Vitamin D and Ceramide Metabolomic Profile in Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Population
2.2. HPLC-MS/MS Analysis for Ceramide Assessment
2.2.1. Plasma Processing for Ceramide Assessment
2.2.2. Chemicals and Materials
2.2.3. Cer(d18:1/17:0) Calibration Curve Preparation
2.3. 25(OH)D Quantitative Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations of this Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Della Nera, G.; Sabatino, L.; Gaggini, M.; Gorini, F.; Vassalle, C. Vitamin D Determinants, Status, and Antioxidant/Anti-Inflammatory-Related Effects in Cardiovascular Risk and Disease: Not the Last Word in the Controversy. Antioxidants 2023, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Serpico, D.; Cantelli, M.; Di Sarno, A.; Dalia, C.; Arianna, R.; Lavorgna, M.; Colao, A.; Di Somma, C. Osteoporosis and Dermatoporosis: A Review on the Role of Vitamin D. Front. Endocrinol. 2023, 14, 1231580. [Google Scholar] [CrossRef] [PubMed]
- Wakle, K.S.; Mokale, S.N.; Sakle, N.S. Emerging Perspectives: Unraveling the Anticancer Potential of Vitamin D3; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Máčová, L.; Kancheva, R.; Bičíková, M. Molecular Regulation of the CNS by Vitamin D. Physiol. Res. 2023, 72, S339–S356. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Machulsky, N.F.; Barchuk, M.; Gagliardi, J.; González, D.; Lombardo, M.; Escudero, A.G.; Gigena, G.; Blanco, F.; Schreier, L.; Fabre, B.; et al. Vitamin D Is Related to Markers of Vulnerable Plaque in Acute Myocardial Infarction. Curr. Vasc. Pharmacol. 2017, 15, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, V.; De Metrio, M.; Cosentino, N.; Marenzi, G.; Tremoli, E. Vitamin D and Acute Myocardial Infarction. World J. Cardiol. 2017, 9, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Legarth, C.; Grimm, D.; Krüger, M.; Wehland, M.; Infanger, M. Potential Beneficial Effects of Vitamin d in Coronary Artery Disease. Nutrients 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Mastorci, F.; Berti, S.; Sabatino, L.; Palmieri, C.; Iervasi, G.; Vassalle, C. Hypovitaminosis D and Low T3 Syndrome: A Link for Therapeutic Challenges in Patients with Acute Myocardial Infarction. J. Clin. Med. 2021, 10, 5267. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Manson, J.A.E.; Pilz, S.; März, W.; Michaëlsson, K.; Lundqvist, A.; Jassal, S.K.; Barrett-Connor, E.; Zhang, C.; et al. Circulating 25-Hydroxy-Vitamin D and Risk of Cardiovascular Disease: A Meta-Analysis of Prospective Studies. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 819–829. [Google Scholar] [CrossRef]
- Jani, R.; Mhaskar, K.; Tsiampalis, T.; Kassaw, N.A.; González, M.Á.M.; Panagiotakos, D.B. Circulating 25-Hydroxy-Vitamin D and the Risk of Cardiovascular Diseases. Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3282–3304. [Google Scholar] [CrossRef]
- Fenizia, S.; Gaggini, M.; Vassalle, C. The Sphingolipid-Signaling Pathway as a Modulator of Infection by SARS-CoV-2. Curr. Issues Mol. Biol. 2023, 45, 7956–7973. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Fenizia, S.; Vassalle, C. Sphingolipid Levels and Signaling via Resveratrol and Antioxidant Actions in Cardiometabolic Risk and Disease. Antioxidants 2023, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Borodzicz-Jażdżyk, S.; Jażdżyk, P.; Łysik, W.; Cudnoch-Jȩdrzejewska, A.; Czarzasta, K. Sphingolipid Metabolism and Signaling in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 915961. [Google Scholar] [CrossRef] [PubMed]
- Balram, A.; Thapa, S.; Chatterjee, S. Glycosphingolipids in Diabetes, Oxidative Stress, and Cardiovascular Disease: Prevention in Experimental Animal Models. Int. J. Mol. Sci. 2022, 23, 15442. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Cirillo, F.; Ghiroldi, A.; Rota, P.; Coviello, S.; Tarantino, A.; La Rocca, P.; Lavota, I.; Creo, P.; Signorelli, P.; et al. Sphingolipids and Atherosclerosis: The Dual Role of Ceramide and Sphingosine-1-Phosphate. Antioxidants 2023, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, E.; Rocchiccioli, S.; Gaggini, M.; Ndreu, R.; Berti, S.; Vassalle, C. Ceramides and Cardiovascular Risk Factors, Inflammatory Parameters and Left Ventricular Function in AMI Patients. Biomedicines 2022, 10, 429. [Google Scholar] [CrossRef]
- Gencer, B.; Morrow, D.A.; Braunwald, E.; Goodrich, E.L.; Hilvo, M.; Kauhanen, D.; Sabatine, M.S.; Laaksonen, R.; O’Donoghue, M.L. Plasma Ceramide and Phospholipid-Based Risk Score and the Risk of Cardiovascular Death in Patients after Acute Coronary Syndrome. Eur. J. Prev. Cardiol. 2022, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wang, Y.; Xu, D.; Liu, X.; Shen, C.; Hu, W.; Wang, Z.; Wu, R.; Tang, X.; Sun, A.; et al. Effect of Combined Testing of Ceramides with High-Sensitive Troponin T on the Detection of Acute Coronary Syndrome in Patients with Chest Pain in China: A Prospective Observational Study. BMJ Open 2019, 9, e028211. [Google Scholar] [CrossRef] [PubMed]
- Edsfeldt, A.; Dunér, P.; Stahlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O.; et al. Sphingolipids Contribute to Human Atherosclerotic Plaque Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef]
- Hadas, Y.; Vincek, A.S.; Youssef, E.; Żak, M.M.; Chepurko, E.; Sultana, N.; Sharkar, M.T.K.; Guo, N.; Komargodski, R.; Kurian, A.A.; et al. Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response after Myocardial Infarction. Circulation 2020, 141, 916–930. [Google Scholar] [CrossRef]
- Fenizia, S.; Gaggini, M.; Vassalle, C. Interplay between Vitamin D and Sphingolipids in Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 17123. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, M.; Pierucci, F.; Vestri, A.; Meacci, E. Crosstalk between Sphingolipids and Vitamin D3: Potential Role in the Nervous System. Br. J. Pharmacol. 2017, 174, 605–627. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van De Werf, F.; et al. Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, E.; Di Giorgi, N.; Finamore, F.; Smit, J.M.; Scholte, A.J.H.A.; Signore, G.; Rocchiccioli, S. Lipid Biomarkers in Statin Users with Coronary Artery Disease Annotated by Coronary Computed Tomography Angiography. Sci. Rep. 2021, 11, 12899. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Maffei, S.; Prontera, C.; Battaglia, D.; Vassalle, C. Preanalytical, Analytical (DiaSorin LIAISON) and Clinical Variables Potentially Affecting the 25-OH Vitamin D Estimation. Clin. Biochem. 2012, 45, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma Lipidomic Analysis of Stable and Unstable Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Uchida, Y.; Kobayashi, T.; Shirai, S.; Hiruta, N.; Shimoyama, E.; Tabata, T. Detection of Ceramide, a Risk Factor for Coronary Artery Disease, in Human Coronary Plaques by Fluorescent Angioscopy. Circ. J. 2017, 81, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma Ceramides Predict Cardiovascular Death in Patients with Stable Coronary Artery Disease and Acute Coronary Syndromes beyond LDL-Cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K. Ceramides Predict CV Death in Stable CAD and ACS. Nat. Rev. Cardiol. 2016, 13, 381. [Google Scholar] [CrossRef]
- Pan, W.; Sun, M.; Wu, J.; Dong, H.; Liu, J.; Gao, R.; Fang, S.; Xing, L.; Hu, S.; Yu, B. Relationship between Elevated Plasma Ceramides and Plaque Rupture in Patients with ST-Segment Elevation Myocardial Infarction. Atherosclerosis 2020, 302, 8–14. [Google Scholar] [CrossRef]
- Pan, W.; Li, L.; Sun, M.; Wang, C.; Fang, S.; Yu, B. Plasma Ceramides Are Associated with Coronary Atherosclerotic Burden in Patients with ST-Segment Elevation Myocardial Infarction. Int. J. Cardiol. 2020, 320, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma Ceramides a Novel Predictor of Major Adverse Cardiovascular Events after Coronary Angiography. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M.; Suoniemi, M.; Kardys, I.; Vihervaara, T.; de Boer, S.P.M.; Akkerhuis, K.M.; Sysi-Aho, M.; Ekroos, K.; Garcia-Garcia, H.M.; Oemrawsingh, R.M.; et al. Plasma Concentrations of Molecular Lipid Species in Relation to Coronary Plaque Characteristics and Cardiovascular Outcome: Results of the ATHEROREMO-IVUS Study. Atherosclerosis 2015, 243, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, X.; Deik, A.A.; Hanna, D.B.; Wang, T.; Haberlen, S.A.; Shah, S.J.; Lazar, J.M.; Hodis, H.N.; Landay, A.L.; et al. Elevated Plasma Ceramides Are Associated with Antiretroviral Therapy Use and Progression of Carotid Artery Atherosclerosis in HIV Infection. Circulation 2019, 139, 2003–2011. [Google Scholar] [CrossRef]
- Saulnier-Blache, J.S.; Wilson, R.; Klavins, K.; Graham, D.; Alesutan, I.; Kastenmüller, G.; Wang-Sattler, R.; Adamski, J.; Roden, M.; Rathmann, W.; et al. Ldlr−/− and ApoE−/− Mice Better Mimic the Human Metabolite Signature of Increased Carotid Intima Media Thickness Compared to Other Animal Models of Cardiovascular Disease. Atherosclerosis 2018, 276, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Fang, Z.; Li, S.; Xu, M.; Zhang, J.; Han, D.; Hu, W.; Yan, L.; Wang, Y.; Fan, L.; et al. Circulating Ceramide: A New Cardiometabolic Biomarker in Patients with Comorbid Acute Coronary Syndrome and Type 2 Diabetes Mellitus. Front. Physiol. 2020, 11, 1104. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Salonurmi, T.; Havulinna, A.S.; Kauhanen, D.; Pedersen, E.R.; Tell, G.S.; Meyer, K.; Teeriniemi, A.M.; Laatikainen, T.; Jousilahti, P.; et al. Ceramide Stearic to Palmitic Acid Ratio Predicts Incident Diabetes. Diabetologia 2018, 61, 1424–1434. [Google Scholar] [CrossRef]
- Warshauer, J.T.; Lopez, X.; Gordillo, R.; Hicks, J.; Holland, W.L.; Anuwe, E.; Blankfard, M.B.; Scherer, P.E.; Lingvay, I. Effect of Pioglitazone on Plasma Ceramides in Adults with Metabolic Syndrome. Diabetes Metab. Res. Rev. 2015, 31, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.W.K.; Ooi, E.M.M.; Watts, G.F.; Chan, D.C.; Weir, J.M.; Meikle, P.J.; Barrett, P.H.R. Dose-Dependent Effects of Rosuvastatin on the Plasma Sphingolipidome and Phospholipidome in the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E2335–E2340. [Google Scholar] [CrossRef]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvänne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular Lipids Identify Cardiovascular Risk and Are Efficiently Lowered by Simvastatin and PCSK9 Deficiency. J. Clin. Endocrinol. Metab. 2014, 99, 45–52. [Google Scholar] [CrossRef]
- Hilvo, M.; Simolin, H.; Metso, J.; Ruuth, M.; Öörni, K.; Jauhiainen, M.; Laaksonen, R.; Baruch, A. PCSK9 Inhibition Alters the Lipidome of Plasma and Lipoprotein Fractions. Atherosclerosis 2018, 269, 159–165. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Alvarez, U.; Avioli, L.V.; Hruska, K.A. Effect of 1,25-Dihydroxyvitamin D3 on Phospholipid Composition of Rat Renal Brush Border Membrane. Am. J. Physiol. 1985, 249, 117–123. [Google Scholar] [CrossRef]
- Magrassi, L.; Adorni, L.; Montorfano, G.; Rapelli, S.; Butti, G.; Berra, B.; Milanesi, G. Vitamin D Metabolites Activate the Sphingomyelin Pathway and Induce Death of Glioblastoma Cells. Acta Neurochir. 1998, 140, 707–713. [Google Scholar] [CrossRef]
- Conte, C.; Cataldi, S.; Arcuri, C.; Mirarchi, A.; Lazzarini, A.; Garcia-Gil, M.; Beccari, T.; Curcio, F.; Albi, E. Vitamin D3 Enriches Ceramide Content in Exosomes Released by Embryonic Hippocampal Cells. Int. J. Mol. Sci. 2021, 22, 9287. [Google Scholar] [CrossRef]
- Nejatian, N.; Trautmann, S.; Thomas, D.; Pfeilschifter, J.; Badenhoop, K.; Koch, A.; Penna-Martinez, M. Vitamin D Effects on Sphingosine 1-Phosphate Signaling and Metabolism in Monocytes from Type 2 Diabetes Patients and Controls. J. Steroid Biochem. Mol. Biol. 2019, 186, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, H.; Shankar, G.; Huang, M.C.; Bikle, D.D.; Goetzl, E.J. Biochemical Regulation of Breast Cancer Cell Expression of S1P2 (Edg-5) and S1P3 (Edg-3) G Protein-Coupled Receptors for Sphingosine 1-Phosphate. J. Cell. Biochem. 2003, 88, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Kamocki, K.; Poirier, C.; Pewzner-Jung, Y.; Laviad, E.L.; Schweitzer, K.S.; Van Demark, M.; Justice, M.J.; Hubbard, W.C.; Futerman, A.H. Ceramide Synthases Expression and Role of Ceramide Synthase-2 in the Lung: Insight from Human Lung Cells and Mouse Models. PLoS ONE 2013, 8, e62968. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Öhman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 Haploinsufficiency Inhibits β-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Bartoccini, E.; Marini, F.; Damaskopoulou, E.; Lazzarini, R.; Cataldi, S.; Cascianelli, G.; Garcia, M.G.; Albi, E. Nuclear Lipid Microdomains Regulate Nuclear Vitamin D3 Uptake and Influence Embryonic Hippocampal Cell Differentiation. Mol. Biol. Cell 2011, 22, 3022–3031. [Google Scholar] [CrossRef]
- Lazzarini, A.; Macchiarulo, A.; Floridi, A.; Coletti, A.; Cataldi, S.; Codini, M.; Lazzarini, R.; Bartoccini, E.; Cascianelli, G.; Ambesi-Impiombato, F.S.; et al. Very Long Chain Fatty Acid Sphingomyelin in Nuclear Lipid Microdomains of Hepatocytes and Hepatoma Cells: Can the Exchange from C24:0 To C16:0 Affect Signal Proteins and Vitamin D Receptor? Mol. Biol. Cell 2015, 1, 2418–2425. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.H.; Song, J.H.; Chang, Y.T.; Hwang, S.Y.; Kim, T.S. Enhancing Effects of Ceramide Derivatives on 1,25-Dihydroxyvitamin D3-Induced Differentiation of Human HL-60 Leukemia Cells. Life Sci. 2007, 81, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Xie, L.; Wang, Z.; Zhang, L.; Wu, H.; Ni, W.; Li, C.; Li, L.; Zeng, Y. Association between Ceramides and Coronary Artery Stenosis in Patients with Coronary Artery Disease. Lipids Health Dis. 2020, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.P.; Tan, S.H.; Ow, G.S.; Tang, Z.; Ching, J.; Kovalik, J.P.; Poh, S.C.; Chin, C.T.; Richards, A.M.; Martinez, E.C.; et al. Plasma Ceramides as Prognostic Biomarkers and Their Arterial and Myocardial Tissue Correlates in Acute Myocardial Infarction. JACC Basic Transl. Sci. 2018, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Pingitore, A.; Vassalle, C. Plasma Ceramides Pathophysiology, Measurements, Challenges, and Opportunities. Metabolites 2021, 11, 719. [Google Scholar] [CrossRef]
- Ponnaiah, M.; Zakiev, E.; Lhomme, M.; Rached, F.; Camont, L.; Serrano, C.V.; Santos, R.D.; Chapman, M.J.; Orekhov, A.; Kontush, A. Acute Myocardial Infarction Preferentially Alters Low-Abundant, Long-Chain Unsaturated Phospholipid and Sphingolipid Species in Plasma High-Density Lipoprotein Subpopulations. Atheroscler. Plus 2024, 55, 21–30. [Google Scholar] [CrossRef]

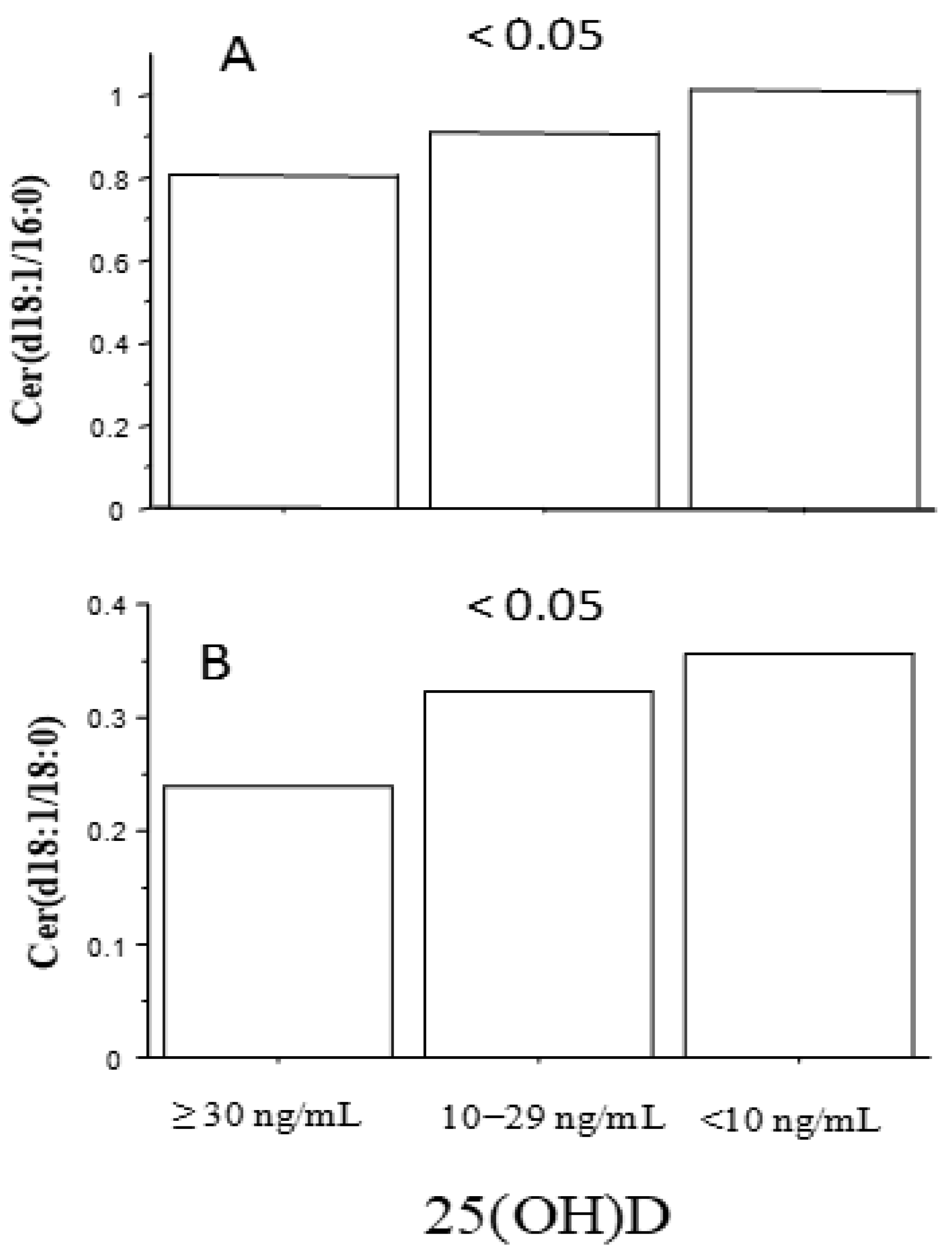
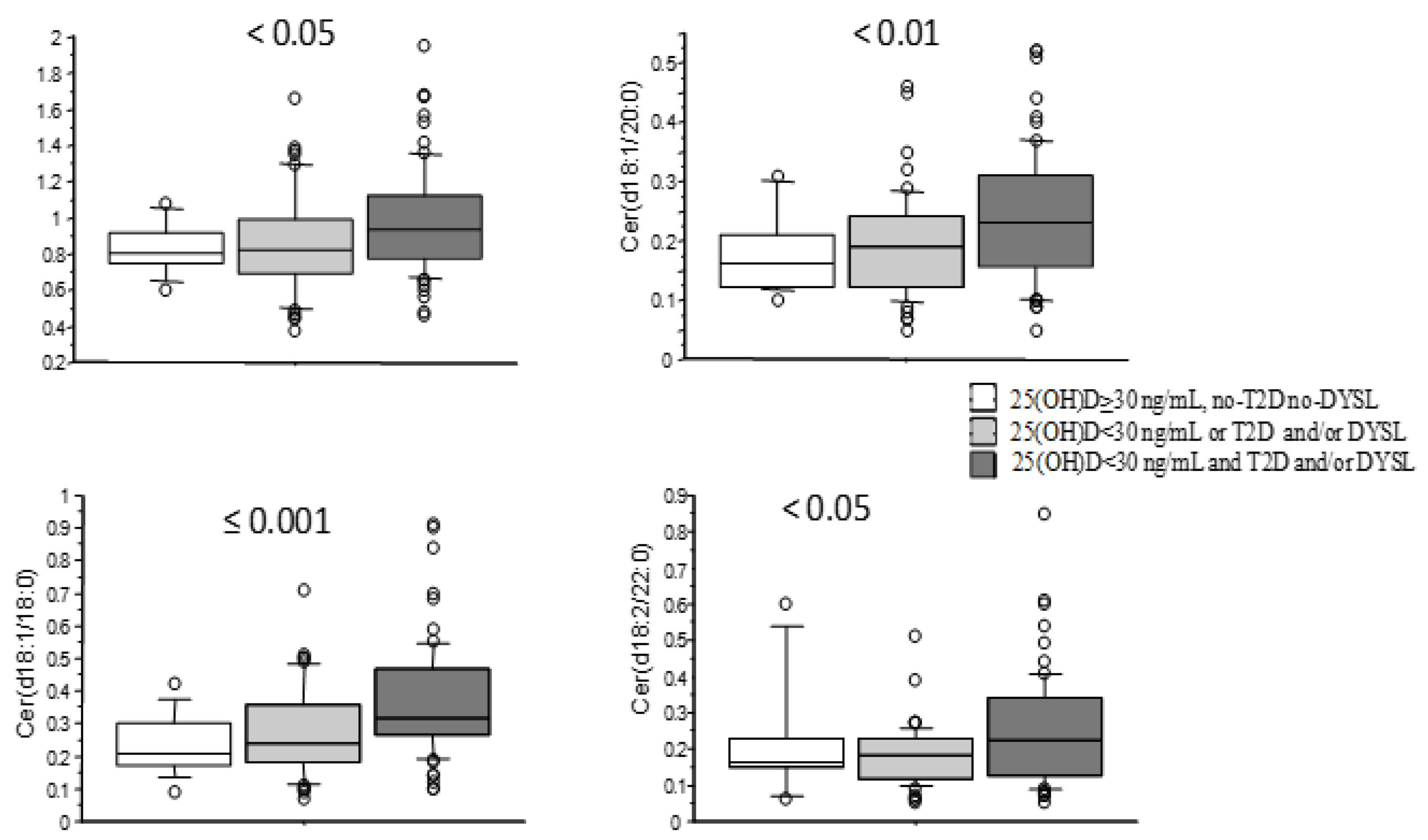
| Number | 134 |
|---|---|
| Age (>50th percentile, 69 y) | 59 (44) |
| Females | 37 (28) |
| T2D or dyslipidemia | 77 (58) |
| Hypertension | 82 (62) |
| Smoking history (current/ex-smokers) | 60 (45) |
| Overweight/obesity (BMI > 25 kg/m2) | 64 (48) |
| 25(OH)D | ||
|---|---|---|
| r (Correlation Coefficient) | p Value (Significance) | |
| Cer(d18:1/16:0) | 0.18 | <0.05 |
| Cer(d18:1/18:0) | 0.2 | <0.05 |
| Cer(d18:1/20:0) | 0.13 | ns |
| Cer(d18:1/22:0) | 0.11 | ns |
| Cer(d18:1/23:0) | 0.05 | ns |
| Cer(d18:1/24:0) | 0.01 | ns |
| Cer(d18:1/24:1) | 0.01 | ns |
| Cer(d18:1/25:0) | 0.01 | ns |
| Cer(d18:2/22:0) | 0.05 | ns |
| Variable | STD Coeff | t-Value | p-Value (Significance) |
|---|---|---|---|
| Age (yrs) | 0.19 | 1.8 | ns |
| Male gender | −0.09 | −1 | ns |
| T2D | −0.08 | −0.9 | ns |
| DYSL | 0.19 | 2.2 | <0.05 |
| Hypertension | 0.08 | 0.9 | ns |
| Smoking history | 0.1 | 1 | ns |
| Overweight/obesity | −0.12 | −1.4 | ns |
| 25(OH9D | −0.18 | −2 | ≤0.05 |
| Variable | STD Coeff | t-Value | p-Value (Significance) |
|---|---|---|---|
| Age (yrs) | −0.01 | −0.13 | ns |
| Male gender | −0.17 | −2 | ≤0.05 |
| T2D | 0.03 | −0.3 | ns |
| DYSL | 0.23 | 2.6 | <0.01 |
| Hypertension | 0.1 | 1.1 | ns |
| Smoking history | 0.04 | 0.4 | ns |
| Overweight/obesity | −0.07 | −0.8 | ns |
| 25(OH9D | −0.2 | −2.2 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaggini, M.; Marchi, F.; Pylypiv, N.; Parlanti, A.; Storti, S.; Paradossi, U.; Berti, S.; Vassalle, C. Vitamin D and Ceramide Metabolomic Profile in Acute Myocardial Infarction. Metabolites 2024, 14, 233. https://doi.org/10.3390/metabo14040233
Gaggini M, Marchi F, Pylypiv N, Parlanti A, Storti S, Paradossi U, Berti S, Vassalle C. Vitamin D and Ceramide Metabolomic Profile in Acute Myocardial Infarction. Metabolites. 2024; 14(4):233. https://doi.org/10.3390/metabo14040233
Chicago/Turabian StyleGaggini, Melania, Federica Marchi, Nataliya Pylypiv, Alessandra Parlanti, Simona Storti, Umberto Paradossi, Sergio Berti, and Cristina Vassalle. 2024. "Vitamin D and Ceramide Metabolomic Profile in Acute Myocardial Infarction" Metabolites 14, no. 4: 233. https://doi.org/10.3390/metabo14040233





