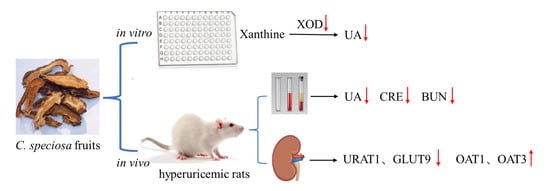
Anti-Hyperuricemic Effects of Extracts from Chaenomeles speciosa (Sweet) Nakai Fruits on Hyperuricemic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of C. speciosa Fruits Extracts
2.3. Inhibitory Assays of C. speciosa Fruits Extracts on XOD Activity In Vitro
2.4. Animals in Experimental Design
2.4.1. Animal Ethics and Preparation
2.4.2. Acute Oral Toxicity Assessment
2.4.3. Anti-Hyperuricemia Activity of CSFTE in the Rat Model
2.5. Biochemical Assay
2.6. Western Blot
2.7. H&E Staining
2.8. Statistical Analysis
3. Results
3.1. Inhibitory Properties of C. speciosa Fruits Extracts on XOD Activity In Vitro
3.2. Acute Oral Toxicity Effects of CSFTE
3.3. Effects of CSFTE on Biochemical Parameters
3.4. CSFTE Down-Regulated Renal Protein Levels of URAT1 and GLUT9 and Up-Regulated Renal Protein Levels of OAT1 and OAT3 in Hyperuricemic Rats
3.5. Effects of CSFTE on Improving Renal Dysfunction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, B.; Ren, P.; Yang, R.; Gao, Y.; Tang, Q.; Xue, C.; Wang, Y. Ameliorative effect of mannuronate oligosaccharides on hyperuricemic mice via promoting uric acid excretion and modulating gut microbiota. Nutrients 2023, 15, 417. [Google Scholar] [CrossRef]
- Boffetta, P.; Nordenvall, C.; Nyrén, O.; Ye, W. A prospective study of gout and cancer. Eur. J. Cancer Prev. 2009, 18, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Danve, A.; Sehra, S.T.; Neogi, T. Role of diet in hyperuricemia and gout. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101723. [Google Scholar] [CrossRef]
- Huang, J.; Ma, Z.F.; Zhang, Y.; Wan, Z.; Li, Y.; Zhou, H.; Chu, A.; Lee, Y.Y. Geographical distribution of hyperuricemia in mainland China: A comprehensive systematic review and meta-analysis. Glob. Health Res. Policy 2020, 5, 52. [Google Scholar] [CrossRef]
- Wang, R.; Halimulati, M.; Huang, X.; Ma, Y.; Li, L.; Zhang, Z. Sulforaphane-driven reprogramming of gut microbiome and metabolome ameliorates the progression of hyperuricemia. J. Adv. Res. 2023, 52, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lin, G.; Yu, Q.; Li, Q.; Mai, L.; Cheng, J.; Xie, J.; Liu, Y.; Su, Z.; Li, Y. Anti-hyperuricemic and nephroprotective effects of dihydroberberine in potassium oxonate- and hypoxanthine-induced hyperuricemic mice. Front. Pharmacol. 2021, 12, 645879. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Schumacher, H.R., Jr.; Wortmann, R.L.; MacDonald, P.A.; Eustace, D.; Palo, W.A.; Streit, J.; Joseph-Ridge, N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N. Engl. J. Med. 2005, 353, 2450–2461. [Google Scholar] [CrossRef]
- Strilchuk, L.; Fogacci, F.; Cicero, A.F. Safety and tolerability of available urate-lowering drugs: A critical review. Expert Opin. Drug Saf. 2019, 18, 261–271. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, N.; Chen, J. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed. Pharmacother. 2016, 83, 975–988. [Google Scholar] [CrossRef]
- Hagos, Y.; Stein, D.; Ugele, B.; Burckhardt, G.; Bahn, A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J. Am. Soc. Nephrol. 2007, 18, 430–439. [Google Scholar] [CrossRef]
- Uchino, H.; Tamai, I.; Yamashita, K.; Minemoto, Y.; Sai, Y.; Yabuuchi, H.; Miyamoto, K.; Takeda, E.; Tsuji, A. P-aminohippuric acid transport at renal apical membrane mediated by human inorganic phosphate transporter NPT1. Biochem. Biophys. Res. Commun. 2000, 270, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Van Aubel, R.A.; Smeets, P.H.; van den Heuvel, J.J.; Russel, F.G. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am. J. Physiol. Renal. Physiol. 2005, 288, F327–F333. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, L.; Yang, T.; Xiong, C.; Xu, L.; Shi, Y.; Bao, W.; Chin, Y.E.; Cheng, S.B.; Yan, H.; et al. EGF receptor inhibition alleviates hyperuricemic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 2716–2729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhan, S.; Li, S.; Zhu, Z.; He, J.; Lorenzo, J.M.; Barba, F.J. Anti-hyperuricemic and nephroprotective effects of extracts from Chaenomeles sinensis (Thouin) Koehne in hyperuricemic mice. Food Funct. 2018, 9, 5778–5790. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, J.; Li, H.; Zhong, P.X.; Xu, Y.J.; Li, C.H.; Ji, K.X.; Wang, L.S. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef]
- Tian, S.; Guo, H.; Zhang, M.; Yan, H.; Wang, X.; Zhao, H. Rapid authentication of Chaenomeles species by visual volatile components fingerprints based on headspace gas chromatography-ion mobility spectrometry combined with chemometric analysis. Phytochem. Anal. 2022, 33, 1198–1204. [Google Scholar] [CrossRef]
- Miao, J.; Zhao, C.; Li, X.; Chen, X.; Mao, X.; Huang, H.; Wang, T.; Gao, W. Chemical composition and bioactivities of two common Chaenomeles fruits in China: Chaenomeles speciosa and Chaenomeles sinensis. J. Food Sci. 2016, 81, H2049–H2058. [Google Scholar] [CrossRef]
- Chinese Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China, 11th ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 62–63. [Google Scholar]
- Wang, Z.J.; Jin, D.N.; Zhou, Y.; Sang, X.Y.; Zhu, Y.Y.; He, Y.J.; Xie, T.Z.; Dai, Z.; Zhao, Y.L.; Luo, X.D. Bioactivity ingredients of Chaenomeles speciosa against microbes: Characterization by LC-MS and activity evaluation. J. Agric. Food Chem. 2021, 69, 4686–4696. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Han, L.Y.; Zhang, H.; Xin, H.L. Chaenomeles speciosa: A review of chemistry and pharmacology. Biomed. Rep. 2014, 2, 12–18. [Google Scholar] [CrossRef]
- Huang, W.; He, J.; Nisar, M.F.; Li, H.; Wan, C. Phytochemical and pharmacological properties of Chaenomeles speciosa: An edible medicinal Chinese Mugua. Evid. Based Complement. Alternat. Med. 2018, 2018, 9591845. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, H.; Zhang, P.; Gao, L.; Yan, N.; Li, P.; Liu, X.; Du, Y.; Shen, G. Chemical composition, antioxidant activity and α-glucosidase inhibitory activity of Chaenomeles Speciosa from four production areas in China. Molecules 2018, 23, 2518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wei, W. Effects and mechanisms of glucosides of Chaenomeles speciosa on collagen-induced arthritis in rats. Int. Immunopharmacol. 2003, 3, 593–608. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.R.; de Paula, C.A.; Pereira de Resende, M.L.; Grabe-Guimarães, A.; de Souza Filho, J.D.; Saúde-Guimarães, D.A. Pharmacological basis for use of Lychnophora trichocarpha in gouty arthritis: Anti-hyperuricemic and anti-inflammatory effects of its extract, fraction and constituents. J. Ethnopharmacol. 2012, 142, 845–850. [Google Scholar] [CrossRef]
- Hall, I.H.; Scoville, J.P.; Reynolds, D.J.; Simlot, R.; Duncan, P. Substituted cyclic imides as potential anti-gout agents. Life Sci. 1990, 46, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, A.; Shaari, M.R.; Ahmad Sayuti, N.S.; Reduan, M.F.H.; Sithambaram, S.; Noordin, M.M.; Shaari, K.; Hamzah, H. Subacute oral administration of Clinacanthus nutans ethanolic leaf extract induced liver and kidney toxicities in ICR mice. Molecules 2020, 25, 263. [Google Scholar] [CrossRef]
- Liu, L.M.; Cheng, S.F.; Shieh, P.C.; Lee, J.C.; Chen, J.J.; Ho, C.T.; Kuo, S.C.; Kuo, D.H.; Huang, L.J.; Way, T.D. The methanol extract of Euonymus laxiflorus, Rubia lanceolata and Gardenia jasminoides inhibits xanthine oxidase and reduce serum uric acid level in rats. Food Chem. Toxicol. 2014, 70, 179–184. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, M.; Chen, D.; Shuai, O.; Chen, S.; Su, J.; Jiao, C.; Feng, D.; Xie, Y. Actions of water extract from Cordyceps militaris in hyperuricemic mice induced by potassium oxonate combined with hypoxanthine. J. Ethnopharmacol. 2016, 194, 403–411. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, Y.; Tang, Z.; Guo, C.; Li, N.; Huang, W.; Ding, G.; Wang, Z.; Xiao, W.; Yang, Z. Anti-hyperuricemic and nephroprotective effects of rhein in hyperuricemic mice. Planta Med. 2015, 81, 279–285. [Google Scholar] [CrossRef]
- McInnes, G.T.; Lawson, D.H.; Jick, H. Acute adverse reactions attributed to allopurinol in hospitalised patients. Ann. Rheum. Dis. 1981, 40, 245–249. [Google Scholar] [CrossRef]
- Dawson, J.; Quinn, T.; Walters, M. Uric acid reduction: A new paradigm in the management of cardiovascular risk? Curr. Med. Chem. 2007, 14, 1879–1886. [Google Scholar] [CrossRef]
- Iseki, K.; Oshiro, S.; Tozawa, M.; Iseki, C.; Ikemiya, Y.; Takishita, S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens. Res. 2001, 24, 691–697. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, H.Y.; Chou, P. The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. J. Rheumatol. 2000, 27, 1501–1505. [Google Scholar]
- Iseki, K.; Ikemiya, Y.; Inoue, T.; Iseki, C.; Kinjo, K.; Takishita, S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am. J. Kidney Dis. 2004, 44, 642–650. [Google Scholar] [CrossRef]
- Ishizaka, N.; Ishizaka, Y.; Toda, E.; Nagai, R.; Yamakado, M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arter. Thromb. Vasc. Biol. 2005, 25, 1038–1044. [Google Scholar] [CrossRef]
- Anzai, N.; Enomoto, A.; Endou, H. Renal urate handling: Clinical relevance of recent advances. Curr. Rheumatol. Rep. 2005, 7, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Hille, R.; Hall, J.; Basu, P. The mononuclear molybdenum enzymes. Chem. Rev. 2014, 114, 3963–4038. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhou, J.; Wen, Y.; Li, J.; Cheng, C.H. Aesculin possesses potent hypouricemic action in rodents but is devoid of xanthine oxidase/dehydrogenase inhibitory activity. Planta Med. 2002, 68, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Su, J.; Shuai, O.; Jiao, C.; Zuo, D. Hypouricemic effects of Ganoderma applanatum in hyperuricemia mice through OAT1 and GLUT9. Front. Pharmacol. 2017, 8, 996. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Fuchs, T.C.; Henzler, T.; Matheis, K.A.; Herget, T.; Dekant, W.; Hewitt, P.; Mally, A. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology 2010, 277, 49–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, Y.; Hu, X.; Hu, X.; Lv, W.; Lv, D.; Chen, J.; Wu, M.; Song, Q.; Shentu, J. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: A review. J. Ethnopharmacol. 2018, 224, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, L.; Niu, Y.; Zhou, Y.; Lin, H.; Jiang, J.; Kong, X.; Liu, X.; Li, L. Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biol. Pharm. Bull. 2015, 38, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wang, X.; Luo, J.; Liu, Y.; Pang, J.; Zhang, H.; Xu, Z.; Xie, J.; Jiang, X.; Ling, W. Hypouricemic and nephroprotective roles of anthocyanins in hyperuricemic mice. Food Funct. 2019, 10, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mellado, J.; Morales, E.M.; Pacheco-Tena, C.; Burgos-Vargas, R. Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann. Rheum. Dis. 2001, 60, 981–983. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Deng, P.; Ma, Y.; Li, K.; Ren, F.; Li, N. Anti-Hyperuricemic Effects of Extracts from Chaenomeles speciosa (Sweet) Nakai Fruits on Hyperuricemic Rats. Metabolites 2024, 14, 117. https://doi.org/10.3390/metabo14020117
Xu R, Deng P, Ma Y, Li K, Ren F, Li N. Anti-Hyperuricemic Effects of Extracts from Chaenomeles speciosa (Sweet) Nakai Fruits on Hyperuricemic Rats. Metabolites. 2024; 14(2):117. https://doi.org/10.3390/metabo14020117
Chicago/Turabian StyleXu, Ruoling, Peng Deng, Yiren Ma, Kui Li, Fucai Ren, and Ning Li. 2024. "Anti-Hyperuricemic Effects of Extracts from Chaenomeles speciosa (Sweet) Nakai Fruits on Hyperuricemic Rats" Metabolites 14, no. 2: 117. https://doi.org/10.3390/metabo14020117