzer, J.; Buettner, A. Monitoring aroma changes during human milk storage at −19 °C by quantification experiments. Food Res. Int. 2013, 51, 250–256. [Google Scholar] [CrossRef]
- Spitzer, J.; Klos, K.; Buettner, A. Monitoring aroma changes during human milk storage at +4 °C by sensory and quantification experiments. Clin. Nutr. 2013, 32, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, F.; Beauchamp, J.; Buettner, A. Time-dependent aroma changes in breast milk after oral intake of a pharmacological preparation containing 1,8-cineole. Clin. Nutr. 2012, 31, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, F.; Horst, K.; Röhrig, W.; Rychlik, M.; Buettner, A. Tracing metabolite profiles in human milk: Studies on the odorant 1,8-cineole transferred into breast milk after oral intake. Metabolomics 2012, 9, 483–496. [Google Scholar] [CrossRef]
- Hausner, H.; Bredie, W.L.P.; Mølgaard, C.; Petersen, M.A.; Møller, P. Differential transfer of dietary flavour compounds into human breast milk. Physiol. Behav. 2008, 95, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Beauchamp, G.K. The effects of repeated exposure to garlic-flavored milk on the nursling’s behavior. Pediatr. Res. 1993, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Buhr, K.; Eisgruber, K.; Kiefl, J.; Schieberle, P. Garlic Breath Sampling and Monitoring by Proton Transfer Reaction-Mass Spectrometry. In Proceedings of the 4th PTR-MS Conference, Innsbruck, Austria, 16–21 February 2009; pp. 203–207.
- Cai, X.J.; Block, E.; Uden, P.C.; Quimby, B.D.; Sullivan, J.J. Allium chemistry—Identification of natural-abundance organoselenium compounds in human breath after ingestion of garlic using gas-chromatography with atomic-emission detection. J. Agric. Food Chem. 1995, 43, 1751–1753. [Google Scholar] [CrossRef]
- Hansanugrum, A.; Barringer, S.A. Effect of milk on the deodorization of malodorous breath after garlic ingestion. J. Food Sci. 2010, 75, C549–C558. [Google Scholar] [CrossRef] [PubMed]
- Munch, R.; Barringer, S.A. Deodorization of garlic breath volatiles by food and food components. J. Food Sci. 2014, 79, C526–C533. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.Y.; Lech, J.; Rosen, S.L.; Hartman, T.G. The determination of metabolites of garlic preparations in breath and human plasma. Biofactors 2000, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.Y.; Lech, J.; Rosen, S.L.; Hartman, T.G. Determination of allicin, s-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J. Nutr. 2001, 131, 968S–971S. [Google Scholar] [PubMed]
- Ruiz, R.; Hartman, T.G.; Karmas, K.; Lech, J.; Rosen, R.T. Breath analysis of garlic-borne phytochemicals in human-subjects—Combined adsorbent trapping and short-path thermal-desorption gas chromatography-mass spectrometry. In Food Phytochemicals for Cancer Prevention I: Fruits and Vegetables; Huang, M.T., Osawa, T., Ho, C.T., Rosen, R.T., Eds.; American Chemical Society: Washington, DC, USA, 1994; Volume 546, pp. 102–119. [Google Scholar]
- Suarez, F.; Springfield, J.; Furne, J.; Levitt, M. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am. J. Physiol. Gastroint. Liver Physiol. 1999, 276, G425–G430. [Google Scholar]
- Taucher, J.; Hansel, A.; Jordan, A.; Lindinger, W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J. Agric. Food Chem. 1996, 44, 3778–3782. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wang, Z.J. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: Use in measuring allicin bloavailability. J. Agric. Food Chem. 2005, 53, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A.; Pappas, C.S.; Polissiou, M.G. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, D.; Wang, Z.; Jiang, J.; Jiang, T.; Cui, F.; Fan, X. Effect of ultrahigh pressure treatment on volatile compounds in garlic. J. Food Process Eng. 2011, 34, 1915–1930. [Google Scholar] [CrossRef]
- Tokarska, B.; Karwowska, K. The role of sulfur-compounds in evaluation of flavoring value of some plant raw-materials. Nahrung 1983, 27, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Vernin, G.; Metzger, J.; Fraisse, D.; Scharff, C. GC-MS (EI, PCI, NCI) computer-analysis of volatile sulfur-compounds in garlic essential oils—Application of the mass fragmentometry sim technique. Planta Medica 1986, 52, 96–101. [Google Scholar] [CrossRef]
- Yu, T.H.; Wu, C.M.; Liou, Y.C. Volatile compounds from garlic. J. Agric. Food Chem. 1989, 37, 725–730. [Google Scholar] [CrossRef]
- Germain, E.; Auger, J.; Ginies, C.; Siess, M.H.; Teyssier, C. In vivo metabolism of diallyl disulphide in the rat: Identification of two new metabolites. Xenobiotica 2002, 32, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Seppanen-Laakso, T.; Hiltunen, R.; Muller, B.; Jansen, H.; Knobloch, K. Volatile garlic odor components: Gas phases and adsorbed exhaled air analysed by headspace gas chromatography-mass spectrometry. Planta Medica 1989, 55, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Boku, T.; Inada, K.; Morita, M.; Okazaki, Y. Odor components of human breath after the ingestion of grated raw garlic. J. Food Sci. 1989, 54, 763–765. [Google Scholar] [CrossRef]
- Lee, S.N.; Kim, N.S.; Lee, D.S. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2003, 377, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Ciaravolo, S.; Chiricosta, G.; Celli, S. Volatile flavour components from ripening and mature garlic bulbs. Flavour Fragr. J. 1992, 7, 111–116. [Google Scholar] [CrossRef]
- Mondy, N.; Naudin, A.; Christides, J.P.; Mandon, N.; Auger, J. Comparison of GC-MS and HPLC for the analysis of allium volatiles. Chromatographia 2001, 53, S356–S360. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Dembicki, J.W.; Goldshlag, P.; Hanus, L.O.; Dembitsky, V.M. The use of the ’cryogenic’ GC/MS and on-column injection for study of organosulfur compounds of the allium sativum. J. Food Compos. Anal. 2004, 17, 235–245. [Google Scholar] [CrossRef]
- Sandgruber, S.; Much, D.; Amann-Gassner, U.; Hauner, H.; Buettner, A. Sensory and molecular characterisation of the protective effect of storage at −80 °C on the odour profiles of human milk. Food Chem. 2012, 130, 236–242. [Google Scholar] [CrossRef]
- Schieberle, P. New developments in methods for analysis of volatile flavor compounds and their precursors. In Characterization of Food: Emerging Methods; Gaonkar, A.G., Ed.; Elsevier: Amsterdam, the Netherlands, 1995; pp. 403–431. [Google Scholar]
- Buettner, A.; Schieberle, P. Application of a comparative aroma extract dilution analysis to monitor changes in orange juice aroma compounds during processing. In Gas Chromatography-Olfactometry; American Chemical Society: Washington, DC, USA, 2001; Volume 782, pp. 33–45. [Google Scholar]
- Buettner, A. A selective and sensitive approach to characterize odour-active and volatile constituents in small-scale human milk samples. Flavour Fragr. J. 2007, 22, 465–473. [Google Scholar] [CrossRef]
- Bachour, P.; Yafawi, R.; Jaber, F.; Choueiri, E.; Abdel-Razzak, Z. Effects of smoking, mother’s age, body mass index, and parity number on lipid, protein, and secretory immunoglobulin a concentrations of human milk. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2012, 7, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Nikolic, V.; Nikolic, L.; Stankovic, M.; Stanojevic, L.; Cakic, M. Allicin and related compounds: Biosynthesis, synthesis and pharmacological activity. Facta Univ. Ser. Phys. Chem. Technol. 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Iberl, B.; Winkler, G.; Knobloch, K. Products of allicin transformation—Ajoenes and dithiins, characterization and their determination by hplc. Planta Medica 1990, 56, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.F.; Ishizaki, H.; Fukuto, J.M.; Lin, M.C.; Fadel, A.; Gapac, J.M.; Yang, C.S. Inhibition of cytochrome p-450 2e1 by diallyl sulfide and its metabolites. Chem. Res. Toxicol. 1991, 4, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.X.; Baillie, T.A. Metabolism of the chemoprotective agent diallyl sulfide to glutathione conjugates in rats. Chem. Res. Toxicol. 1997, 10, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Kirsch, F.; Buettner, A. Real-time breath gas analysis for pharmacokinetics: Monitoring exhaled breath by on-line proton-transfer-reaction mass spectrometry after ingestion of eucalyptol-containing capsules. J. Breath Res. 2010, 4, 026006. [Google Scholar] [CrossRef] [PubMed]
- Iberl, B.; Winkler, G.; Muller, B.; Knobloch, K. Quantitative-determination of allicin and alliin from garlic by hplc. Planta Medica 1990, 56, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.D.; Wood, S.G.; Hughes, B.G. Hplc analysis of allicin and other thiosulfinates in garlic clove homogenates. Planta Medica 1991, 57, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Egen-Schwind, C.; Eckard, R.; Kemper, F.H. Metabolism of garlic constituents in the isolated perfused rat liver. Planta Medica 1992, 58, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Egen-Schwind, C.; Eckard, R.; Jekat, F.W.; Winterhoff, H. Pharmacokinetics of vinyldithiins, transformation products of allicin. Planta Medica 1992, 58, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Sheen, L.Y.; Wu, C.C.; Lii, C.K.; Tsai, S.J. Metabolites of diallyl disulfide and diallyl sulfide in primary rat hepatocytes. Food Chem. Toxicol. 1999, 37, 1139–1146. [Google Scholar] [CrossRef]
- Hartmann, C.; Mayenzet, F.; Larcinese, J.-P.; Haefliger, O.P.; Buettner, A.; Starkenmann, C. Development of an analytical approach for identification and quantification of 5-α-androst-16-en-3-one in human milk. Steroids 2013, 78, 156–160. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, B.M.; Boogaard, P.J.; Rijksen, D.A.; Commandeur, J.N.M.; Vermeulen, N.P.E. Urinary excretion of N-acetyl-S-allyl-l-cysteine upon garlic consumption by human volunteers. Arch. Toxicol. 1996, 70, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Jandke, J.; Spiteller, G. Unusual conjugates in biological profiles originating from consumption of onions and garlic. J. Chromatogr. B Biomed. Sci. Appl. 1987, 421, 1–8. [Google Scholar] [CrossRef]
- Chowdhury, S.; Samuel, P.M.; Das, I.; Roy, S. B12 mimicry in a weak ligand environment: Oxidation and alkylation of thiols. J. Chem. Soc. Chem. Commun. 1994, 1993–1994. [Google Scholar] [CrossRef]
- Oae, S.; Takata, T.; Kim, Y.H. Oxidation of unsymmetrical disulfide and thiosulfinic s-esters with peroxy acids. Search for formation of α-disulfoxide as an intermediate in the electrophilic oxidation of thiosulfinic s-ester. Bull. Chem. Soc. Jpn. 1982, 55, 2484–2494. [Google Scholar] [CrossRef]
- Bland, J.M.; Stammer, C.H. An intramolecular bromonium to thiiranium ion rearrangement. J. Organ. Chem. 1983, 48, 4393–4394. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Abbasi, M. A one-pot, efficient, and odorless synthesis of symmetrical disulfides using organic halides and thiourea in the presence of manganese dioxide and wet polyethylene glycol (peg-200). Tetrahedron Lett. 2010, 51, 508–509. [Google Scholar] [CrossRef]
- Lu, G.-P.; Cai, C. An odorless and efficient synthesis of symmetrical thioethers using organic halides and thiourea in triton x10 aqueous micelles. Green Chem. Lett. Rev. 2012, 5, 481–485. [Google Scholar] [CrossRef]
- Mokhtary, M.; Qandalee, M.; Niaki, M.R. Highly efficient selective oxygenation of sulfides to sulfoxides by oxalic acid dihydrate in the presence of H2O2. E J. Chem. 2012, 9, 863–868. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Sheikh Arabi, M. Tapc-promoted oxidation of sulfides and deoxygenation of sulfoxides. J. Organ. Chem. 2010, 75, 6208–6213. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Niu, S.; Segi, M.; Tanaka, K.; Nakajima, T.; Zingaro, R.A.; Reibenspies, J.H.; Hall, M.B. On the behavior of α,β-unsaturated thioaldehydes and thioketones in the diels−alder reaction. J. Organ. Chem. 2000, 65, 6601–6612. [Google Scholar] [CrossRef]
- Ren, F.-K.; He, X.-Y.; Deng, L.; Li, B.-H.; Shin, D.-S.; Li, Z.-B. Synthesis and antibacterial activity of 1,3-diallyltrisulfane derivatives. Bull. Korean Chem. Soc. 2009, 30, 687–690. [Google Scholar]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Bemelmans, J.M.H. Review of isolation and concentration techniques. In Progress in Flavour Research, Proceedings of the 2nd Weurman Flavour Research Symposium, Norwich, UK, 2–6 April 1978; Land, D.G., Nursten, H.E., Eds.; Applied Science Publisher: London, UK, 1979; pp. 79–98. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]

| Substance (Abbreviation) | Retention Index (RI) | Identified in Milk after Garlic Intake a | Odor Quality | Previously Detected in/Described as | Reference | |
|---|---|---|---|---|---|---|
| FFAP | DB-5 | |||||
| Allyl methyl sulfide (AMS) | <1000 | 715 | + b | garlic-like c,d | Human breath after garlic consumption | [17,18,19,20,21,22,23,24,25,26] |
| Human urine after garlic consumption | [24] | |||||
| Garlic | [27,28,29,30,31] | |||||
| Allyl methyl sufloxide (AMSO) | 1742 | 1018 | + | odorless e | Identified in rat stomach, liver, plasma and urine after administration of diallyl disulfide (DADS) | [32] |
| Garlic metabolite in the human body | ||||||
| Allyl methyl sulfone (AMSO2) | 1983 | 1061 | + | odorless e | Identified in rat stomach, liver, plasma and urine after administration of DADS | [26,32] |
| Garlic metabolite in the human body | ||||||
| Diallyl sulfoxide (DASO) | 1889 | 1163 | - | garlic-like e | Potential garlic metabolite in the human body | |
| Diallyl sulfone (DASO2) | 2079 | 1289 | - | odorless e | Potential garlic metabolite in the human body | [26] |
| Diallyl disulfide (DADS) | 1462 | 1083 | - | garlic-like c,d | Human breath after garlic consumption | [17,19,20,21,22,23,24,25,33,34] |
| pungent d | Garlic | [28,29,30,35,36,37] | ||||
| Allyl methyl disulfide (AMDS) | 1265 | 921 | - | garlic-like, cooked garlic-like d | Human breath after garlic consumption | [18,19,20,24,25] |
| Garlic | [27,28,29,30,31,38] | |||||
| Dimethyl disulfide (DMDS) | 1071 | 751 | - | cabbage-like c cooked garlic-like, onion-like, rubber-like d | Human breath after garlic consumption | [18] |
| Garlic | [27,28,29,30,31,36] | |||||
| Dimethyl trisulfide (DMTS) | 1362 | 973 | - | garlic-like c | Garlic | [27,29,30,31,36] |
| burnt garlic-like, diffusive, penetrating, sulfury d | ||||||
| Diallyl trisulfide (DATS) | 1771 | 1308 | - | garlic-like c | Human breath after garlic consumption | [18,25] |
| garlic-like, onion-like d | Garlic | [27,28,29,30,31,35,36,37,38] | ||||
| Diallyl sulfide (DAS) | 1138 | 868 | - | garlic-like c | Human breath after garlic consumption | [18,21,22,23,25] |
| Garlic | [27,28,29,30,31,35,36,37] | |||||
| 2-Vinyl-4H-1,3-dithiin | 1827 | 1222 | - | garlic-like c | Garlic | [27,31,36,37,38] |
| 3-Vinyl-4H-1,2-dithiin | 1720 | 1194 | - | Pungent garlic‑like c | Garlic | [27,31,36,37,38] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheffler, L.; Sauermann, Y.; Zeh, G.; Hauf, K.; Heinlein, A.; Sharapa, C.; Buettner, A. Detection of Volatile Metabolites of Garlic in Human Breast Milk. Metabolites 2016, 6, 18. https://doi.org/10.3390/metabo6020018
Scheffler L, Sauermann Y, Zeh G, Hauf K, Heinlein A, Sharapa C, Buettner A. Detection of Volatile Metabolites of Garlic in Human Breast Milk. Metabolites. 2016; 6(2):18. https://doi.org/10.3390/metabo6020018
Chicago/Turabian StyleScheffler, Laura, Yvonne Sauermann, Gina Zeh, Katharina Hauf, Anja Heinlein, Constanze Sharapa, and Andrea Buettner. 2016. "Detection of Volatile Metabolites of Garlic in Human Breast Milk" Metabolites 6, no. 2: 18. https://doi.org/10.3390/metabo6020018