Metabolomic Reconfiguration in Primed Barley (Hordeum vulgare) Plants in Response to Pyrenophora teres f. teres Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Barley Plant Material and Growth Conditions
2.2. Fungal Isolate, Culture, and Sporulation
2.3. Plant Treatment/Inoculation and Experimental Design
2.4. Metabolite Extraction and Sample Preparation
2.5. Ultra-High Performance Liquid Chromatography–High Definition Mass Spectrometry (UHPLC-HDMS) for Untargeted Metabolomics
2.6. LC-ESI-QqQ-MS System for Targeted Metabolite Analyses
2.7. Data Processing and Data Mining
2.8. Metabolite Annotation and Metabolic Pathway and Network Analyses
3. Results and Discussion
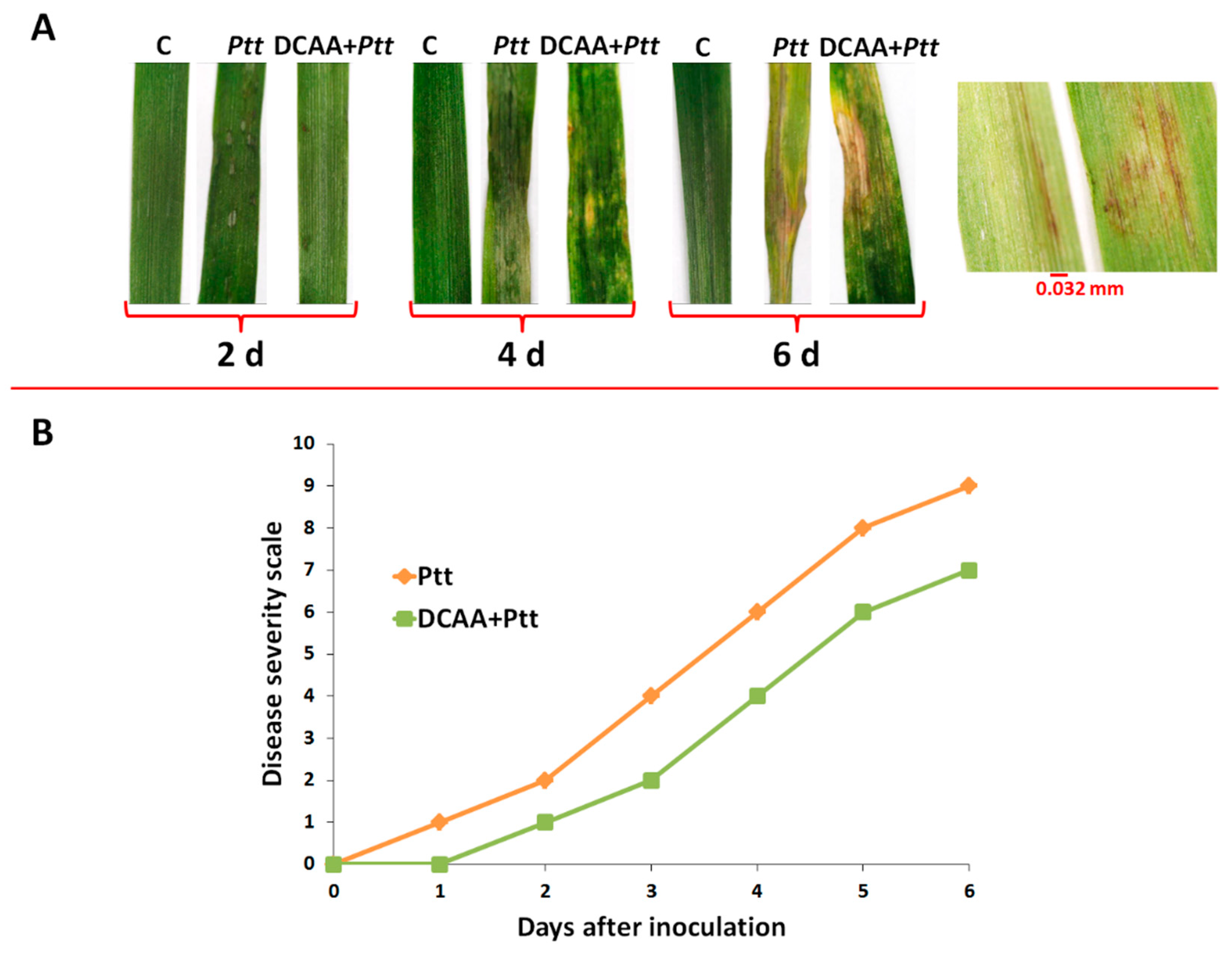
3.1. Evaluation of the Net Blotch Net Form Disease on Barley
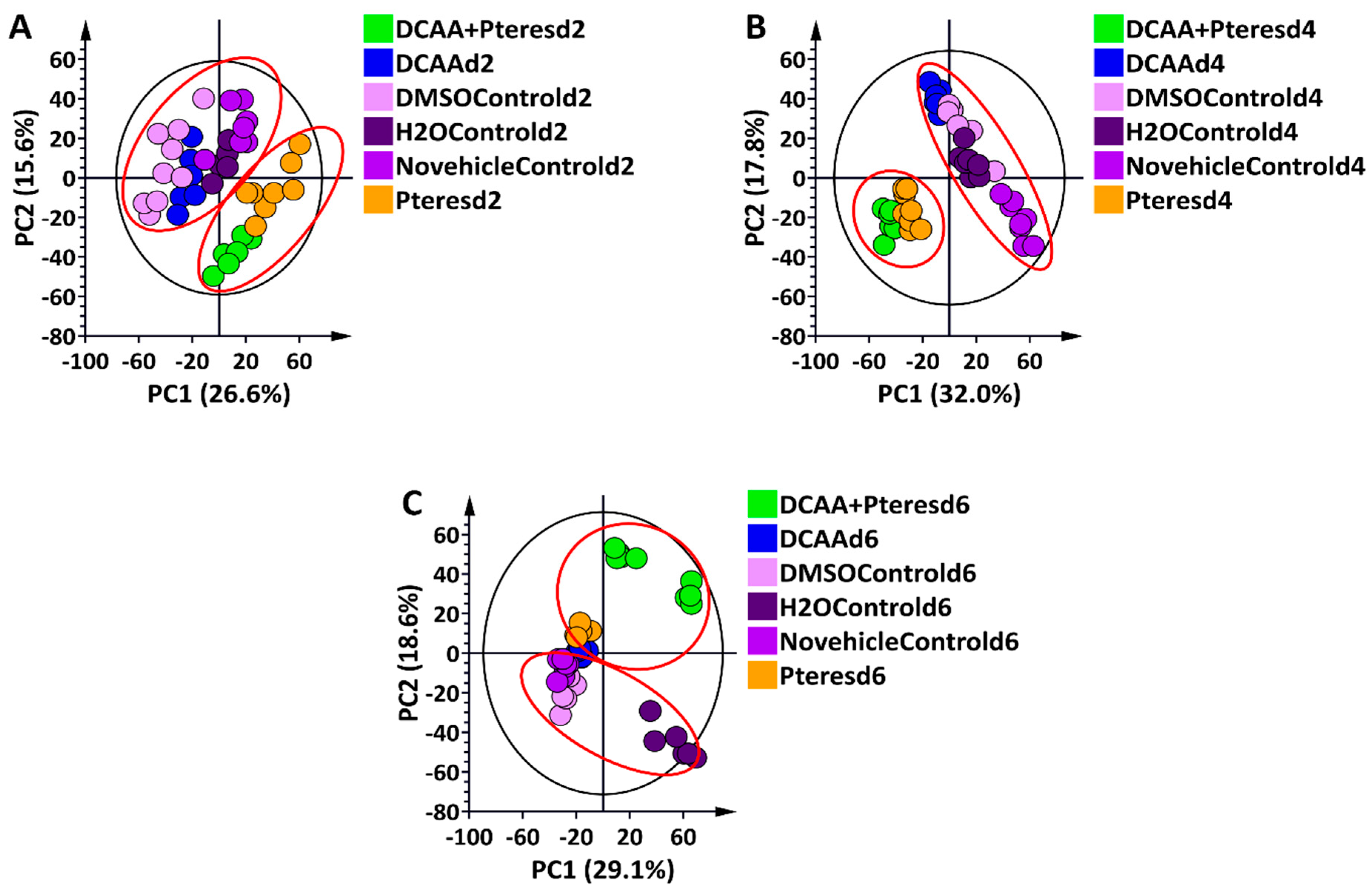
3.2. Chromatographic and Chemometric Profiling of Primed and Naïve Barley Plants Following Infection by the Fungus P. teres f. teres
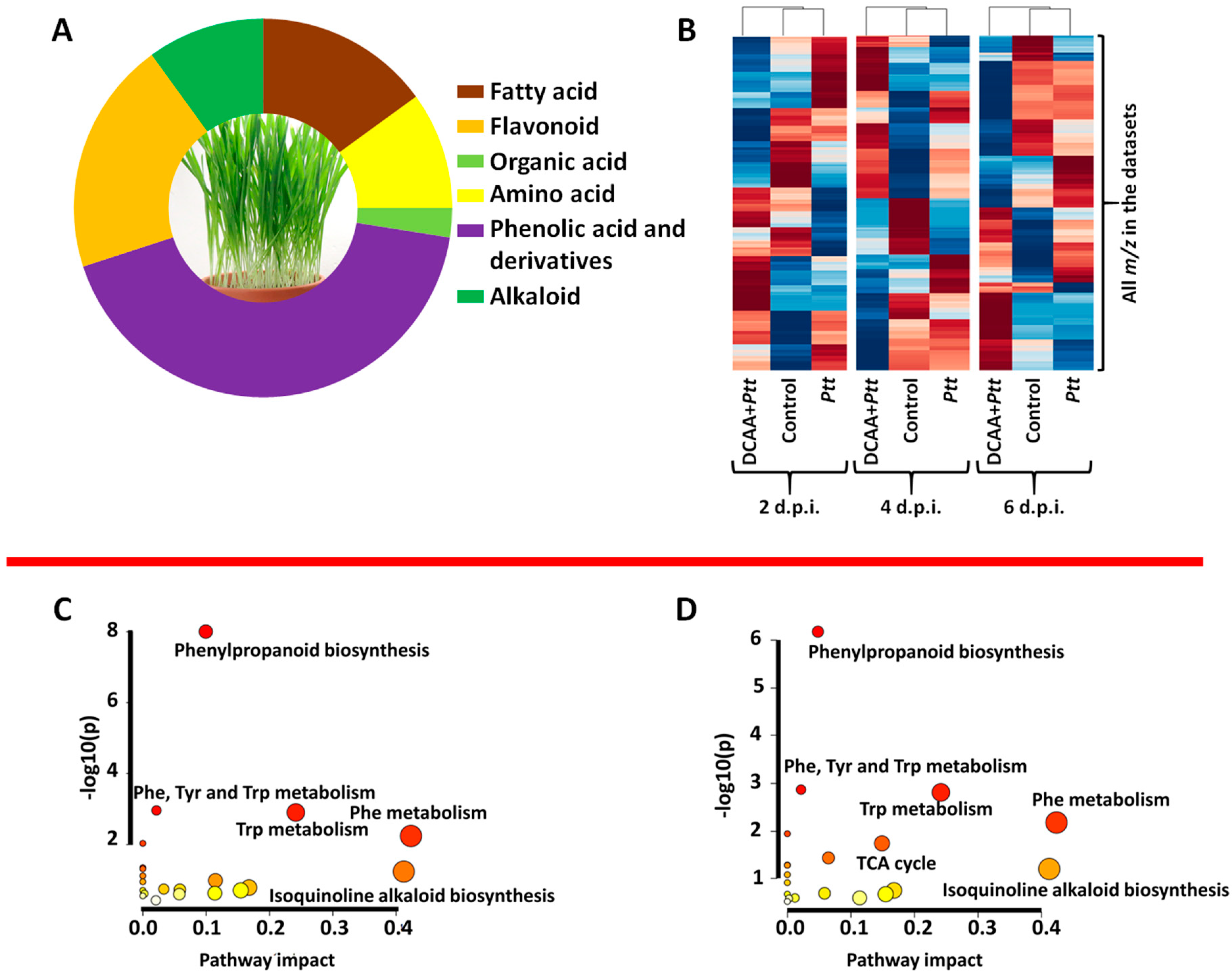
3.3. Defence-Related Metabolic Reprogramming in 3,5-DCAA-Primed and Naïve Barley Plants Following Infection by the Fungus P. teres f. teres
3.3.1. Amino Acids
3.3.2. Tricarboxylates and Fatty Acids
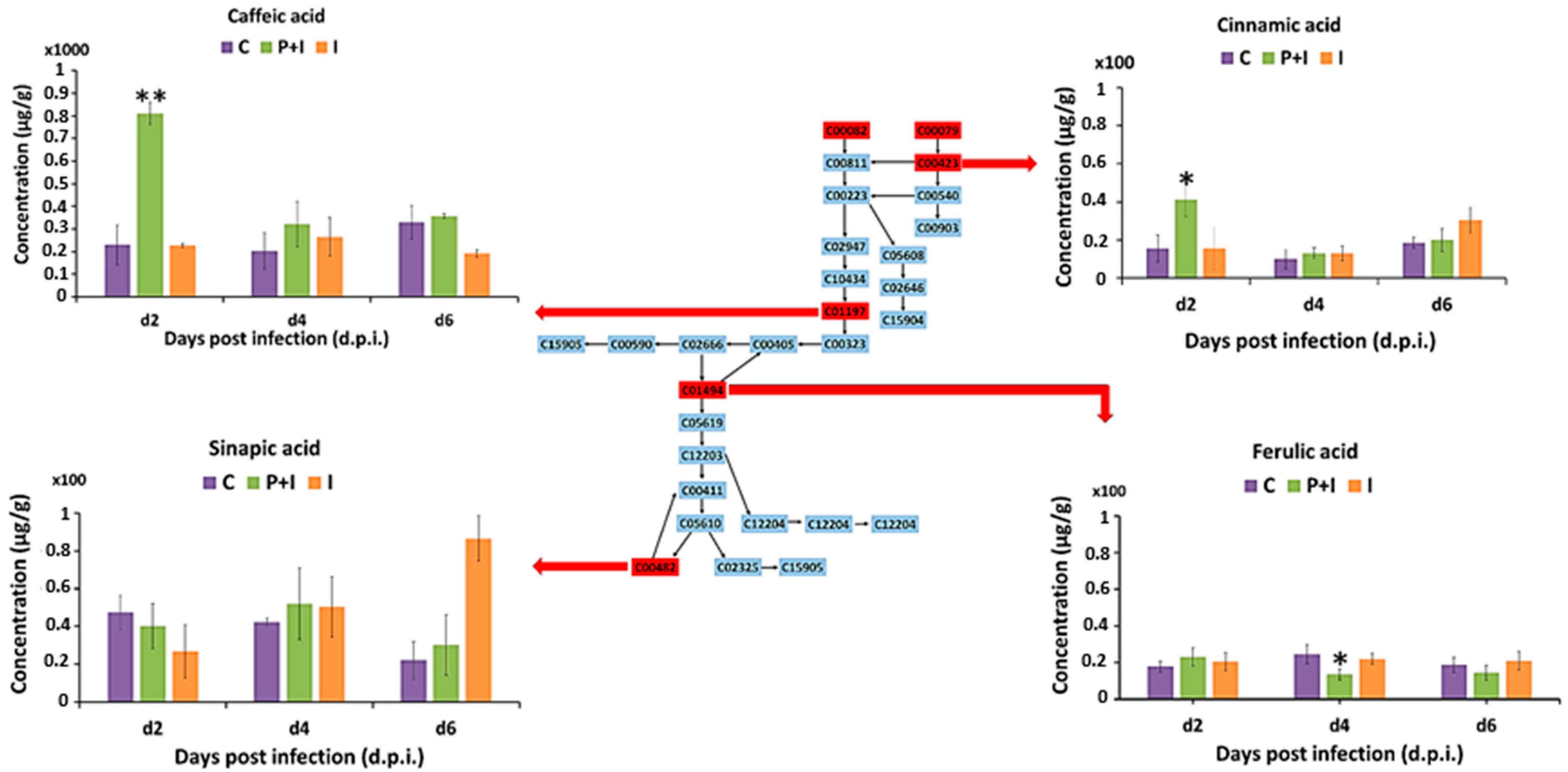
3.3.3. Hydroxycinnamic Acids
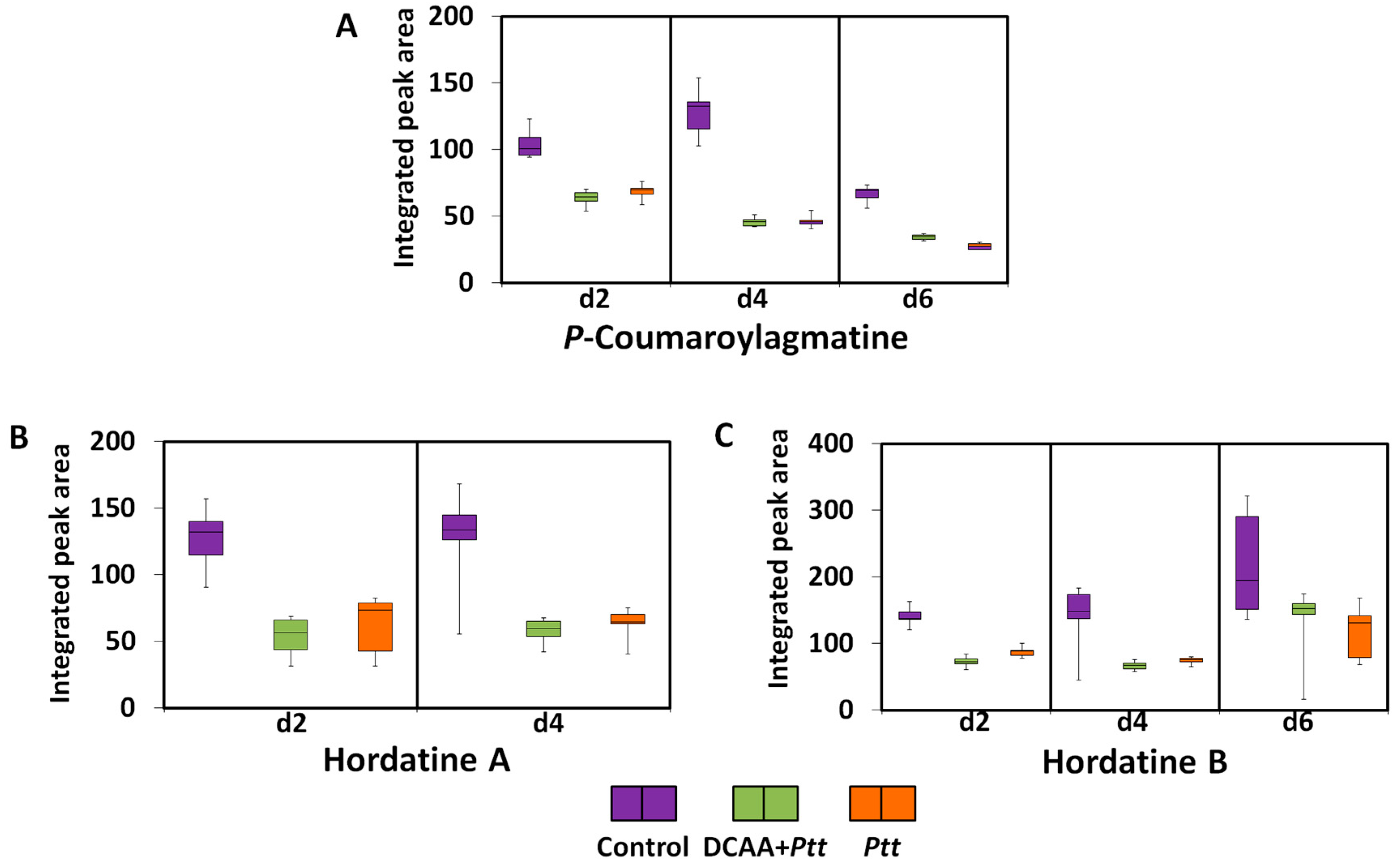
3.3.4. Hydroxycinnamic Acid Amides and Hordatines
3.3.5. Flavonoids
3.3.6. Alkaloids
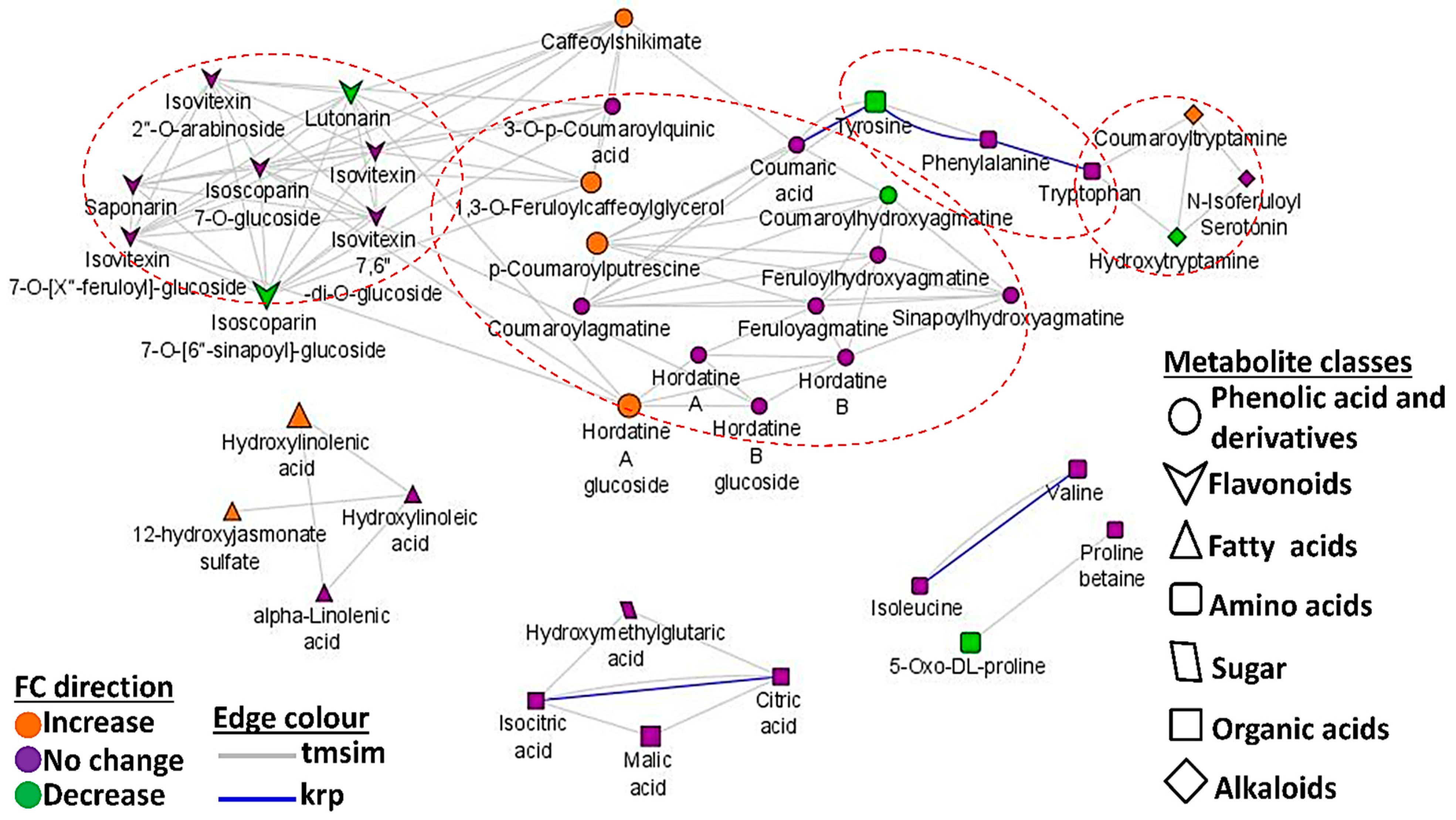
3.4. Mapped Metabolic Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The ‘prime-ome’: Towards a holistic approach to priming. Trends Plant Sci. 2015, 20, 443–452. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.T.; Maake, M.P.; Piater, L.A.; Dubery, I.A. Isonitrosoacetophenone drives transcriptional reprogramming in Nicotiana tabacum cells in support of defense. PLoS ONE 2015, 10, e0117377. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, S.R. Transposable element genomic fissuring in Pyrenophora teres is associated with genome expansion and dynamics of host–pathogen genetic interactions. Front. Genet. 2018, 9, 130. [Google Scholar]
- Liu, Z.; Ellwood, S.R.; Oliver, R.P.; Friesen, T.L. Pyrenophora teres: Profile of an increasingly damaging barley pathogen. Mol. Plant Pathol. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Wallwork, H.; Butt, M.; Capio, E. Pathogen diversity and screening for minor gene resistance to Pyrenophora teres f. teres in barley and its use for plant breeding. Australas. Plant Pathol. 2016, 45, 527–531. [Google Scholar] [CrossRef]
- Backes, A.; Hausman, J.F.; Renaut, J.; Ait Barka, E.; Jacquard, C.; Guerriero, G. Expression analysis of cell wall-related genes in the plant pathogenic fungus Drechslera teres. Genes 2020, 11, 300. [Google Scholar] [CrossRef]
- Clare, S.J.; Wyatt, N.A.; Brueggeman, R.S.; Friesen, T.L. Research advances in the Pyrenophora teres–barley interaction. Mol. Plant Pathol. 2020, 21, 272–288. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, E.; Fedele, G.; Salinari, F.; Rossi, V. A general model for the effect of crop management on plant disease epidemics at different scales of complexity. Agronomy 2020, 10, 462. [Google Scholar] [CrossRef]
- Islam, W. Plant disease epidemiology: Disease triangle and forecasting mechanisms in highlights. Hosts Viruses 2018, 5, 7–11. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Primed plants do not forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- De Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.H.; Mauch-Mani, B.; Pastor, V.; Pozo, M.J.; Pieterse, C.M.; Ton, J.; et al. The induced resistance lexicon: Do’s and don’ts. Trends Plant Sci. 2021, 26, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Schwachtje, J.; Whitcomb, S.J.; Firmino, A.A.P.; Zuther, E.; Hincha, D.K.; Kopka, J. Induced, imprinted, and primed responses to changing environments: Does metabolism store and process information? Front. Plant Sci. 2019, 10, 106. [Google Scholar] [CrossRef]
- Tugizimana, F.; Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. Metabolomics in plant priming research: The way forward? Int. J. Mol. Sci. 2018, 19, 1759. [Google Scholar]
- Allwood, J.W.; Heald, J.; Lloyd, A.J.; Goodacre, R.; Mur, L.A. Separating the Inseparable: The Metabolomic Analysis of Plant–Pathogen Interactions. In Plant Metabolomics. Methods in Molecular Biology; Hardy, N., Hall, R., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 860, pp. 31–49. [Google Scholar] [CrossRef]
- Hamany Djande, C.Y.; Steenkamp, P.A.; Piater, L.A.; Tugizimana, F.; Dubery, I.A. Metabolic Reprogramming of Barley in Response to Foliar Application of Dichlorinated Functional Analogues of Salicylic Acid as Priming Agents and Inducers of Plant Defence. Metabolites 2023, 13, 666. [Google Scholar] [CrossRef]
- Balmer, D.; Flors, V.; Glauser, G.; Mauch-Mani, B. Metabolomics of cereals under biotic stress: Current knowledge and techniques. Front. Plant Sci. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an emerging tool for the study of plant–pathogen interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Hamany Djande, C.Y.; Piater, L.A.; Steenkamp, P.A.; Tugizimana, F.; Dubery, I.A. A metabolomics approach and chemometric tools for differentiation of barley cultivars and biomarker discovery. Metabolites 2021, 11, 578. [Google Scholar] [CrossRef]
- Muria-Gonzalez, M.J.; Zulak, K.G.; Allegaert, E.; Oliver, R.P.; Ellwood, S.R. Profile of the in vitro secretome of the barley net blotch fungus, Pyrenophora teres f. teres. Physiol. Mol. Plant Pathol. 2020, 109, 101451. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Hamany Djande, C.Y.; Steenkamp, P.A.; Piater, L.A.; Tugizimana, F.; Dubery, I.A. Hordatines and associated precursors dominate metabolite profiles of barley (Hordeum vulgare L.) seedlings: A metabolomics study of five cultivars. Metabolites 2022, 12, 310. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 28 April 2022).
- ChemSpider. Available online: http://www.chemspider.com/ (accessed on 3 March 2022).
- Lipidmaps. Available online: https://www.lipidmaps.org (accessed on 18 March 2022).
- Dictionary of Natural Products. Available online: www.dnp.chemnetbase.com (accessed on 10 January 2022).
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Kyoto Encyclopedia of Genes and Genomics. Available online: https://www.genome.jp (accessed on 20 February 2022).
- Barupal, D.K.; Haldiya, P.K.; Wohlgemuth, G.; Kind, T.; Kothari, S.L.; Pinkerton, K.E.; Fiehn, O. MetaMapp: Mapping and visualizing metabolomic data by integrating information from biochemical pathways and chemical and mass spectral similarity. BMC Bioinform. 2012, 13, 1–15. [Google Scholar] [CrossRef]
- Cytoscape. Available online: https://cytoscape.org. (accessed on 20 February 2022).
- Tekauz, A. A numerical scale to classify reactions of barley to Pyrenophora teres. Can. J. Plant Pathol. 1985, 7, 181–183. [Google Scholar] [CrossRef]
- Lightfoot, D.J.; Able, A.J. Growth of Pyrenophora teres in planta during barley net blotch disease. Australas. Plant Pathol. 2010, 39, 499–507. [Google Scholar] [CrossRef]
- Masi, M.; Zorrilla, J.G.; Meyer, S. Bioactive metabolite production in the genus Pyrenophora (Pleosporaceae, Pleosporales). Toxins 2022, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.A.; Able, A.J. Gene expression profiling of virulence-associated proteins in planta during net blotch disease of barley. Physiol. Mol. Plant Pathol. 2017, 98, 69–79. [Google Scholar] [CrossRef]
- Backes, A.; Guerriero, G.; Barka, E.A.; Jacquard, C. Pyrenophora teres: Taxonomy, morphology, interaction with barley, and mode of control. Front. Plant Sci. 2021, 12, 614951. [Google Scholar] [CrossRef] [PubMed]
- Balmer, A.; Pastor, V.; Glauser, G.; Mauch-Mani, B. Tricarboxylates induce defense priming against bacteria in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1221. [Google Scholar] [CrossRef]
- Wang, M.; Ding, Y.; Wang, Q.; Wang, P.; Han, Y.; Gu, Z.; Yang, R. NaCl treatment on physio-biochemical metabolism and phenolics accumulation in barley seedlings. Food Chem. 2020, 331, 127282. [Google Scholar] [CrossRef]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology—Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2022; p. 102873. [Google Scholar]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Labuschagne, N.; Dubery, I.A. Metabolomic evaluation of tissue-specific defense responses in tomato plants modulated by PGPR-priming against Phytophthora capsici infection. Plants 2021, 8, 1530. [Google Scholar] [CrossRef]
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Labuschagne, N.; Dubery, I.A. Unravelling the metabolic reconfiguration of the post-challenge primed state in Sorghum bicolor responding to Colletotrichum sublineolum infection. Metabolites 2019, 9, 194. [Google Scholar] [CrossRef]
- Othibeng, K.; Nephali, L.; Ramabulana, A.T.; Steenkamp, P.; Petras, D.; Kang, K.B.; Opperman, H.; Huyser, J.; Tugizimana, F. A metabolic choreography of maize plants treated with a humic substance-based biostimulant under normal and starved conditions. Metabolites 2021, 11, 403. [Google Scholar] [CrossRef]
- Guidea, A.; Zăgrean-Tuza, C.; Moț, A.C.; Sârbu, C. Comprehensive evaluation of radical scavenging, reducing power and chelating capacity of free proteinogenic amino acids using spectroscopic assays and multivariate exploratory techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 233, 118158. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- da Camara, N.; Dubery, I.A.; Piater, L.A. Proteome Analysis of Nicotiana tabacum Cells following Isonitrosoacetophenone Treatment Reveals Defence-Related Responses Associated with Priming. Plants 2023, 12, 1137. [Google Scholar] [CrossRef]
- Pretorius, C.J.; Zeiss, D.R.; Dubery, I.A. The presence of oxygenated lipids in plant defence in response to biotic stress: A metabolomics appraisal. Plant Signal. Behav. 2021, 16, 1989215. [Google Scholar] [CrossRef]
- Walley, J.W.; Kliebenstein, D.J.; Bostock, R.M.; Dehesh, K. Fatty acids and early detection of pathogens. Curr. Opin. Plant Biol. 2013, 16, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Avis, T.J.; Bélanger, R.R. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Venâncio, A. The potential of fatty acids and their derivatives as antifungal agents: A review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar]
- Atta, B.M.; Saleem, M.; Ali, H.; Bilal, M.; Fayyaz, M. Application of Fluorescence Spectroscopy in Wheat Crop: Early Disease Detection and Associated Molecular Changes. J. Fluoresc. 2020, 30, 801–810. [Google Scholar] [CrossRef]
- Aoun, M. Host defense mechanisms during fungal pathogenesis and how these are overcome in susceptible plants: A review. Int. J. Bot. 2017, 13, 82–102. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R.A. Phenolic compound induction in plant-microbe and plant-insect interactions: A meta-analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef] [PubMed]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Lajayer, B.A.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 1, e09094. [Google Scholar] [CrossRef] [PubMed]
- Zeiss, D.R.; Piater, L.A.; Dubery, I.A. Hydroxycinnamate amides: Intriguing conjugates of plant protective metabolites. Trends Plant Sci. 2021, 26, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Chen, P.; Zhou, X.; Li, Y.; Ma, K.; Li, S.; Liu, H.; Li, L. Arms Race between the Host and Pathogen Associated with Fusarium Head Blight of Wheat. Cells 2022, 11, 2275. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity. Front. Plant Sci. 2022, 13, 922119. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, L.; Su, J.; Tian, B.; Fang, A.; Yu, Y.; Bi, C.; Yang, Y. Integrated Metabolo-transcriptomics Reveals the Defense Response of Homogentisic Acid in Wheat against Puccinia striiformis f. sp. tritici. J. Agric. Food Chem. 2022, 70, 3719–3729. [Google Scholar] [CrossRef]
- Campos, L.; Lison, P.; López-Gresa, M.P.; Rodrigo, I.; Zacares, L.; Conejero, V.; Bellés, J.M. Transgenic tomato plants overexpressing tyramine N-hydroxycinnamoyltransferase exhibit elevated hydroxycinnamic acid amide levels and enhanced resistance to Pseudomonas syringae. Mol. Plant-Microbe Interact. 2014, 27, 1159–1169. [Google Scholar] [CrossRef]
- Stoessl, A. The antifungal factors in barley. IV. Isolation, structure, and synthesis of the hordatines. Can. J. Chem. 1967, 45, 1745–1760. [Google Scholar] [CrossRef]
- Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S. Identification of metabolites related to mechanisms of resistance in barley against Fusarium graminearum, based on mass spectrometry. Plant Mol. Biol. 2011, 77, 355–370. [Google Scholar] [CrossRef]
- Stoessl, A.; Unwin, C.H. The antifungal factors in barley. V. antifungal activity of the hordatines. Can. J. Bot. 1970, 48, 465–470. [Google Scholar] [CrossRef]
- Mikkelsen, B.L.; Olsen, C.E.; Lyngkjær, M.F. Accumulation of secondary metabolites in healthy and diseased barley, grown under future climate levels of CO2, ozone and temperature. Phytochemistry 2015, 118, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Heo, S.I.; Wang, M.H. HPLC analysis and antioxidant activity of Ulmus davidiana and some flavonoids. Food Chem. 2010, 120, 313–318. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid mediated selective cross-talk between plants and beneficial soil microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the study of the function and mechanism of the action of flavonoids in plants under environmental stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef]
- Koga, R.; Meng, T.; Nakamura, E.; Miura, C.; Irino, N.; Yahara, S.; Kondo, R. Model examination for the effect of treading stress on young green barley (Hordeum vulgare). Am. J. Plant Sci. 2013, 4, 2013. [Google Scholar] [CrossRef]
- Khlestkina, E. The adaptive role of flavonoids: Emphasis on cereals. Cereal Res. Commun. 2013, 41, 185–198. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Buśko, M.; Góral, T.; Ostrowska, A.; Matysiak, A.; Walentyn-Góral, D.; Perkowski, J. The effect of Fusarium inoculation and fungicide application on concentrations of flavonoids (apigenin, kaempferol, luteolin, naringenin, quercetin, rutin, vitexin) in winter wheat cultivars. Am. J. Plant Sci. 2014, 5, 3727–3736. [Google Scholar] [CrossRef]
- Venturini, G.; Toffolatti, S.L.; Assante, G.; Babazadeh, L.; Campia, P.; Fasoli, E.; Salomoni, D.; Vercesi, A. The influence of flavonoids in maize pericarp on Fusarium ear rot symptoms and fumonisin accumulation under field conditions. Plant Pathol. 2015, 64, 671–679. [Google Scholar] [CrossRef]
- Lovett, J.V.; Hoult, A.H.C. Biological activity of barley secondary metabolites. In Proceeding of the 7th Australian Society of Agronomy Conference, Adelaide, Australia, 19–24 September 1993; pp. 158–161. [Google Scholar]
- Ube, N.; Yabuta, Y.; Tohnooka, T.; Ueno, K.; Taketa, S.; Ishihara, A. Biosynthesis of phenylamide phytoalexins in pathogen-infected barley. Int. J. Mol. Sci. 2019, 20, 5541. [Google Scholar] [CrossRef] [PubMed]
- Macoy, D.M.J.; Uddin, S.; Ahn, G.; Peseth, S.; Ryu, G.R.; Cha, J.Y.; Lee, J.Y.; Bae, D.; Paek, S.M.; Chung, H.J.; et al. Effect of Hydroxycinnamic Acid Amides, Coumaroyl Tyramine and Coumaroyl Tryptamine on Biotic Stress Response in Arabidopsis. J. Plant Biol. 2022, 65, 145–155. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamany Djande, C.Y.; Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic Reconfiguration in Primed Barley (Hordeum vulgare) Plants in Response to Pyrenophora teres f. teres Infection. Metabolites 2023, 13, 997. https://doi.org/10.3390/metabo13090997
Hamany Djande CY, Tugizimana F, Steenkamp PA, Piater LA, Dubery IA. Metabolomic Reconfiguration in Primed Barley (Hordeum vulgare) Plants in Response to Pyrenophora teres f. teres Infection. Metabolites. 2023; 13(9):997. https://doi.org/10.3390/metabo13090997
Chicago/Turabian StyleHamany Djande, Claude Y., Fidele Tugizimana, Paul A. Steenkamp, Lizelle A. Piater, and Ian A. Dubery. 2023. "Metabolomic Reconfiguration in Primed Barley (Hordeum vulgare) Plants in Response to Pyrenophora teres f. teres Infection" Metabolites 13, no. 9: 997. https://doi.org/10.3390/metabo13090997