Liver Fat Storage Is a Better Predictor of Coronary Artery Disease than Visceral Fat
Abstract
:1. Introduction
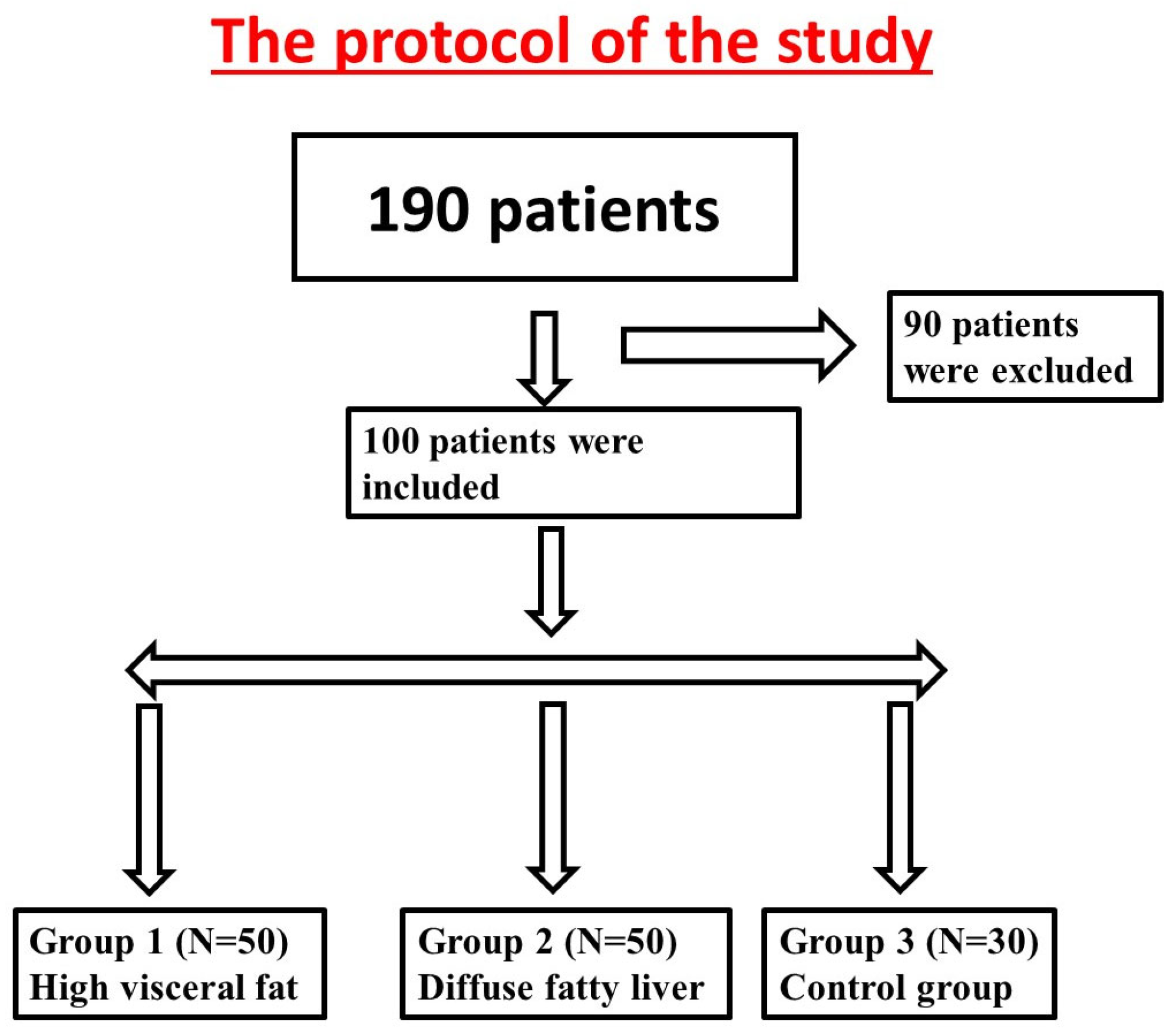
2. Materials and Methods
2.1. Study Population
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
- BMI > 35.
- Acute illness.
- Previous ischemic heart disease or cerebrovascular disease.
- Patients suffered from typical chest pain.
- Previous CAD, conventional coronary angiography, coronary bypass grafting, renal failure, percutaneous intervention, or cancer.
- Hepatic steatosis agents like steroids, estrogens, methotrexate, and procor.
- Cardiac CT exclusions: presence of multiple ectopic beats, heart rate of more than 75/min despite therapy, atrial fibrillation, severe lung disease, and a history of allergic reaction to iodine-containing contrast agents.
2.3. Study Design
2.4. Hepatic Steatosis
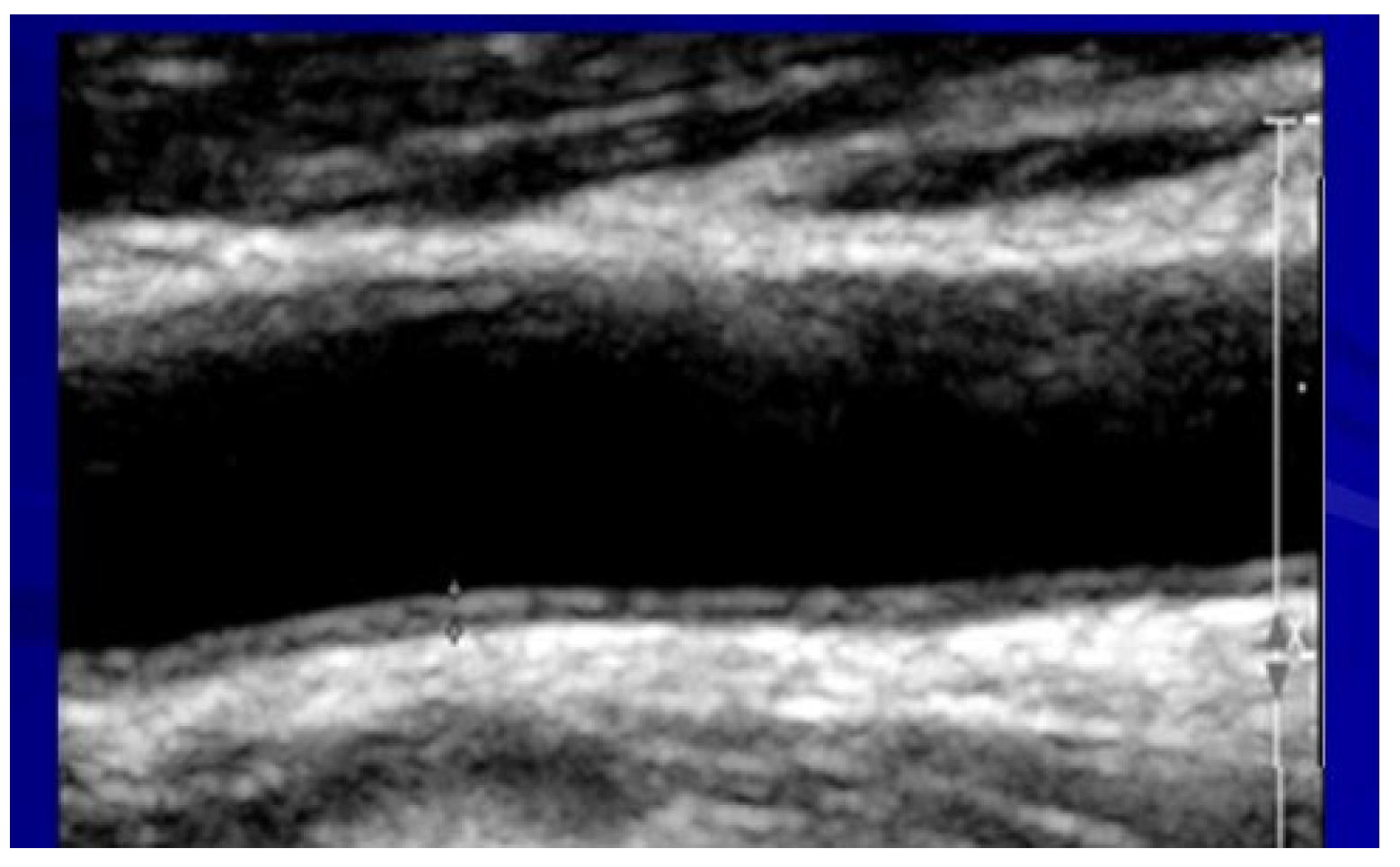
2.5. Assessment of Carotid Atherosclerosis
2.6. Assessment of Visceral Fat
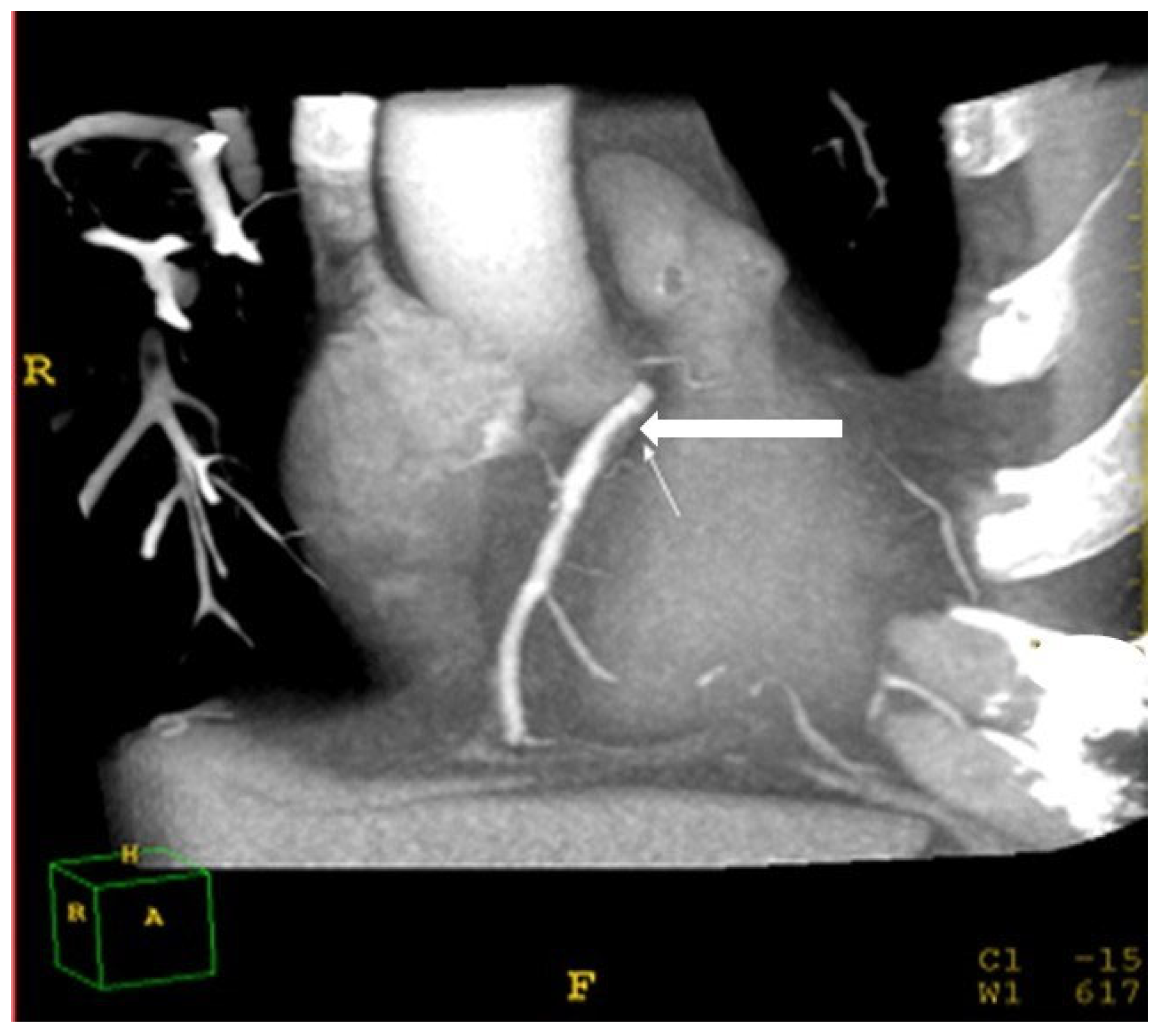
2.7. Assessment of Coronary Atherosclerosis
2.8. Ethics
2.9. Statistics
3. Results
3.1. Clinical Characteristics of the Study Participants
3.2. Differences in Coronary Plaques, Coronary Stenosis, Carotid Atherosclerosis, Biomarkers of Insulin Resistance, Inflammation, and Oxidative Stress among the Three Groups
3.3. Multivariate Logistic Regression Analysis for Predicting Coronary Artery Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Jaberi, S.; Cohen, A.; Saeed, Z.; Ojha, S.; Singh, J. Obesity: Molecular Mechanisms, Epidemiology, Complications and Pharmacotherapy. Cell. Biochem. Mech. Obes. 2021, 23, 249–266. [Google Scholar]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [PubMed]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front. Nutr. 2016, 3, 10. [Google Scholar] [PubMed] [Green Version]
- He, Q.; Horlick, M.; Thornton, J.; Wang, J.; Pierson, R.N.; Heshka, S.; Gallagher, D. Sex-specific fat distribution is not linear across pubertal groups in a multiethnic study. Obes. Res. 2004, 12, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar]
- He, Y.; Pan, A.; Wang, Y.; Yang, Y.; Xu, J.; Zhang, Y.; Liu, D.; Wang, Q.; Shen, H.; Zhang, Y.; et al. Prevalence of overweight and obesity in 15.8 million men aged 15–49 years in rural China from 2010 to 2014. Sci. Rep. 2017, 7, 5012. [Google Scholar]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2015, 64, 73–84. [Google Scholar]
- Shields, W.W.; Thompson, K.E.; Grice, G.A.; Harrison, S.A.; Coyle, W.J. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH):A Pilot Trial. Ther. Adv. Gastroenterol. 2009, 2, 157–163. [Google Scholar]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar]
- Zhang, J.-Z.; Yu, Y.; She, Z.-G.; Li, H. Nonalcoholic Fatty Liver Disease: An Update on the Diagnosis. Gene Expr. 2019, 19, 187–198. [Google Scholar] [PubMed]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targher, G.; Arcaro, G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007, 191, 240. [Google Scholar]
- Mantovani, A. Nonalcoholic fatty liver disease (NAFLD) and risk of cardiac arrhythmias: A new aspect of the liver-heart axis. J. Clin. Transl. Hepatol. 2017, 5, 134–141. [Google Scholar]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [PubMed] [Green Version]
- Haffner, S.M.; Kennedy, E.; Gonzalez, C.; Stern, M.P.; Miettinen, H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care 1996, 19, 1138–1141. [Google Scholar] [CrossRef]
- Toh, J.Z.K.; Pan, X.-H.; Tay, P.W.L.; Ng, C.H.; Yong, J.N.; Xiao, J.; Koh, J.H.; Tan, E.Y.; Tan, E.X.X.; Dan, Y.Y.; et al. A Meta-Analysis on the Global Prevalence, Risk factors and Screening of Coronary Heart Disease in Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2021, 20, 2462–2473.e10. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults (Adult Panel III) Final Report. Circulation 2002, 106, 3143–3421.
- Montagne, P.; Laroche, P.; Cuillière, M.L.; Varcin, P.; Pau, B.; Duheille, J. Microparticle-enhanced nephelometric immunoassay for human C-reactive protein. J. Clin. Lab. Anal. 1992, 6, 24–29. [Google Scholar] [CrossRef]
- Gan, K.N.; Smolen, A.; Eckerson, H.W.; La Du, B.N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab. Dispos. 1991, 19, 100–106. [Google Scholar]
- Baker, H.; Frank, O.; De Angelis, B.; Feingold, S. Plasma tocopherol in man at various time intervals after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 1980, 21, 531–536. [Google Scholar]
- Yagi, K. Lipid peroxides and human diseases. Chem. Phys. Lipids 1987, 45, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.E.; Saverymuttu, S.H.; Al-Sam, S.; Cook, M.G.; Maxwell, J.D. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin. Radiol. 1991, 43, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Yadav, J.S. Carotid artery intima- media thickness: Indicator of atherosclerosis burden and response to risk factor modification. Am. Heart J. 2002, 144, 753–759. [Google Scholar] [PubMed]
- Schroeder, S.; Kopp, A.F.; Baumbach, A.; Meisner, C.; Kuettner, A.; Georg, C.; Ohnesorge, B.; Herdeg, C.; Claussen, C.D.; Karsch, K.R. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J. Am. Coll. Cardiol. 2001, 37, 1430–1435. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Levin, D.C.; Halpern, E.J.; Fischman, D.; Savage, M.; Walinsky, P. Accuracy of MDCT in assessing the degree of stenosis caused by calcified coronary artery plaques. AJR Am. J. Roentgenol. 2008, 191, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Hoeing, M.R.; Crowin, G.; Buckley, R.; McHenery, C.; Coulthard, A. Liver fat percent is associated with metabolic risk factors and the metabolic syndrome in a high- risk vascular cohort. Nutr. Metab. 2010, 16, 7–50. [Google Scholar]
- Clifford, S.M.; Murphy, D.J. Non-alcoholic fatty liver disease and coronary atherosclerosis-does myocardial glucose metabolism provide the missing link? J. Nucl. Cardiol. 2021, 28, 621–623. [Google Scholar] [CrossRef] [Green Version]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar]
- Polyzos, S.A.; Kechagias, S.; Tsochatzis, E.A. Review article: Non-alcoholic fatty liver disease and cardiovascular diseases: Associations and treatment considerations. Aliment. Pharmacol. Ther. 2021, 54, 1013–1025. [Google Scholar]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Tamboli, R.A.; Magkos, F.; Marks–Shulman, P.A.; Eckhauser, A.W.; Richards, W.O.; Klein, S.; Abumrad, N.N. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 2010, 139, 448–455. [Google Scholar] [PubMed] [Green Version]
- Lee, J.; Chung, D.-S.; Kang, J.-H.; Yu, B.-Y. Comparison of Visceral Fat and Liver Fat as Risk Factors of Metabolic Syndrome. J. Korean Med. Sci. 2012, 27, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Schulz, E.; Anter, E.; Keaney, J.F., Jr. Oxidative stress, antioxidants, and endothelial function. Curr. Med. Chem. 2004, 11, 1093–1104. [Google Scholar] [CrossRef]
- Khamaysi, I.; William, N.; Olga, A.; Alex, I.; Vladimir, M.; Kamal, D.; Nimer, A. Sub-clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: A randomized controlled study. J. Hepatol. 2011, 54, 72–77. [Google Scholar]
- Abid, A.; Taha, O.; Nseir, W.; Farah, R.; Grosovski, M.; Assy, N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J. Hepatol. 2009, 51, 918–924. [Google Scholar] [CrossRef]
- Stephan, N.; Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar]
- Pilz, S.; Mangge, H.; Wellnitz, B.; Seelhorst, U.; Winkelmann, B.R.; Tiran, B.; Boehm, B.O.; Ma, W. Adiponectin and mortality in patients undergoing coronary angiography. J. Clin. Endocrinol. Metab. 2006, 91, 4277–4286. [Google Scholar] [CrossRef] [Green Version]
- Stefan, N.; Kantartzis, K.; Häring, H.-U. Causes and metabolic consequences of Fatty liver. Endocr. Rev. 2008, 29, 939–960. [Google Scholar]
- Hyun, J.; Jung, Y. DNA Methylation in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 8138. [Google Scholar] [CrossRef]
- Lafontan, M.; Girad, J. Impact of visceral adipose tissue on liver metabolism and insulin resistance. Part I: Heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008, 34, 317–327. [Google Scholar]
- Ouchi, N.; Ohishi, M.; Kihara, S.; Funahashi, T.; Nakamura, T.; Nagaretani, H.; Kumada, M.; Ohashi, K.; Okamoto, Y.; Nishizawa, H.; et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 2003, 42, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [PubMed] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 8–11. [Google Scholar]
- Boden, G. Role of fatty liver in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10. [Google Scholar] [CrossRef]
- Bjorntorp, P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990, 10, 493–496. [Google Scholar] [PubMed]
- Nielssen, S.; Guo, Z.; Johnson, C.M.; Hendsrud, D.D.; Jensen, M.D. Body Fat Distribution, Adipocyte Size, and Metabolic Characteristics of Nondiabetic. Adults J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [PubMed] [Green Version]
- Jon, O.E.; Michael, D.J. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar]
- Cherukuri, L.; Birudaraju, D.; Budoff, M.J. Coronary artery calcium score: Pivotal role as a predictor for detecting coronary artery disease in symptomatic patients. Coron. Artery Dis. 2021, 32, 578–585. [Google Scholar]
- National Cholesterol Education Program (US). Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Denk, H.; Abuja, P.M.; Zatloukal, K. Animal models of NAFLD from the pathologist’s point of view. Biochim. Biophys. Acta 2019, 1865, 929–942. [Google Scholar] [CrossRef] [PubMed]


| Excessive Visceral Fat Storage N = 30 | Fatty Liver Storage N = 70 | Controls N = 30 | p-Value FL vs. Controls | |
|---|---|---|---|---|
| Age (yrs.) | 51 ± 8.7 | 50 ± 9.4 | 50 ± 10 | 0.3 |
| Male (% of total) | 68 | 74 | 75 | 0.1 |
| Hypertension (% of total) | 20 | 50 | 24 | 0.02 |
| Diabetes mellitus (% of total) | 27 | 52 | 13 | 0.003 |
| Alcohol intake (g/day) | 5 ± 10 | 7.0 ± 11 | 5 ± 4 | 0.3 |
| BMI * | 32 ± 5 | 32 ± 5 | 30 ± 4 | 0.08 |
| Waist circumference (cm) | 111 ± 11 | 113 ± 10 | 103 ± 11 | 0.003 |
| Metabolic syndrome (%) | 44% | 57% | 0% | 0.001 |
| ALT (IU/L) | 31 ± 17 | 38 ± 21 | 25 ± 10 | 0.005 |
| GGT (IU/L) | 34 ± 19 | 38 ± 17 | 30 ± 18 | 0.05 |
| Triglycerides (mg/dL) | 182 ± 90 | 196 ± 103 | 145 ± 60 | 0.005 |
| LDL cholesterol (mg/dL) | 117 ± 32 | 106 ± 29 | 115 ± 30 | 0.4 |
| HDL cholesterol (mg/dL) | 43 ± 9 | 41 ± 10 | 45 ± 11 | 0.1 |
| Metabolic syndrome | 44% | 57% | 0% | 0.001 |
| Delta liver density–spleen density (HU) | +1 ± 7 | −20 ± 4 | +7 ± 6 | 0.001 |
| Visceral lipids | 330–463>330 ± 99 | 197–330 | 74–230 | 0.001 |
| Visceral Fat Storage N = 30 | Liver Fat Storage N = 70 | Healthy Controls N = 30 | p-Value FL vs. Controls | |
|---|---|---|---|---|
| Coronary soft plaques (% of total) | 38 | 50 | 25 | 0.001 |
| CAD: stenosis > 50% (% of total) | 22 | 30 | 11 | 0.001 |
| IMT * | 0.86 ± 0.1 | 0.98 ± 0.3 | 0.83 ± 0.1 | 0.01 |
| HOMA | 3.0 ± 1.0 | 4.5 ± 3.0 | 1.5 ± 1.2 | 0.005 |
| CRP(mg/dL) | 0.4 ± 0.40 | 0.4 ± 0.3 | 0.3 ± 0.5 | 0.02 |
| MDA(micmol/L) | 69.8 ± 30 | 55.6 ± 35 | 50.0 ± 15 | 0.01 |
| Paraoxonase (nmol/min) | 0.2 ± 0.07 | 0.2 ± 0.06 | 0.2 ± 0.01 | 0.1 |
| OR * | ±95% CI | p-Value | |
|---|---|---|---|
| Diffuse fatty liver (LD-SD > −10HU) | 2.75 | 2.3–4.9 | 0.001 |
| Excessive visceral fat (>330 cm2) | 2.01 | 1.2–3.8 | 0.001 |
| Subcutaneous fat (>230 cm2) | 1.15 | 1.0–3.6 | 0.2 |
| CRP (>2 mg/dL) | 1.20 | 0.5–1.9 | 0.4 |
| HOMA (>2.5) | 0.85 | 0.5–3.0 | 0.1 |
| Metabolic syndrome (yes/no) | 1.52 | 0.8–3.5 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basheer, M.; Saad, E.; Jeries, H.; Assy, N. Liver Fat Storage Is a Better Predictor of Coronary Artery Disease than Visceral Fat. Metabolites 2023, 13, 896. https://doi.org/10.3390/metabo13080896
Basheer M, Saad E, Jeries H, Assy N. Liver Fat Storage Is a Better Predictor of Coronary Artery Disease than Visceral Fat. Metabolites. 2023; 13(8):896. https://doi.org/10.3390/metabo13080896
Chicago/Turabian StyleBasheer, Maamoun, Elias Saad, Helena Jeries, and Nimer Assy. 2023. "Liver Fat Storage Is a Better Predictor of Coronary Artery Disease than Visceral Fat" Metabolites 13, no. 8: 896. https://doi.org/10.3390/metabo13080896





