Urinary Metabolomic Differentiation of Infants Fed on Human Breastmilk and Formulated Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Extraction for Instrumental Analysis
2.3. Instrumental Analysis
2.4. Statistical Analysis
3. Results
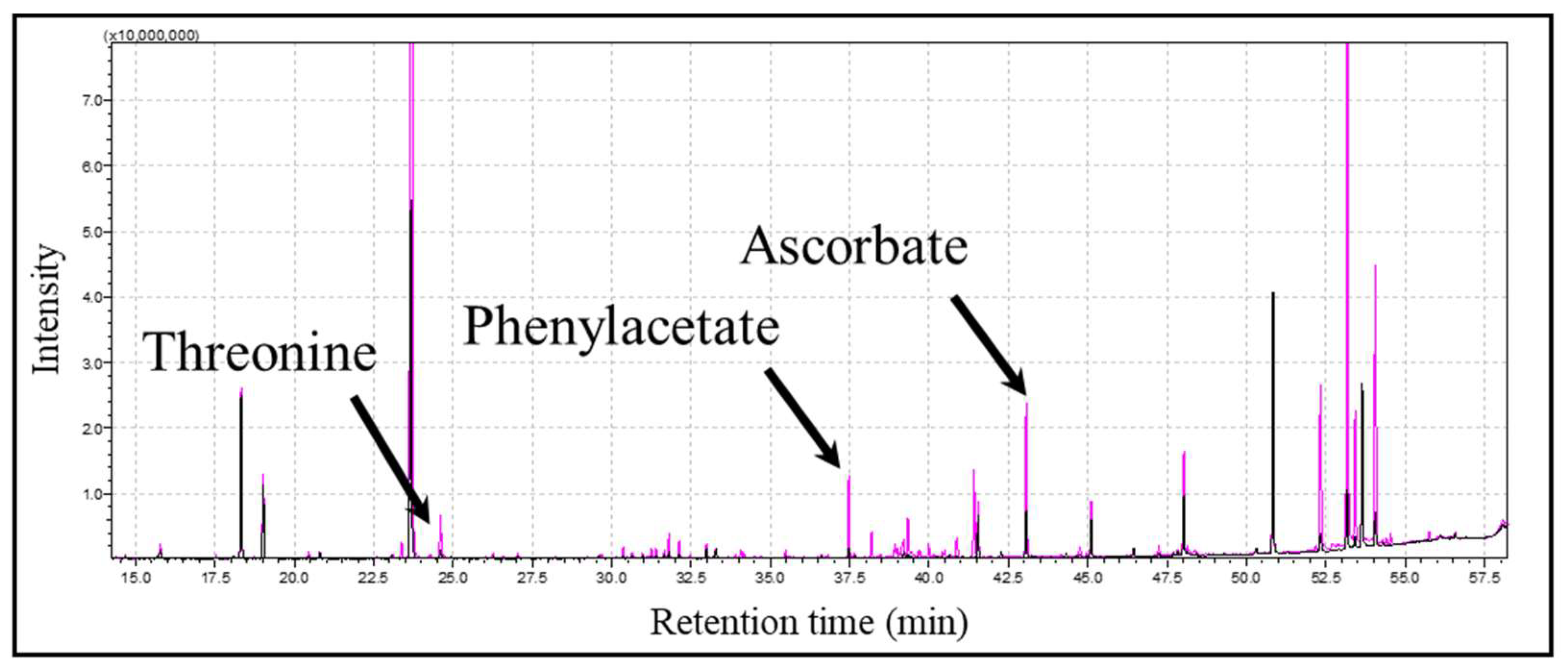
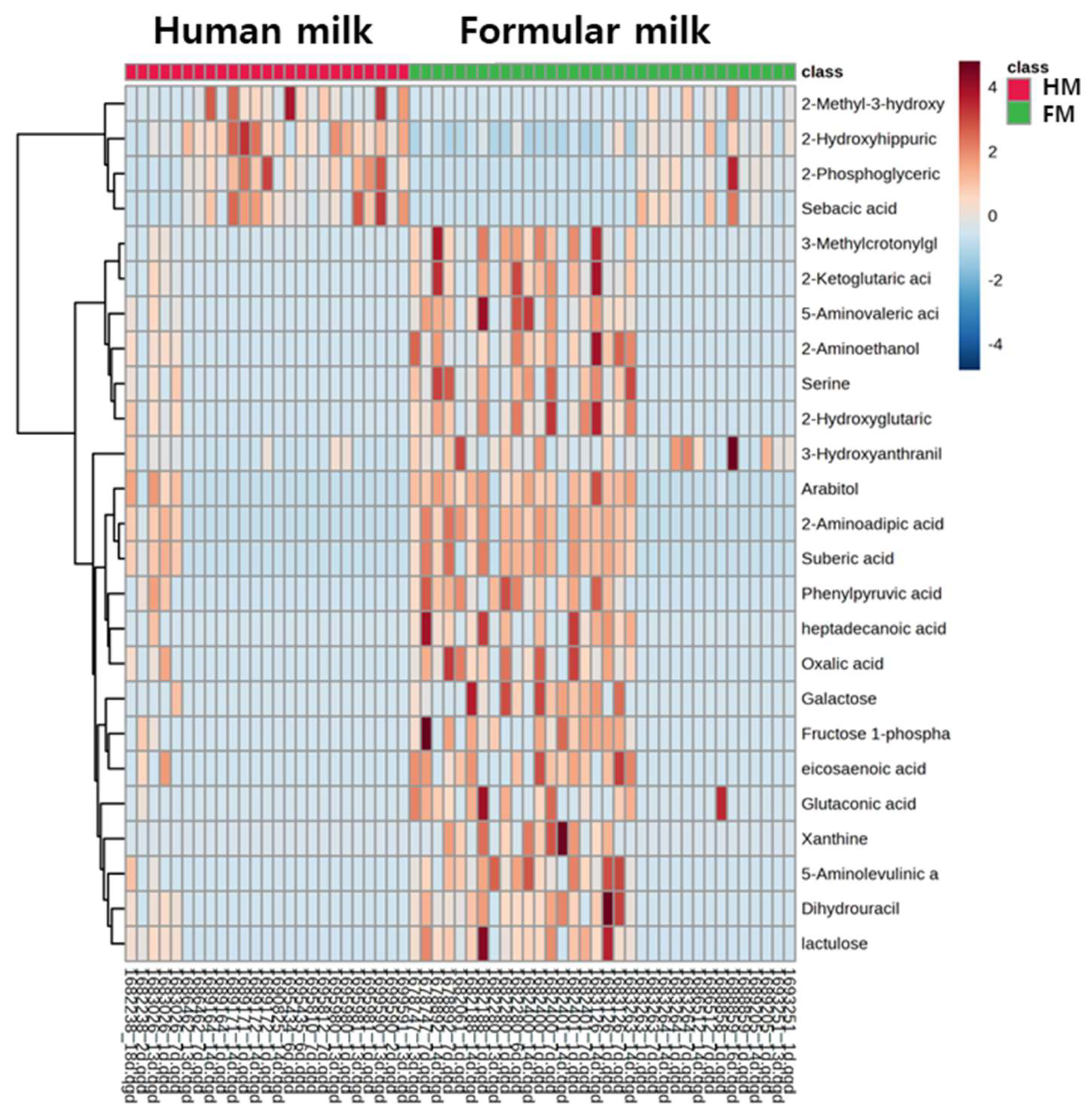
3.1. Untargeted Metabolomics Using GC-MS
3.2. Targeted Metabolomics and Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Kim, K.-U.; Kim, W.-H.; Jeong, C.H.; Yi, D.Y.; Min, H. More than nutrition: Therapeutic potential of breast milk-derived exosomes in cancer. Int. J. Mol. Sci. 2020, 21, 7327. [Google Scholar] [CrossRef]
- Sánchez, C.; Franco, L.; Regal, P.; Lamas, A.; Cepeda, A.; Fente, C. Breast milk: A source of functional compounds with potential application in nutrition and therapy. Nutrients 2021, 13, 1026. [Google Scholar] [CrossRef]
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive compounds in infant formula and their effects on infant nutrition and health: A systematic literature review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef] [PubMed]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Martín, M.; Arboleya, S.; Gueimonde, M.; González, S. Nutritional composition of processed baby foods targeted at infants from 0–12 months. J. Food Compos. Anal. 2019, 79, 55–62. [Google Scholar] [CrossRef]
- Viriato, R.L.S.; Queiros, M.d.S.; Macedo, G.A.; Ribeiro, A.P.B.; Gigante, M.L. Design of new lipids from bovine milk fat for baby nutrition. Crit. Rev. Food Sci. Nutr. 2022, 62, 145–159. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Amaro, F.; Bastos, M.d.L.; Carvalho, M.; Guedes de Pinho, P. Advances and perspectives in prostate cancer biomarker discovery in the last 5 years through tissue and urine metabolomics. Metabolites 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; González-Domínguez, Á.; Sayago, A.; Fernández-Recamales, Á. Recommendations and best practices for standardizing the pre-analytical processing of blood and urine samples in metabolomics. Metabolites 2020, 10, 229. [Google Scholar] [CrossRef]
- Miller, I.J.; Peters, S.R.; Overmyer, K.A.; Paulson, B.R.; Westphall, M.S.; Coon, J.J. Real-time health monitoring through urine metabolomics. NPJ Digit. Med. 2019, 2, 109. [Google Scholar] [CrossRef]
- Khodadadi, M.; Pourfarzam, M. A review of strategies for untargeted urinary metabolomic analysis using gas chromatography–mass spectrometry. Metabolomics 2020, 16, 66. [Google Scholar] [CrossRef]
- Dinges, S.S.; Hohm, A.; Vandergrift, L.A.; Nowak, J.; Habbel, P.; Kaltashov, I.A.; Cheng, L.L. Cancer metabolomic markers in urine: Evidence, techniques and recommendations. Nat. Rev. Urol. 2019, 16, 339–362. [Google Scholar] [CrossRef]
- Khamis, M.M.; Adamko, D.J.; El-Aneed, A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom. Rev. 2017, 36, 115–134. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Fernández-Peralbo, M.; De Castro, M.L. Preparation of urine samples prior to targeted or untargeted metabolomics mass-spectrometry analysis. TrAC Trends Anal. Chem. 2012, 41, 75–85. [Google Scholar] [CrossRef]
- Barbosa, S.; Saurina, J.; Puignou, L.; Núñez, O. Classification and authentication of paprika by UHPLC-HRMS fingerprinting and multivariate calibration methods (PCA and PLS-DA). Foods 2020, 9, 486. [Google Scholar] [CrossRef]
- Walkowiak, A.; Ledziński, Ł.; Zapadka, M.; Kupcewicz, B. Detection of adulterants in dietary supplements with Ginkgo biloba extract by attenuated total reflectance Fourier transform infrared spectroscopy and multivariate methods PLS-DA and PCA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kremer, D.M.; Sajjakulnukit, P.; Zhang, L.; Lyssiotis, C.A. A large-scale analysis of targeted metabolomics data from heterogeneous biological samples provides insights into metabolite dynamics. Metabolomics 2019, 15, 103. [Google Scholar] [CrossRef]
- Gladine, C.; Ostermann, A.I.; Newman, J.W.; Schebb, N.H. MS-based targeted metabolomics of eicosanoids and other oxylipins: Analytical and inter-individual variabilities. Free Radic. Biol. Med. 2019, 144, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, L.; Johnson, M.; Mandal, R.; Wishart, D.S. Comprehensive targeted metabolomic assay for urine analysis. Anal. Chem. 2020, 92, 10627–10634. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; LeVatte, M.A.; Wishart, D.S. A fast and accurate colorimetric assay for quantifying hippuric acid in human urine. Anal. Biochem. 2023, 680, 115303. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, D.; Wang, Y.; Zeng, T.; Peng, Y. Urinary 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, 3-hydroxyphenylacetic acid, and 3-hydroxyhippuric acid are elevated in children with autism spectrum disorders. BioMed Res. Int. 2016, 2016, 9485412. [Google Scholar] [CrossRef]
- Toromanović, J.; Kovač-Bešović, E.; Šapčanin, A.; Tahirović, I.; Rimpapa, Z.; Kroyer, G.; Sofić, E. Urinary hippuric acid after ingestion of edible fruits. Bosn. J. Basic Med. Sci. 2008, 8, 38. [Google Scholar] [CrossRef]
- Krupp, D.; Doberstein, N.; Shi, L.; Remer, T. Hippuric acid in 24-hour urine collections is a potential biomarker for fruit and vegetable consumption in healthy children and adolescents. J. Nutr. 2012, 142, 1314–1320. [Google Scholar] [CrossRef]
- Penczynski, K.J.; Krupp, D.; Bring, A.; Bolzenius, K.; Remer, T.; Buyken, A.E. Relative validation of 24-h urinary hippuric acid excretion as a biomarker for dietary flavonoid intake from fruit and vegetables in healthy adolescents. Eur. J. Nutr. 2017, 56, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chan, Y.-T.; Lo, K.K.-H.; Wong, V.W.-S.; Ng, Y.-F.; Li, S.-Y.; Ho, W.-W.; Wong, M.-S.; Zhao, D. Levels of polyphenols and phenolic metabolites in breast milk and their association with plant-based food intake in Hong Kong lactating women. Food Funct. 2021, 12, 12683–12695. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, H.; Martineau, R.; Hanigan, M.; Van Lingen, H.; Kebreab, E.; Spek, J.; Ouellet, D. Impact of protein and energy supply on the fate of amino acids from absorption to milk protein in dairy cows. Animal 2020, 14, s87–s102. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.-W.; Song, M.-H.; Lee, J.-H.; Song, J.-H.; Hahn, W.-H.; Keum, Y.-S.; Kang, N.M. Urinary Metabolomic Differentiation of Infants Fed on Human Breastmilk and Formulated Milk. Metabolites 2024, 14, 128. https://doi.org/10.3390/metabo14020128
Yu J-W, Song M-H, Lee J-H, Song J-H, Hahn W-H, Keum Y-S, Kang NM. Urinary Metabolomic Differentiation of Infants Fed on Human Breastmilk and Formulated Milk. Metabolites. 2024; 14(2):128. https://doi.org/10.3390/metabo14020128
Chicago/Turabian StyleYu, Ji-Woo, Min-Ho Song, Ji-Ho Lee, Jun-Hwan Song, Won-Ho Hahn, Young-Soo Keum, and Nam Mi Kang. 2024. "Urinary Metabolomic Differentiation of Infants Fed on Human Breastmilk and Formulated Milk" Metabolites 14, no. 2: 128. https://doi.org/10.3390/metabo14020128





