Resurrection Plants—A Valuable Source of Natural Bioactive Compounds: From Word-of-Mouth to Scientifically Proven Sustainable Use
Abstract
:1. Introduction
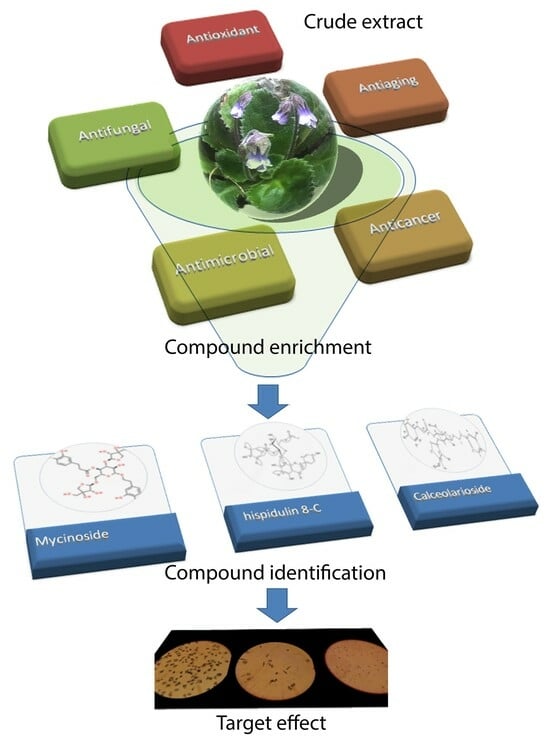
2. Metabolite Profiling and Application of Resurrection Plant Extracts as Bioactive Compounds
3. Potential Mechanisms of Action of the Phenolic Glycoside Myconoside
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bistgani, Z.E.; Barker, A.V.; Hashemi, M. Physiology of Medicinal and Aromatic Plants under Drought Stress. Crop J. 2024. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Gaff, D.F.; Oliver, M. The Evolution of Desiccation Tolerance in Angiosperm Plants: A Rare yet Common Phenomenon. Funct. Plant Biol. 2013, 40, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, C.; Bartels, D. Desiccation Tolerance in Resurrection Plants: New Insights from Transcriptome, Proteome and Metabolome Analysis. Front. Plant Sci. 2013, 4, 482. [Google Scholar] [CrossRef]
- Suguiyama, V.; da Silva, E.; Meirelles, S.; Centeno, D.; Braga, M. Leaf Metabolite Profile of the Brazilian Resurrection Plant Barbacenia purpurea Hook. (Velloziaceae) Shows Two Time-Dependent Responses during Desiccation and Recovering. Front. Plant Sci. 2014, 5, 96. [Google Scholar] [CrossRef]
- Legardón, A.; García-Plazaola, J.I. Gesneriads, a Source of Resurrection and Double-Tolerant Species: Proposal of New Desiccation- and Freezing-Tolerant Plants and Their Physiological Adaptations. Biology 2023, 12, 107. [Google Scholar] [CrossRef]
- Rakic, T.; Lazarevic, M.; Jovanovic, Z.; Radovic, S.; Siljak-Yakovlev, S.; Stevanovic, B.; Stevanovic, V. Resurrection Plants of the Genus Ramonda: Prospective Survival Strategies—Unlock Further Capacity of Adaptation, or Embark on the Path of Evolution? Front. Plant Sci. 2014, 4, 550. [Google Scholar] [CrossRef] [PubMed]
- Okemo, P.A.; Njaci, I.; Kim, Y.-M.; McClure, R.S.; Peterson, M.J.; Beliaev, A.S.; Hixson, K.K.; Mundree, S.; Williams, B. Tripogon loliiformis Tolerates Rapid Desiccation after Metabolic and Transcriptional Priming during Initial Drying. Sci. Rep. 2023, 13, 20613. [Google Scholar] [CrossRef]
- Mihailova, G.; Solti, Á.; Sárvári, É.; Hunyadi-Gulyás, É.; Georgieva, K. Protein Changes in Shade and Sun Haberlea rhodopensis Leaves during Dehydration at Optimal and Low Temperatures. Plants 2023, 12, 401. [Google Scholar] [CrossRef]
- Mihaylova, D.; Bahchevanska, S.; Toneva, V. Examination of the Antioxidant Activity of Haberlea rhodopensis Leaf Extracts and Their Phenolic Constituents. J. Food Biochem. 2013, 37, 255–261. [Google Scholar] [CrossRef]
- Gechev, T.; Lyall, R.; Petrov, V.; Bartels, D. Systems Biology of Resurrection Plants. Cell. Mol. Life Sci. 2021, 78, 6365–6394. [Google Scholar] [CrossRef]
- Gechev, T.S.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Hille, J.; Temanni, M.-R.; Marriott, A.S.; Bergström, E.; et al. Molecular Mechanisms of Desiccation Tolerance in the Resurrection Glacial Relic Haberlea rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709. [Google Scholar] [CrossRef]
- Liu, J.; Moyankova, D.; Lin, C.-T.; Mladenov, P.; Sun, R.-Z.; Djilianov, D.; Deng, X. Transcriptome Reprogramming during Severe Dehydration Contributes to Physiological and Metabolic Changes in the Resurrection Plant Haberlea rhodopensis. BMC Plant Biol. 2018, 18, 351. [Google Scholar] [CrossRef] [PubMed]
- Tebele, S.M.; Marks, R.A.; Farrant, J.M. Two Decades of Desiccation Biology: A Systematic Review of the Best Studied Angiosperm Resurrection Plants. Plants 2021, 10, 2784. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Moyankova, D.; Djilianov, D.; Deng, X. Common and Specific Mechanisms of Desiccation Tolerance in Two Gesneriaceae Resurrection Plants. Multiomics Evidences. Front. Plant Sci. 2019, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, P.; Zasheva, D.; Planchon, S.; Leclercq, C.C.; Falconet, D.; Moyet, L.; Brugière, S.; Moyankova, D.; Tchorbadjieva, M.; Ferro, M.; et al. Proteomics Evidence of a Systemic Response to Desiccation in the Resurrection Plant Haberlea rhodopensis. Int. J. Mol. Sci. 2022, 23, 8520. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, P.; Wang, X.; Yang, Z.; Djilianov, D.; Deng, X. Dynamics of Chromatin Accessibility and Genome Wide Control of Desiccation Tolerance in the Resurrection Plant Haberlea rhodopensis. BMC Plant Biol. 2023, 23, 654. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.M.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation Tolerance: Avoiding Cellular Damage During Drying and Rehydration. Ann. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Blomstedt, C.K.; Griffiths, C.A.; Gaff, D.F.; Hamill, J.D.; Neale, A.D. Plant Desiccation Tolerance and Its Regulation in the Foliage of Resurrection “Flowering-Plant” Species. Agronomy 2018, 8, 146. [Google Scholar] [CrossRef]
- Dace, H.J.W.; Adetunji, A.E.; Moore, J.P.; Farrant, J.M.; Hilhorst, H.W.M. A Review of the Role of Metabolites in Vegetative Desiccation Tolerance of Angiosperms. Curr. Opin. Plant Biol. 2023, 75, 102410. [Google Scholar] [CrossRef] [PubMed]
- Moyankova, D.; Djilianov, D. Time- and Space-Saving Procedure to Obtain Extracts with Antioxidative Properties from Haberlea rhodopensis. C. R. L’académie Bulg. Sci. 2016, 69, 879–884. [Google Scholar]
- Georgiev, Y.N.; Ognyanov, M.H.; Denev, P.N. The Ancient Thracian Endemic Plant Haberlea rhodopensis Friv. and Related Species: A Review. J. Ethnopharmacol. 2020, 249, 112359. [Google Scholar] [CrossRef] [PubMed]
- Rigat, M.; Bonet, M.À.; Garcia, S.; Garnatje, T.; Vallès, J. Studies on Pharmaceutical Ethnobotany in the High River Ter Valley (Pyrenees, Catalonia, Iberian Peninsula). J. Ethnopharmacol. 2007, 113, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Agelet, A.; Vallès, J. Studies on Pharmaceutical Ethnobotany in the Region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or Very Rare Uses of Previously Known Medicinal Plants. J. Ethnopharmacol. 2003, 84, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Nantapo, C.W.T.; Marume, U. Exploring the Potential of Myrothamnus flabellifolius Welw. (Resurrection Tree) as a Phytogenic Feed Additive in Animal Nutrition. Animals 2022, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Gahamanyi, N.; Munyaneza, E.; Dukuzimana, E.; Tuyiringire, N.; Pan, C.-H.; Komba, E.V.G. Ethnobotany, Ethnopharmacology, and Phytochemistry of Medicinal Plants Used for Treating Human Diarrheal Cases in Rwanda: A Review. Antibiotics 2021, 10, 1231. [Google Scholar] [CrossRef]
- Erhabor, J.O.; Komakech, R.; Kang, Y.; Tang, M.; Matsabisa, M.G. Ethnopharmacological Importance and Medical Applications of Myrothamnus flabellifolius Welw. (Myrothamnaceae)—A Review. J. Ethnopharmacol. 2020, 252, 112576. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J.; Woerdenbag, H.J.; Benina, M.; Mehterov, N.; Toneva, V.; Fernie, A.R.; Mueller-Roeber, B. Natural Products from Resurrection Plants: Potential for Medical Applications. Biotechnol. Adv. 2014, 32, 1091–1101. [Google Scholar] [CrossRef]
- Peters, S.; Mundree, S.G.; Thomson, J.A.; Farrant, J.M.; Keller, F. Protection Mechanisms in the Resurrection Plant Xerophyta viscosa (Baker): Both Sucrose and Raffinose Family Oligosaccharides (RFOs) Accumulate in Leaves in Response to Water Deficit. J. Exp. Bot. 2007, 58, 1947–1956. [Google Scholar] [CrossRef]
- Djilianov, D.; Ende, W.V.D.; Alexieva, V.; Moyankova, D. Sugar Ratios, Glutathione Redox Status and Phenols in the Resurrection Species Haberlea rhodopensis and the Closely Related Non-Resurrection Species Chirita eberhardtii. Plant Biol. 2011, 13, 767–776. [Google Scholar] [CrossRef]
- Ghasempour, H.R.; Gaff, D.F.; Williams, R.P.W.; Gianello, R.D. Contents of Sugars in Leaves of Drying Desiccation Tolerant Flowering Plants, Particularly Grasses. Plant Growth Regul. 1998, 24, 185–191. [Google Scholar] [CrossRef]
- Farrant, J.M.; Cooper, K.; Hilgart, A.; Abdalla, K.O.; Bentley, J.; Thomson, J.A.; Dace, H.J.W.; Peton, N.; Mundree, S.G.; Rafudeen, M.S. A Molecular Physiological Review of Vegetative Desiccation Tolerance in the Resurrection Plant Xerophyta viscosa (Baker). Planta 2015, 242, 407–426. [Google Scholar] [CrossRef]
- Zhang, Q.; Bartels, D. Octulose: A Forgotten Metabolite? J. Exp. Bot. 2017, 68, 5689–5694. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Summers, R.; Kahaka, G. Qualitative and Quantitative Analysis of Phytochemical Compounds in Namibian Myrothamnus flabellifolius. Int. Sci. Technol. J. Namibia 2015, 5, 71–83. [Google Scholar]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Moore, J.P.; Westall, K.L.; Ravenscroft, N.; Farrant, J.M.; Lindsey, G.G.; Brandt, W.F. The Predominant Polyphenol in the Leaves of the Resurrection Plant Myrothamnus flabellifolius, 3,4,5 Tri-O-Galloylquinic Acid, Protects Membranes against Desiccation and Free Radical-Induced Oxidation. Biochem. J. 2004, 385, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Veljovic-Jovanovic, S.; Kukavica, B.; Navari-Izzo, F. Characterization of Polyphenol Oxidase Changes Induced by Desiccation of Ramonda serbica Leaves. Physiol. Plant 2008, 132, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-S.; Li, Y.-J.; Zheng, X.-K.; Wang, Y.-Z.; Su, F.-Y. Chemical Constituents of Boea hygrometrica. Chin. J. Nat. Med. 2011, 9, 406–409. [Google Scholar]
- Moyankova, D.; Mladenov, P.; Berkov, S.; Peshev, D.; Georgieva, D.; Djilianov, D. Metabolic Profiling of the Resurrection Plant Haberlea rhodopensis during Desiccation and Recovery. Physiol. Plant 2014, 152, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.; Battisti, I.; Pantelić, A.; Morina, F.; Arrigoni, G.; Masi, A.; Jovanović, S.V. Desiccation Tolerance in Ramonda serbica Panc.: An Integrative Transcriptomic, Proteomic, Metabolite and Photosynthetic Study. Plants 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Gođevac, D.; Ivanović, S.; Simić, K.; Anđelković, B.; Jovanović, Ž.; Rakić, T. Metabolomics Study of the Desiccation and Recovery Process in the Resurrection Plants Ramonda serbica and R. nathaliae. Phytochem. Anal. 2022, 33, 961–970. [Google Scholar] [CrossRef]
- Passon, M.; Weber, F.; Jung, N.U.; Bartels, D. Profiling of Phenolic Compounds in Desiccation-Tolerant and Non-Desiccation-Tolerant Linderniaceae. Phytochem. Anal. 2021, 32, 521–529. [Google Scholar] [CrossRef]
- Cañigueral, S.; Salvía, M.J.; Vila, R.; Iglesias, J.; Virgili, A.; Parella, T. New Polyphenol Glycosides from Ramonda myconi. J. Nat. Prod. 1996, 59, 419–422. [Google Scholar] [CrossRef]
- Jensen, S.R. Caffeoyl Phenylethanoid Glycosides in Sanango racemosum and in the Gesneriaceae. Phytochemistry 1996, 43, 777–783. [Google Scholar] [CrossRef]
- Ebrahimi, S.N.; Gafner, F.; Dell’Acqua, G.; Schweikert, K.; Hamburger, M. Flavone 8-C-Glycosides from Haberlea rhodopensis Friv. (Gesneriaceae). Helv. Chim. Acta 2011, 94, 38–45. [Google Scholar] [CrossRef]
- Nyalo, P.; Omwenga, G.; Ngugi, M. Quantitative Phytochemical Profile and In Vitro Antioxidant Properties of Ethyl Acetate Extracts of Xerophyta spekei (Baker) and Grewia tembensis (Fresen). J. Evid. Based Complement. Altern. Med. 2023, 28, 2515690X231165096. [Google Scholar] [CrossRef]
- Da Costa, D.J.; Leitão, A.; Faria, R.X.; Anholeti, M.C.; Nunes, M.A.; Oliveira, M.B.P.; Da Costa Santos, W.; De Barros Machado, T. Preliminary Phytochemical Analysis of the Ethanolic Extract of Xerophyta stenophylla Baker. Res. Soc. Dev. 2022, 11, e38211528319. [Google Scholar] [CrossRef]
- Dhillon, J.; Miller, V.; Carter, J.; Badiab, A.; Tang, C.N.; Huynh, A.; Peethambaran, B. Apoptosis-Inducing Potential of Myrothamnus flabellifolius, an Edible Medicinal Plant, on Human Myeloid Leukemia HL-60 Cells. Int. J. Appl. Res. Nat. Prod. 2014, 7, 28–32. [Google Scholar]
- Georgieva, M.; Moyankova, D.; Djilianov, D.; Uzunova, K.; Miloshev, G. Methanol Extracts from the Resurrection Plant Haberlea rhodopensis Ameliorate Cellular Vitality in Chronologically Ageing Saccharomyces Cerevisiae Cells. Biogerontology 2015, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Popov, B.; Georgieva, S.; Oblakova, M.; Bonev, G. Effects of Haberlea rhodopensis Extract on Antioxidation and Lipid Peroxidation in Rabbits after Exposure to 60Co-γ-Rays. Arch. Biol. Sci. 2013, 65, 91–97. [Google Scholar]
- Popov, B.; Radev, R.; Georgieva, S. In Vitro Incidence of Chromosome Aberrations in Gamma-Irradiated Rabbit Lymphocytes, Treated with Haberlea rhodopensis Extract and Vitamin C. Bulg. J. Vet. Med. 2010, 13, 148–153. [Google Scholar]
- Dobreva, Z.G.; Popov, B.N.; Georgieva, S.Y.; Stanilova, S.A. Immunostimulatory Activities of Haberlea rhodopensis Leaf Extract on the Specific Antibody Response: Protective Effects against γ-Radiation-Induced Immunosuppression. Food Agric. Immunol. 2015, 26, 381–393. [Google Scholar] [CrossRef]
- Popov, B.; Georgieva, S.; Gadjeva, V. Modulatory Effects of Total Extract of Haberlea rhodopensis against the Cyclophosphamide Induced Genotoxicity in Rabbit Lymphocytes in Vivo. Trakia J. Sci. 2011, 9, 51–57. [Google Scholar]
- Georgieva, S.; Popov, B.; Bonev, G. Radioprotective Effect of Haberlea rhodopensis (Friv.) Leaf Extract on Gamma-Radiation-Induced DNA Damage, Lipid Peroxidation and Antioxidant Levels in Rabbit Blood. Indian. J. Exp. Biol. 2013, 51, 29–36. [Google Scholar]
- Staneva, D.; Dimitrova, N.; Popov, B.; Alexandrova, A.; Georgieva, M.; Miloshev, G. Haberlea rhodopensis Extract Tunes the Cellular Response to Stress by Modulating DNA Damage, Redox Components, and Gene Expression. Int. J. Mol. Sci. 2023, 24, 15964. [Google Scholar] [CrossRef]
- Kostadinova, A.; Doumanov, J.; Moyankova, D.; Ivanov, S.; Mladenova, K.; Djilianov, D.; Topuzova-Hristova, T. Haberlea rhodopensis Extracts Affect Cell Periphery of Keratinocytes. C. R. L’académie Bulg. Sci. 2016, 69, 439–448. [Google Scholar]
- Moyankova, D.; Hinkov, A.; Shishkov, S.; Djilianov, D. Inhibitory Effect of Extracts from Haberlea rhodopensis Friv. against Herpes Simplex Virus. C. R. L’académie Bulg. Sci. 2014, 76, 1369–1376. [Google Scholar]
- Moyankova, D.; Lyubenova, A.; Slavov, S.; Djilianov, D. Extracts of the Endemic Resurrection Plant Haberlea rhodopensis Stimulate In Vitro Growth of Various Phytophthora Spp. Pathogens. Eur. J. Plant Pathol. 2014, 138, 149–155. [Google Scholar] [CrossRef]
- Hayrabedyan, S.; Todorova, K.; Zasheva, D.; Moyankova, D.; Georgieva, D.; Todorova, J.; Djilianov, D. Haberlea rhodopensis Has Potential as a New Drug Source Based on Its Broad Biological Modalities. Biotechnol. Biotechnol. Equip. 2013, 27, 3553–3560. [Google Scholar]
- Spyridopoulou, K.; Kyriakou, S.; Nomikou, A.; Roupas, A.; Ermogenous, A.; Karamanoli, K.; Moyankova, D.; Djilianov, D.; Galanis, A.; Panayiotidis, M.I.; et al. Chemical Profiling, Antiproliferative and Antimigratory Capacity of Haberlea rhodopensis Extracts in an In Vitro Platform of Various Human Cancer Cell Lines. Antioxidants 2022, 11, 2305. [Google Scholar] [CrossRef]
- Radev, R.; Lazarova, G.; Nedialkov, P.; Sokolova, K.; Rukanova, D.; Tsokeva, Z. Study on Antibacterial Activity of Haberlea rhodopensis. Trakia J. Sci. 2008, 7, 34–36. [Google Scholar]
- Moyankova, D.; Georgieva, D.; Batchvarova, R.; Slavov, S.; Djilianov, D. Effect of Extracts from the Resurrection Plant Haberlea rhodopensis on in Vitro Growth of Plant Pathogens. C. R. L’académie Bulg. Sci. 2013, 66, 1269–1272. [Google Scholar]
- Berkov, S.; Nikolova, M.; Hristozova, N.; Momekov, G.; Ionkova, I.; Djilianov, D. GC-MS Profiling of Bioactive Extracts from Haberlea rhodopensis: An Endemic Resurrection Plant. J. Serbian Chem. Soc. 2011, 76, 211–220. [Google Scholar] [CrossRef]
- Brar, J.; Fultang, N.; Askey, K.; Tettamanzi, M.C.; Peethambaran, B. A Novel Anti-Triple Negative Breast Cancer Compound Isolated from Medicinal Herb Myrothamnus flabellifolius. J. Med. Plants Res. 2018, 12, 7–14. [Google Scholar] [CrossRef]
- Zasheva, D.; Mladenov, P.; Rusanov, K.; Simova, S.; Zapryanova, S.; Simova-Stoilova, L.; Moyankova, D.; Djilianov, D. Fractions of Methanol Extracts from the Resurrection Plant Haberlea rhodopensis Have Anti-Breast Cancer Effects in Model Cell Systems. Separations 2023, 10, 388. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, U.; Mitcheva, M. Cytoprotective and Antioxidant Effects of Phenolic Compounds from Haberlea rhodopensis Friv. (Gesneriaceae). Pharmacogn. Mag. 2013, 9, 294–301. [Google Scholar] [CrossRef]
- Amirova, K.M.; Dimitrova, P.A.; Marchev, A.S.; Krustanova, S.V.; Simova, S.D.; Alipieva, K.I.; Georgiev, M.I. Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils. Int. J. Mol. Sci. 2021, 22, 1759. [Google Scholar] [CrossRef] [PubMed]
- Dell’Acqua, G.; Schweikert, K. Skin Benefits of a Myconoside-Rich Extract from Resurrection Plant Haberlea rhodopensis. Int. J. Cosmet. Sci. 2012, 34, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kamng’ona, A.; Moore, J.P.; Lindsey, G.; Brandt, W. Inhibition of HIV-1 and M-MLV Reverse Transcriptases by a Major Polyphenol (3,4,5 Tri-O-Galloylquinic Acid) Present in the Leaves of the South African Resurrection Plant, Myrothamnus flabellifolia. J. Enzym. Inhib. Med. Chem. 2011, 26, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, A.; Staneva, G.; Topouzova-Hristova, T.; Moyankova, D.; Yordanova, V.; Veleva, R.; Nikolova, B.; Momchilova, A.; Djilianov, D.; Hazarosova, R. Myconoside Affects the Viability of Polarized Epithelial MDCKII Cell Line by Interacting with the Plasma Membrane and the Apical Junctional Complexes. Separations 2022, 9, 239. [Google Scholar] [CrossRef]
- Kostadinova, A.; Hazarosova, R.; Topouzova-Hristova, T.; Moyankova, D.; Yordanova, V.; Veleva, R.; Nikolova, B.; Momchilova, A.; Djilianov, D.; Staneva, G. Myconoside Interacts with the Plasma Membranes and the Actin Cytoskeleton and Provokes Cytotoxicity in Human Lung Adenocarcinoma A549 Cells. J. Bioenerg. Biomembr. 2022, 54, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Fultang, N.; Brar, J.; Mercier, I.; Klase, Z.; Peethambaran, B. Myrothamnus flabellifolius Selectively Targets Triple Negative Breast Cancer in Vitro, Restoring Tamoxifen Sensitivity through Modulation of MiRNAs Associated with Estrogen Receptors. Int. J. Appl. Res. Nat. Prod. 2018, 11, 24–33. [Google Scholar]
- Georgieva, S.; Gencheva, D.; Popov, B.; Grozeva, N.; Zhelyazkova, M. Radioprotective Action of Resurrection Plant Haberlea rhodopensis Friv. (Gesneriaceae) and Role of Flavonoids and Phenolic Acids. Bulg. J. Agric. Sci. 2019, 25, 158–168. [Google Scholar]
- Van Dijk, C.; Driessen, A.J.M.; Recourt, K. The Uncoupling Efficiency and Affinity of Flavonoids for Vesicles. Biochem. Pharmacol. 2000, 60, 1593–1600. [Google Scholar] [CrossRef]
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Muzafarov, E.N.; Kim, Y.A. Rafts Making and Rafts Braking: How Plant Flavonoids May Control Membrane Heterogeneity. Mol. Cell Biochem. 2008, 314, 65–71. [Google Scholar] [CrossRef]
- Pragallapati, S.; Manyam, R. Glucose Transporter 1 in Health and Disease. J. Oral Maxillofac. Pathol. 2019, 23, 443. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, W.; Dang, M.; Deng, X.; Shi, X.; Zhang, Y.; Li, K.; Li, C. Targeting Lipid Rafts as a Rapid Screening Strategy for Potential Antiadipogenic Polyphenols along with the Structure-Activity Relationship and Mechanism Elucidation. J. Agric. Food Chem. 2022, 70, 3872–3885. [Google Scholar] [CrossRef]
- Böhl, M.; Tietze, S.; Sokoll, A.; Madathil, S.; Pfennig, F.; Apostolakis, J.; Fahmy, K.; Gutzeit, H.O. Flavonoids Affect Actin Functions in Cytoplasm and Nucleus. Biophys. J. 2007, 93, 2767–2780. [Google Scholar] [CrossRef]
- Zheng, Y.; Lim, E.J.; Wang, L.; Smart, E.J.; Toborek, M.; Hennig, B. Role of Caveolin-1 in EGCG-Mediated Protection against Linoleic-Acid-Induced Endothelial Cell Activation. J. Nutr. Biochem. 2009, 20, 202–209. [Google Scholar] [CrossRef]
- Li, Y.; Ying, C.; Zuo, X.; Yi, H.; Yi, W.; Meng, Y.; Ikeda, K.; Ye, X.; Yamori, Y.; Sun, X. Green Tea Polyphenols Down-Regulate Caveolin-1 Expression via ERK1/2 and P38MAPK in Endothelial Cells. J. Nutr. Biochem. 2009, 20, 1021–1027. [Google Scholar] [CrossRef]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Djilianov, D.; Genova, G.; Parvanova, D.; Zapryanova, N.; Konstantinova, T.; Atanassov, A. In Vitro Culture of the Resurrection Plant Haberlea rhodopensis. Plant Cell Tiss. Organ. Cult. 2005, 80, 115–118. [Google Scholar] [CrossRef]
- Daskalova, E.; Dontcheva, S.; Zekaj, Z.; Bacu, A.; Sota, V.; Abdullai, K.; Gashi, B.; Minkov, I.; Toneva, V.; Kongjika, E. Initial Determination of Polymorphism and In Vitro Conservation of Some Ramonda serbica and Ramonda nathaliae Populations from Albania, Macedonia and Bulgaria. Biotechnol. Biotechnol. Equip. 2012, 26, 16–25. [Google Scholar] [CrossRef]
- Tóth, S.; Scott, P.; Sorvari, S.; Toldi, O. Effective and Reproducible Protocols for in Vitro Culturing and Plant Regeneration of the Physiological Model Plant Ramonda myconi (L.) Rchb. Plant Sci. 2004, 166, 1027–1034. [Google Scholar] [CrossRef]


| Extract or Compound | Resurrection Species | Biological Effect | Ref |
|---|---|---|---|
| Crude ethanol extracts | Xerophyta spp. | For traditional ethnomedicine; antibacterial activity—S. typhi, B. subtilis, S. aureus, E. coli | [48] |
| Crude ethanol extracts | Xerophyta spp. | Pharmacological application for antioxidant activity | [49] |
| Crude ethanol, methanol and water extracts | Myrothamnus flabellifolius | Source of nutraceuticals | [26] |
| Crude methanol and petroleum ether extracts | Myrothamnus flabellifolius | Methanol extract suppresses human leukemic HL-60, but not non-leukemic TK6 line | [50] |
| Crude methanol extracts | Haberlea rhodopensis | Proliferative, anti-aging, and protective effect on model yeast S. cerevisiae cell line. | [51] |
| Crude ethanol extract | Haberlea rhodopensis | Radioprotective effects | [52,53,54,55,56,57] |
| Crude methanol extracts | Haberlea rhodopensis | Influence on cell periphery, permeabilization of the membrane, and disruption of HaCaT keratinocyte tight junctions. | [58] |
| Crude ethanol, methanol, water extracts, polar/apolar fractions of methanol extracts | Haberlea rhodopensis | Crude methanol extract was the most active in MTT assay modified for HSV. No direct virus inactivating effect. | [59] |
| Crude methanol extracts | Haberlea rhodopensis | Phythophtora spp. isolates were stimulated to grow under in vitro conditions. | [60] |
| Crude methanol extracts | Haberlea rhodopensis | Antioxidative effect in cancer vs. normal cell lines, and differentially modulate distinct cell lines in genotoxic and inflammatory stress. | [61] |
| Crude ethanol and water extracts | Haberlea rhodopensis | The human cancer cell lines A549, HepG2, HT29, and Caco-2 and PC3 and DU145 were treated. Water extracts—no effect. Ethanol extracts—effective to HepG2 and A459 cell lines. | [62] |
| Crude ethanol extracts | Haberlea rhodopensis | Lack of effect on E. coli, S. enterica subsp. enterica, P. aeruginosa, S. aureus, B. subtilis, S. cerevisiae, A. niger, Rhizopus sp., K. pneumonia, L. monocytogenes | [63] |
| Polar/apolar fractions of methanol extracts | Haberlea rhodopensis | The growth of Botrytis cinerea was strongly inhibited, in particular by apolar fractions. Same fraction had stimulating effect on Phytophthora citricola. No effect was found against Alternaria alternata and Fusarium oxysporum. | [64] |
| Polar and apolar fractions of methanol extracts | Haberlea rhodopensis | Polar fractions possessed strong free radical scavenging activity. No effect on HL-60, HL-60/Dox, SKW-3 (KE-37), and MDA-MB-231 | [65] |
| Fractions of methanol extract, novel compound | Myrothamnus flabellifolia | Anti-triple negative breast cancer effect | [66] |
| Fractions rich of myconoside and hispidulin from methanol extracts | Haberlea rhodopensis | Significant influence on the proliferation rate of the hormone receptor expressing MCF7 and the triple negative MDA-MB231 breast cancer cell lines. No significant effects on the benign MCF10A cell line. | [67] |
| Myconoside and hispidulin | Haberlea rhodopensis | Cytoprotective, radical scavenging potential, and lipid peroxidation inhibition in rat hepatocytes. | [68] |
| Myconoside and Calceolarioside E | Haberlea rhodopensis | Increased Nrf2 expression in bone marrow neutrophils. | [69] |
| Myconoside-enriched fraction | Haberlea rhodopensis | Increases skin elasticity. Protection of human dermal fibroblasts against H2O2 damage | [70] |
| 3,4,5 tri-O-galloylquinic acid | Myrothamnus flabellifolius | Inhibition of HIV-1 and M-MLV reverse transcriptases | [71] |
| Myconoside | Haberlea rhodopensis | At low concentrations—increased MDCKII cell viability by enhancing membrane lipid order and adherent junctions. Higher doses—the opposite effect. | [72] |
| Myconoside | Haberlea rhodopensis | Low concentration has no influence on human lung adenocarcinoma A549 cell viability but increases plasma membrane lipid order of the treated cells. Higher concentration inhibits cell viability. | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djilianov, D.; Moyankova, D.; Mladenov, P.; Topouzova-Hristova, T.; Kostadinova, A.; Staneva, G.; Zasheva, D.; Berkov, S.; Simova-Stoilova, L. Resurrection Plants—A Valuable Source of Natural Bioactive Compounds: From Word-of-Mouth to Scientifically Proven Sustainable Use. Metabolites 2024, 14, 113. https://doi.org/10.3390/metabo14020113
Djilianov D, Moyankova D, Mladenov P, Topouzova-Hristova T, Kostadinova A, Staneva G, Zasheva D, Berkov S, Simova-Stoilova L. Resurrection Plants—A Valuable Source of Natural Bioactive Compounds: From Word-of-Mouth to Scientifically Proven Sustainable Use. Metabolites. 2024; 14(2):113. https://doi.org/10.3390/metabo14020113
Chicago/Turabian StyleDjilianov, Dimitar, Daniela Moyankova, Petko Mladenov, Tanya Topouzova-Hristova, Aneliya Kostadinova, Galya Staneva, Diana Zasheva, Strahil Berkov, and Lyudmila Simova-Stoilova. 2024. "Resurrection Plants—A Valuable Source of Natural Bioactive Compounds: From Word-of-Mouth to Scientifically Proven Sustainable Use" Metabolites 14, no. 2: 113. https://doi.org/10.3390/metabo14020113