Comparative Pharmacokinetics of Naringin in Rat after Oral Administration of Chaihu-Shu-Gan-San Aqueous Extract and Naringin Alone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Conditions Optimization
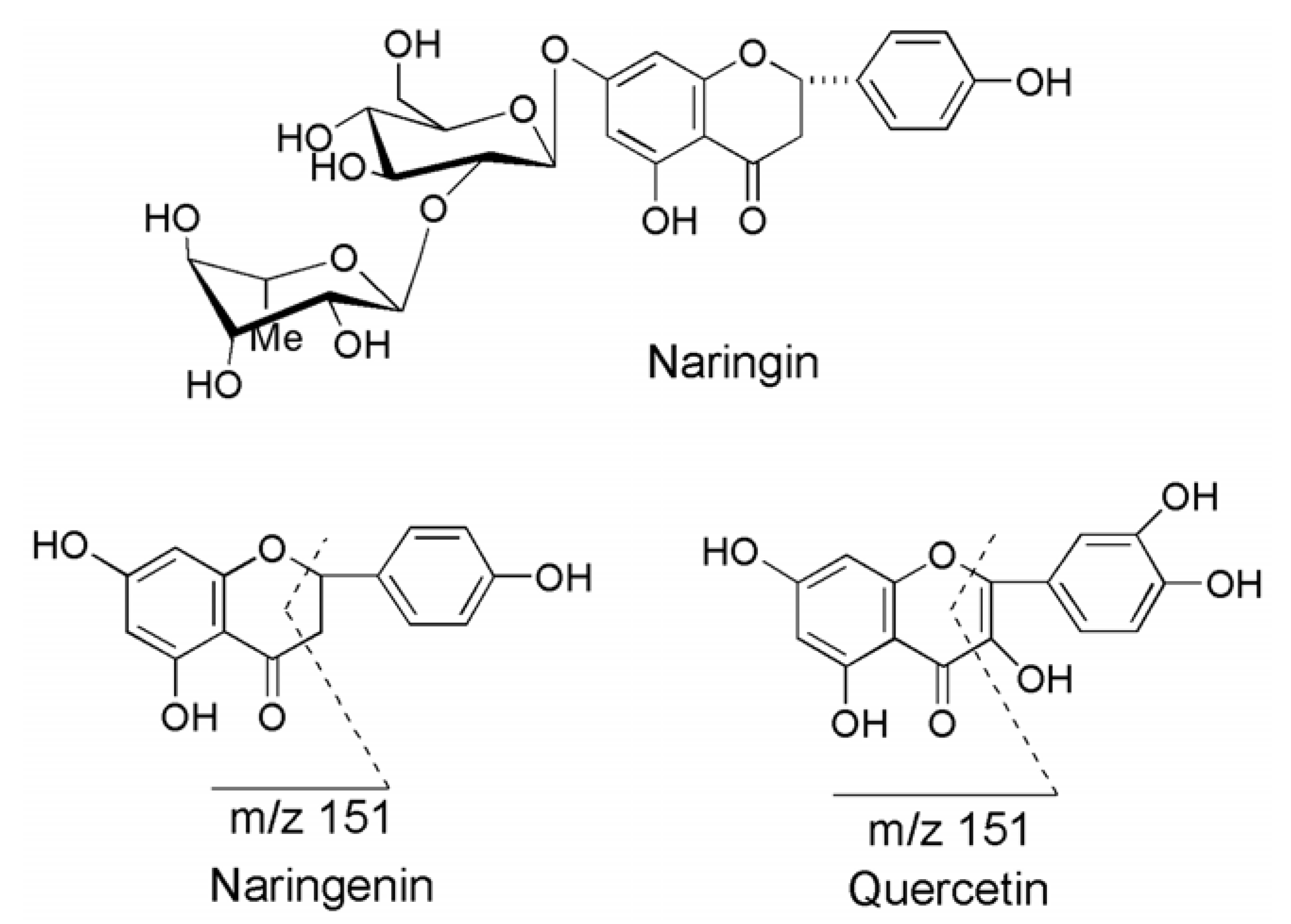
2.2. Method Validation
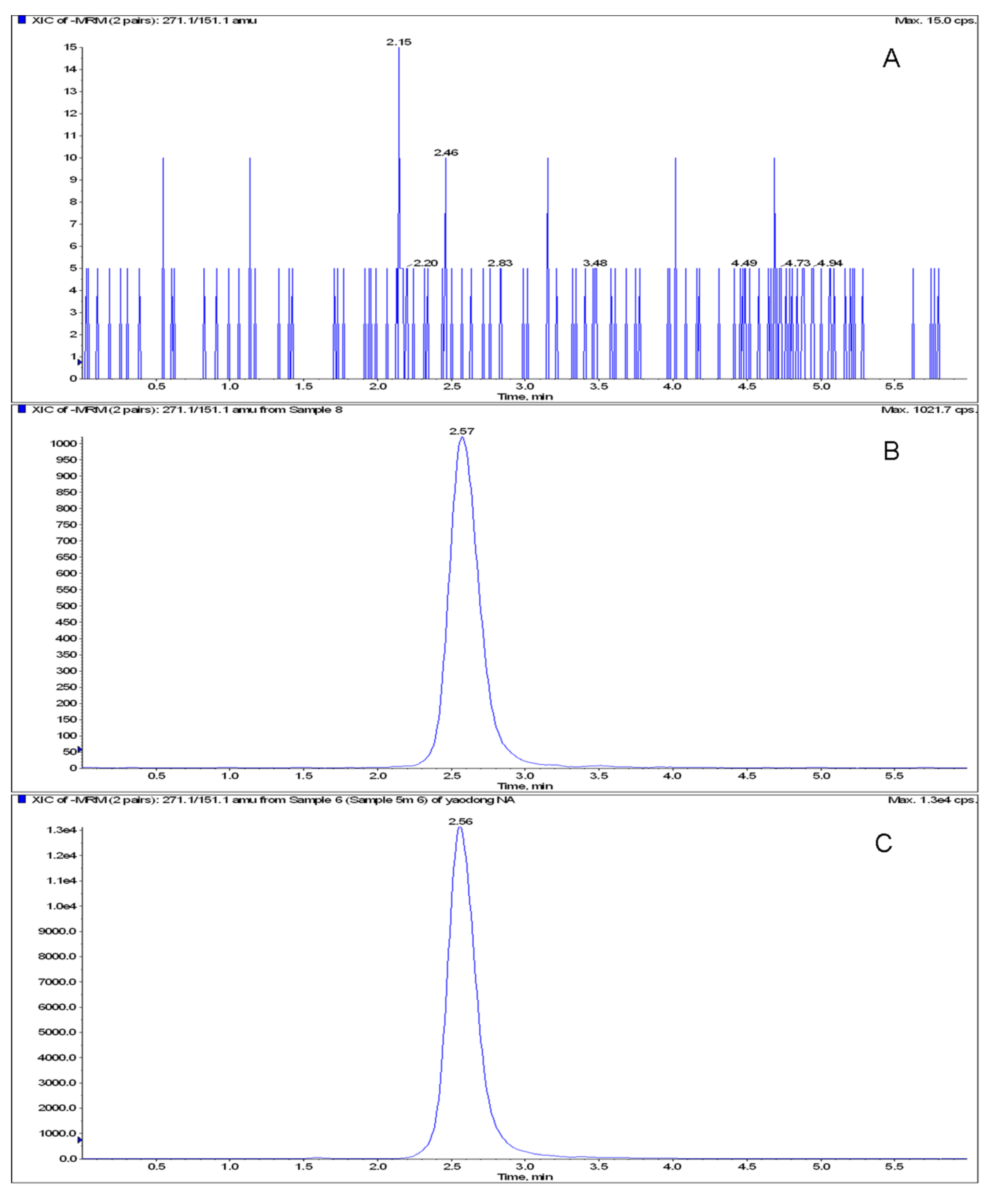
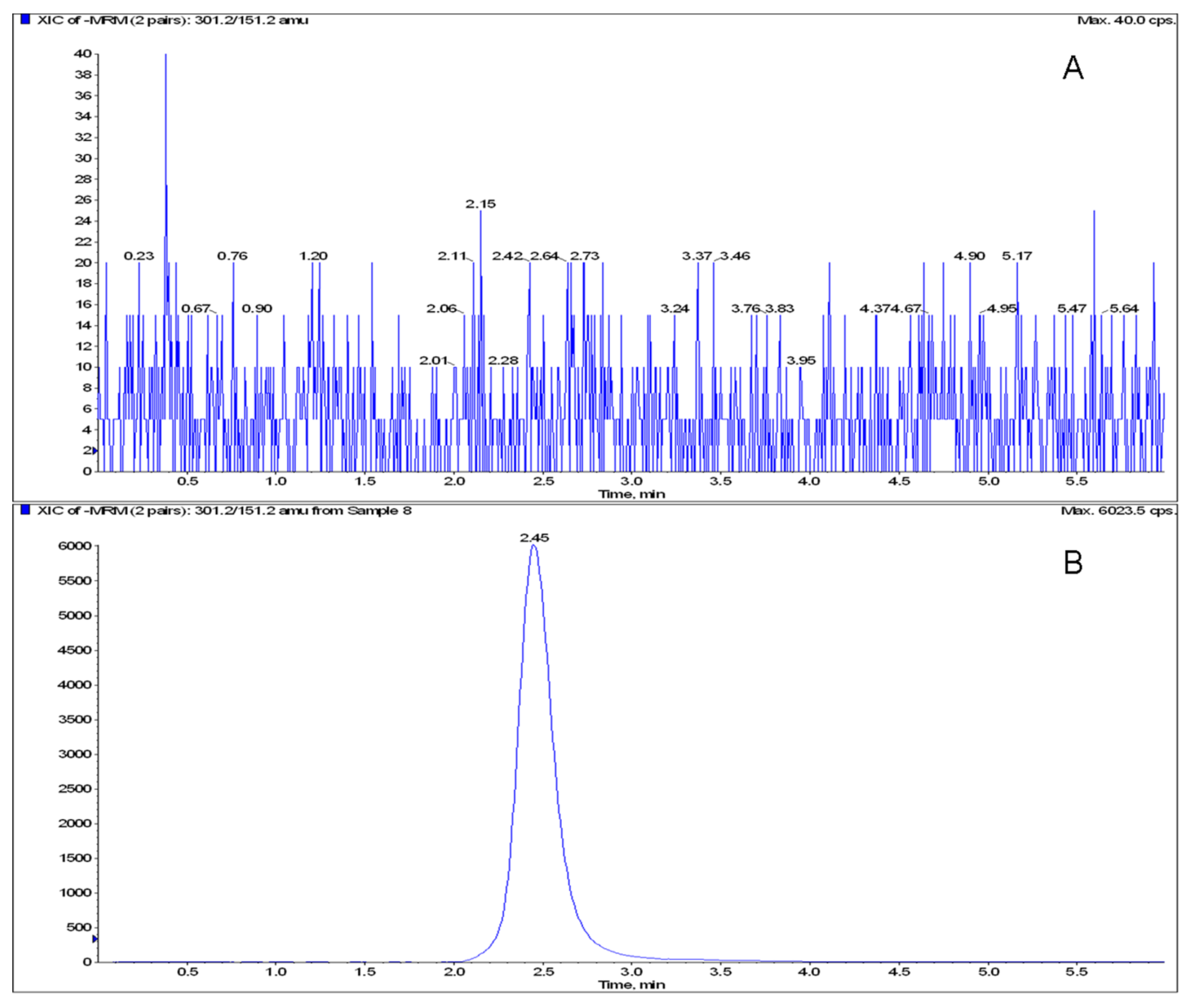
| Spiked concentration (μg·mL-1) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|
| Measured concentration (μg·mL−1) | Precision (%) | Accuracy (%) | Measured concentration (μg·mL−1) | Precision (%) | Accuracy (%) | |
| 0.36 | 0.392 | 3.27 | 104.00 | 0.423 | 7.78 | 105.17 |
| 0.365 | 0.347 | |||||
| 0.372 | 0.389 | |||||
| 0.381 | 0.375 | |||||
| 0.362 | 0.359 | |||||
| 0.374 ± 0.012 | 0.379 ± 0.02 | |||||
| 1.8 | 1.82 | 3.77 | 99.00 | 1.90 | 5.3 | 98.56 |
| 1.77 | 1.83 | |||||
| 1.86 | 1.79 | |||||
| 1.78 | 1.64 | |||||
| 1.68 | 1.71 | |||||
| 1.78 ± 0.07 | 1.77 ± 0.10 | |||||
| 9.0 | 8.96 | 1.22 | 100.69 | 9.08 | 2.18 | 99.89 |
| 9.06 | 8.72 | |||||
| 9.24 | 9.24 | |||||
| 8.98 | 9.02 | |||||
| 9.07 | 8.89 | |||||
| 9.06 ± 0.11 | 8.99 ± 0.20 | |||||
| Concentration (μg·mL−1) | Short term (at room temperature) | Long term (at −80 °C) | Freeze-thaw (cycle number) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 4h | 6h | 10h | RSD (%) | 0 | 5d | 10d | 15d | RSD (%) | 0 | 1 | 2 | 3 | RSD (%) | |
| 0.36 | 0.342 | 0.354 | 0.374 | 0.365 | 3.85 | 0.347 | 0.365 | 0.418 | 0.384 | 8.02 | 0.357 | 0.403 | 0.382 | 0.369 | 5.17 |
| 1.8 | 1.87 | 1.86 | 1.78 | 1.80 | 2.30 | 2.02 | 1.95 | 1.79 | 1.87 | 5.21 | 1.78 | 1.89 | 1.92 | 1.82 | 3.45 |
| 9 | 9.94 | 9.87 | 9.29 | 10.03 | 3.42 | 9.07 | 9.47 | 9.24 | 9.05 | 2.11 | 8.90 | 9.23 | 9.89 | 9.09 | 4.64 |
| Concentration ( μg·mL−1) | Extracted peak area | Control peak area | Absolute recovery (%) | Average recovery (%) | RSD (%) | |
|---|---|---|---|---|---|---|
| 0.36 | 2.08e + 003 | 3.17e + 003 | 78.74 | 77.84 | 4.80 | |
| 2.13e + 003 | 3.22e + 003 | 79.38 | ||||
| 2.18e + 003 | 3.64e + 003 | 71.87 | ||||
| 2.28e + 003 | 3.34e + 003 | 81.92 | ||||
| 2.08e + 003 | 3.23e + 003 | 77.28 | ||||
| 1.8 | 2.15e + 004 | 3.12e + 004 | 82.69 | 83.94 | 4.17 | |
| 2.07e + 004 | 3.05e + 004 | 81.44 | ||||
| 2.22e + 004 | 3.08e + 004 | 86.49 | ||||
| 1.99e + 004 | 2.97e + 004 | 80.40 | ||||
| 2.29e + 004 | 3.10e + 004 | 88.64 | ||||
| 9 | 1.97e + 005 | 2.58e + 005 | 91.63 | 90.16 | 5.17 | |
| 1.67e + 005 | 2.42e + 005 | 82.81 | ||||
| 1.65e + 005 | 2.23e + 005 | 88.79 | ||||
| 1.93e + 005 | 2.44e + 005 | 94.92 | ||||
| 1.83e + 005 | 2.37e + 005 | 92.66 | ||||
| QU (IS) | 5 | 6.34e + 004 | 8.23e + 004 | 92.44 | 86.08 | 5.65 |
| 5.98e + 004 | 9.02e + 004 | 79.56 | ||||
| 5.81e + 004 | 8.32e + 004 | 83.80 | ||||
| 5.87e + 004 | 8.19e + 004 | 86.01 | ||||
| 6.38e + 004 | 8.64e + 004 | 88.61 |
2.3. Pharmacokinetic Study
| Parameters | Units | CSGS | NA |
|---|---|---|---|
| Tmax,1 | min | 360.0000 | 15.0000 |
| Cmax,1 | mg·L-1 | 1.8970 | 0.4840 |
| Tmax,2 | min | 600.0000 | 180.0000 |
| Cmax,2 | mg·L-1 | 1.3040 | 0.7390 |
| Lambda_z | 1·min-1 | 0.0040 | 0.0027 |
| HL_Lambda_z | min | 171.8932 | 255.7001 |
| AUCall | min·mg·L-1 | 840.2175 | 114.0243 |
| Cl_F | L·min-1·kg-1 | 3.0512 | 0.0443 |
| MRT | min | 523.3516 | 274.8070 |

3. Experimental Section
3.1. Chemicals and Reagents
3.2. Preparation of CSGS Aqueous Extract
3.3. Animals
3.4. LC/MS/MS Apparatus and Chromatographic Conditions
3.5. Preparation of Calibration and Quality Control (QC) Samples
3.6. Preparation of Serum Samples
3.7. Method Validation
3.7.1. Specificity
3.7.2. Calibration Curve and Sensitivity
3.7.3. Accuracy and Precision
3.7.4. Extraction Recovery
3.7.5. Stability
3.8. Pharmacokinetic Study
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhang, H.W.; Zou, Z.M. Clinical applications and development of modern researches of Chai-Hu-Shu-Gan-San. Lishizhen Med. Mater. Med. Res. 2007, 18, 1234–1236. [Google Scholar]
- Li, S.Q.; Su, Z.H.; Peng, J.B.; Zou, Z.M.; Yu, C.Y. In vitro and in vivo antioxidant effects and the possible relationship between the antidepression efficacy of traditional Chinese medicine formulation Chaihu Shugan San. Chinese J. Nat. Med. 2010, 8, 353–361. [Google Scholar]
- Su, Z.H.; Zou, G.A.; Preiss, A.; Zhang, H.W.; Zou, Z.M. Online identification of the antioxidant constituents of traditional Chinese medicine formula Chaihu-Shu-Gan-San by LC-LTQ-Orbitrap mass spectrometry and microplate spectrophotometer. J. Pharmaceut. Biomed. 2010, 53, 454–461. [Google Scholar] [CrossRef]
- Su, Z.H.; Li, S.Q.; Zou, G.A.; Yu, C.Y.; Sun, Y.G.; Zhang, H.W.; Gu, Y.; Zou, Z.M. Urinary metabonomics study of anti-depressive effect of Chaihu-Shu-Gan-San on an experimental model of depression induced by chronic variable stress in rats. J. Pharm. Biomed. Anal. 2011, 55, 533–539. [Google Scholar] [CrossRef]
- Jia, H.M.; Su, Z.H.; Long, W.; Liu, Y.T.; Chang, X.; Zhang, H.W.; Ding, G.; Feng, Y.F.; Cai, D.Y.; Zou, Z.M. Metabonomics combined with UPLC-MS chemical profiling for discovery of antidepressant ingredients of a traditional Chinese medicines formula, Chaihu-Shu-Gan-San. Evid. Based. Complement. Alternat. Med. 2013, 2013. Article ID 487158. [Google Scholar]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar]
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol.—Gastr. L. 2000, 279, G1148–G1154. [Google Scholar]
- Fang, T.Z.; Wang, Y.G.; Ma, Y.; Su, W.W.; Bai, Y.; Zhao, P.Y. A rapid LC/MS/MS quantitation assay for naringin and its two metabolites in rats plasma. J. Pharmaceut. Biomed. 2006, 40, 454–459. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Rahman, M.M.; Kashiwada, Y.; Ikeshiro, Y.; Shida, Y.; Hatano, Y.; Matsumoto, H.; Hirayama, M.; Tsuda, T.; Konishi, T. Absorption and metabolism of delphinidin 3-O-β-glucopyranoside in rats. Free Radical Bio. Med. 2004, 36, 930–937. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–498. [Google Scholar] [CrossRef]
- Lai, M.Y.; Hsiu, S.L.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol. 2003, 55, 205–209. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Day, A.J.; Needs, P.W.; Sly, W.S.; O'Brien, N.M.; Williamson, G. Flavonoid glucuronides are substrates for human liver β-glucuronidase. FEBS Lett. 2001, 503, 103–106. [Google Scholar]
- Ameer, B.; Weintraub, R.A.; Johnson, J.V.; Yost, R.A.; Rouseff, R.L. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin. Pharmaco. Ther. 1996, 60, 34–40. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.L.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar]
- Lu, T.; Song, J.; Huang, F.; Deng, Y.X.; Xie, L.; Wang, G.J.; Liu, X.D. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J. Ethnopharmacol. 2007, 110, 412–418. [Google Scholar] [CrossRef]
- Liang, W.Q. Biopharmaceutics and Pharmacokinetics; People’s Medical Publishing House: Beijing, China, 2003; p. 151. [Google Scholar]
- Wan, L.L.; Sun, X.P.; Wang, X.W.; Li, Y.; Yu, Q.; Guo, C. A stereospecific HPLC method and its application in determination of pharmacokinetics profile of two enantiomers of naringenin in rats. J. Chromatogr. Sci. 2011, 49, 316–320. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, S.-Q.; Dong, S.; Su, Z.-H.; Zhang, H.-W.; Peng, J.-B.; Yu, C.-Y.; Zou, Z.-M. Comparative Pharmacokinetics of Naringin in Rat after Oral Administration of Chaihu-Shu-Gan-San Aqueous Extract and Naringin Alone. Metabolites 2013, 3, 867-880. https://doi.org/10.3390/metabo3040867
Li S-Q, Dong S, Su Z-H, Zhang H-W, Peng J-B, Yu C-Y, Zou Z-M. Comparative Pharmacokinetics of Naringin in Rat after Oral Administration of Chaihu-Shu-Gan-San Aqueous Extract and Naringin Alone. Metabolites. 2013; 3(4):867-880. https://doi.org/10.3390/metabo3040867
Chicago/Turabian StyleLi, Shu-Qi, Shu Dong, Zhi-Heng Su, Hong-Wu Zhang, Jing-Bo Peng, Chang-Yuan Yu, and Zhong-Mei Zou. 2013. "Comparative Pharmacokinetics of Naringin in Rat after Oral Administration of Chaihu-Shu-Gan-San Aqueous Extract and Naringin Alone" Metabolites 3, no. 4: 867-880. https://doi.org/10.3390/metabo3040867




