Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis
Abstract
:1. Introduction
2. Materials and Methods
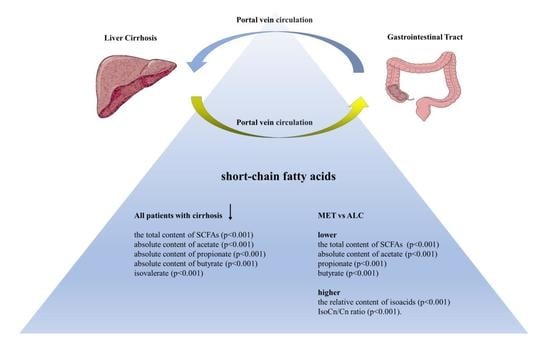
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Kioh, D.Y.; Yap, G.C.; Lee, B.W.; Chan, E.C. A novel LCMSMS method for quantitative measurement of short-chain fatty acids in human stool derivatized with 12C- and 13C-labelled aniline. J. Pharm. Biomed. Anal. 2017, 138, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.J.; Sellmann, C.; Engstler, A.J.; Ziegenhardt, D.; Bergheim, I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br. J. Nut. 2015, 114, 1745–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [Green Version]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Bloom, P.P.; Luévano, J.M., Jr.; Miller, K.J.; Chung, R.T. Deep stool microbiome analysis in cirrhosis reveals an association between short-chain fatty acids and hepatic encephalopathy. Ann. Hepatol. 2021, 25, 100333. [Google Scholar] [CrossRef]
- Jin, M.; Kalainy, S.; Baskota, N.; Chiang, D.; Deehan, E.C.; McDougall, C.; Tandon, P.; Martínez, I.; Cervera, C.; Walter, J.; et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019, 39, 1437–1447. [Google Scholar] [CrossRef]
- Manzoor, R.; Ahmed, W.; Afify, N.; Memon, M.; Yasin, M.; Memon, H.; Rustom, M.; Al Akeel, M.; Alhajri, N. Trust Your Gut: The Association of Gut Microbiota and Liver Disease. Microorganisms 2022, 10, 1045. [Google Scholar] [CrossRef]
- Fang, J.; Yu, C.H.; Li, X.J.; Yao, J.M.; Fang, Z.Y.; Yoon, S.H.; Yu, W.Y. Gut dysbiosis in nonalcoholic fatty liver disease: Pathogenesis, diagnosis, and therapeutic implications. Front. Cell Infect. Microbiol. 2022, 12, 997018. [Google Scholar] [CrossRef]
- Zeng, S.; Schnabl, B. Roles for the mycobiome in liver disease. Liver Int. 2022, 42, 729–741. [Google Scholar] [CrossRef]
- Abenavoli, L.; Maurizi, V.; Rinninella, E.; Tack, J.; Di Berardino, A.; Santori, P.; Rasetti, C.; Procopio, A.C.; Boccuto, L.; Scarpellini, E. Fecal Microbiota Transplantation in NAFLD Treatment. Medicina 2022, 58, 1559. [Google Scholar] [CrossRef]
- Reshetova, M.S.; Zolnikova, O.Y.; Ivashkin, V.T.; Ivashkin, K.V.; Appolonova, S.A.; Lapina, T.L. Gut Microbiota and its Metabolites in Pathogenesis of NAFLD. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2022, 32, 75–88. [Google Scholar] [CrossRef]
- Philips, C.A.; Schnabl, B.; Bajaj, J.S. Gut Microbiome and Alcohol-associated Liver Disease. J. Clin. Exp. Hepatol. 2022, 12, 1349–1359. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Alieva, A.; Kashuh, E.; Tsvetaeva, E.; Poluektova, E.; Shirokova, E.; Ivashkin, K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J. Hepatol. 2021, 13, 557–570. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Mande, S.S. Host-microbiome interactions: Gut-Liver axis and its connection with other organs. NPJ Biofilms Microbiomes 2022, 8, 89. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://patents.google.com/patent/RU2220755C1/ru (accessed on 1 January 2021).
- Xiong, J.; Chen, X.; Zhao, Z.; Liao, Y.; Zhou, T.; Xiang, Q. A potential link between plasma short-chain fatty acids, TNF-α level and disease progression in non-alcoholic fatty liver disease: A retrospective study. Exp. Ther. Med. 2022, 3, 598. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-chain fatty acids: Ready for prime time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H. Short chain fatty acids in the human colon. Gut 1981, 22, 763–779. [Google Scholar] [CrossRef] [Green Version]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Damink, S.W.O.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef]
- Posma, J.M.; Garcia-Perez, I.; Frost, G.; Aljuraiban, G.S.; Chan, Q.; Van Horn, L.; Daviglus, M.; Stamler, J.; Holmes, E.; Elliott, P.; et al. Nutriome–metabolome relationships provide insights into dietary intake and metabolism. Nat. Food 2020, 1, 426–436. [Google Scholar] [CrossRef]
- Kendrick, S.F.W.; O’Boyle, G.; Mann, J.; Zeybel, M.; Palmer, J.; Jones, D.E.J.; Day, C.P. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology 2010, 51, 1988–1997. [Google Scholar] [CrossRef]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 2016, 40, 955–963. [Google Scholar] [CrossRef]

| Patients with Cirrhosis (n = 40) | Healthy Controls (n = 20) | p | |
|---|---|---|---|
| Age, years | 57 [51–64] | 56 [52–59] | 0.712 |
| Male/Female | 21/19 | 9/11 | 0.392 |
| Body mass index, kg/m2 | 29.2 [24.5–32.0] | 26.4 [24.6–26.8] | 0.044 |
| Waist, cm | 113 [102–119] | 87 [81–93] | <0.001 |
| Serum cholesterol, mmol/L | 4.5 [3.4–5.9] | 4.4 [4.2–4.8] | 0.975 |
| Serum triglycerides, mmol/L | 1.57 [1.21–1.72] | 0.83 [0.61–1.27] | <0.001 |
| Serum glucose, mmol/L | 6.1 [5.1–7.4] | 4.8 [4.6–5.0] | <0.001 |
| Alanine aminotransferase, U/L | 42 [22–78] | 19 [17–24] | <0.001 |
| Aspartate aminotransferase, U/L | 50 [38–89] | 16 [11–19] | <0.001 |
| Hemoglobin, g/L | 130 [120–139] | 135 [132–138] | 0.086 |
| Alcohol consumption, doses/ week | 27 [1–36] | 1 [0–2] | 0.001 |
| ALC (n = 24) | MET (n = 16) | p | |
|---|---|---|---|
| Age, years | 55 [44–62] | 60 [54–65] | 0.132 |
| Male/Female | 15/9 | 6/10 | 0.110 |
| Body mass index, kg/m2 | 25.2 [22.9–29.4] | 32.1 [30.7–33.1] | <0.001 |
| Waist, cm | 108 [98–114] | 118 [116–124] | <0.001 |
| Child–Pugh score | 6 [5–7] | 5 [5–7] | 0.610 |
| Child–Pugh class, A/B | 17/7 | 11/5 | 0.580 |
| Esophageal varices, grade 0–1/2–3 | 19/5 | 11/5 | 0.351 |
| Ascites, present/absent | 6/18 | 5/11 | 0.467 |
| Minimal hepatic encephalopathy, present/absent | 4/20 | 4/12 | 0.399 |
| Serum total protein, g/L | 71.0 [67.5–75.5] | 70.5 [67.5–76.5] | 0.978 |
| Serum albumin, g/L | 39.6 [37.2–44.7] | 37.1 [34.2–40.8] | 0.068 |
| Serum total bilirubin, μmol/L | 28.6 [23.8–33.4] | 20.2 [13.6–27.4] | 0.007 |
| Serum cholesterol, mmol/L | 4.5 [2.8–7.0] | 4.4 [3.7–5.2] | 0.934 |
| Serum HDL cholesterol, mmol/L | 1.4 [0.9–2.5] | 1.1 [0.9–1.3] | 0.113 |
| Serum LDL cholesterol, mmol/L | 2.6 [2.1–3.7] | 2.7 [2.0–3.3] | 0.638 |
| Serum triglycerides, mmol/L | 1.32 [1.05–1.69] | 2.14 [1.65–2.65] | <0.001 |
| Serum uric acid | 353 [288–428] | 247 [217–274] | <0.001 |
| Serum glucose, mmol/L | 5.8 [4.6–6.7] | 6.7 [5.8–8.1] | 0.028 |
| Alanine aminotransferase, U/L | 40 [18–75] | 42 [31–78] | 0.294 |
| Aspartate aminotransferase, U/L | 55 [34–112] | 49 [43–70] | 0.782 |
| Gamma glutamyl transferase, U/L | 77 [44–758] | 60 [53–68] | 0.464 |
| Alkaline phosphatase, U/L | 98 [87–120] | 91 [70–105] | 0.163 |
| C-reactive protein, mg/L | 3.6 [1.7–6.9] | 2.5 [1.7–4.4] | 0.214 |
| Fibrinogen, g/L | 3.2 [2.6–3.8] | 4.2 [3.8–4.4] | <0.001 |
| Prothrombin index (Quick test), % | 75 [64–91] | 81 [77–86] | 0.269 |
| IgG, g/L | 10.9 [9.4–13.9] | 15.6 [13.1–17.0] | 0.011 |
| IgM, g/L | 1.6 [1.5–1.8] | 1.2 [1.1–1.3] | <0.001 |
| Hemoglobin, g/L | 130 [115–139] | 130 [124–138] | 0.525 |
| White blood cells, 109/L | 5.5 [4.8–6.1] | 5.8 [4.6–6.2] | 0.751 |
| Neutrophils, 109/L | 2.8 [2.3–4.1] | 2.9 [2.5–3.1] | 0.890 |
| Lymphocytes, 109/L | 1.5 [1.1–2.2] | 2.1 [1.6–2.6] | <0.024 |
| Platelets, 109/L | 145 [99–187] | 147 [103–180] | 0.761 |
| Portal vein diameter, cm | 12.5 [11.7–13.7] | 12.1 [11.4–12.8] | 0.258 |
| Splenic vein diameter, cm | 7.0 [6.4–8.8] | 7.8 [6.6–8.5] | 0.761 |
| Alcohol consumption, doses/week | 36 [32–38] | 1 [0–1] | <0.001 |
| ALC (n = 24) | MET (n = 16) | CON (n = 20) | ALC vs. MET | ALC vs. CON | MET vs. CON | |
|---|---|---|---|---|---|---|
| Fecal SCFA, mg/g | 5.31 [3.65–7.11] | 3.20 [2.13–4.22] | 10.2 [9.76–10.7] | <0.001 | <0.001 | <0.001 |
| Fecal acetate, mg/g | 3.14 [2.30–4.49] | 2.12 [1.03–2.28] | 5.87 [5.65–6.04] | <0.001 | <0.001 | <0.001 |
| Fecal propionate, mg/g | 1.11 [0.78–1.36] | 0.58 [0.46–0.81] | 1.77 [1.70–1.83] | <0.001 | <0.001 | <0.001 |
| Fecal butyrate, mg/g | 0.68 [0.46–1.10] | 0.35 [0.28–0.48] | 1.69 [1.66–1.77] | 0.001 | <0.001 | <0.001 |
| Fecal isoacids, mg/g | 0.27 [0.22–0.33] | 0.23 [0.21–0.31] | 0.62 [0.59–0.64] | 0.276 | <0.001 | <0.001 |
| Fraction of acetate, % | 64.5 [60.2–66.9] | 62.8 [56.3–66.8] | 61.8 [56.0–67.5] | 0.320 | 0.458 | 0.927 |
| Fraction of propionate, % | 21.0 [18.6–24.7] | 22.7 [19.8–26.3] | 19.1 [8.7–19.8] | 0.923 | 0.017 | 0.043 |
| Fraction of butyrate, % | 14.8 [11.3–17.8] | 12.9 [11.3–15.0] | 17.1 [15.3–21.0] | 0.590 | 0.008 | 0.033 |
| Fraction of isoacids, % | 5.8 [3.1–7.0] | 8.2 [7.4–10.0] | 5.9 [5.8–6.0] | <0.001 | 0.860 | <0.001 |
| Isoacid/unbrached acid ratio | 0.06 [0.03–0.08] | 0.08 [0.07–0.11] | 0.07 [0.07–0.07] | <0.001 | 0.564 | <0.001 |
| Alcoholic Fatty Liver Disease | |||
|---|---|---|---|
| Minimal Hepatic Encephalopathy Present (n = 4) | Minimal Hepatic Encephalopathy Absent (n = 20) | p | |
| Fecal SCFA, mg/g | 4.95 [4.11–6.72] | 5.56 [3.65–7.11] | 0.698 |
| Fecal acetate, mg/g | 3.03 [2.30–4.28] | 3.14 [2.29–4.49] | 1.000 |
| Fecal propionate, mg/g | 0.95 [0.63–1.36] | 1.11 [0.88–1.36] | 0.588 |
| Fecal butyrate, mg/g | 0.52 [0.36–1.05] | 0.76 [0.49—1.10] | 0.670 |
| Fecal isoacids, mg/g | 0.28 [0.26–0.36] | 0.25 [0.21–0.33] | 0.373 |
| Non-alcoholic (metabolic-associated) fatty liver disease | |||
| Minimal hepatic encephalo- pathy present (n = 4) | Minimal hepatic encephalopathy absent (n = 12) | p | |
| Fecal SCFA, mg/g | 4.22 [3.75–4.40] | 3.29 [2.32–3.86] | 0.034 |
| Fecal acetate, mg/g | 2.45 [2.17–2.50] | 1.75 [1.26–2.28] | 0.025 |
| Fecal propionate, mg/g | 0.72 [0.63–0.82] | 0.60 [0.43–0.80] | 0.431 |
| Fecal butyrate, mg/g | 0.48 [0.45–0.50] | 0.37 [0.34–0.43] | 0.060 |
| Fecal isoacids, mg/g | 0.38 [0.37–0.42] | 0.27 [0.23–0.33] | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Zolnikova, O.; Maslennikov, R.; Reshetova, M.; Poluektova, E.; Bogacheva, A.; Zharkova, M.; Ivashkin, V. Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis. Metabolites 2023, 13, 859. https://doi.org/10.3390/metabo13070859
Cao X, Zolnikova O, Maslennikov R, Reshetova M, Poluektova E, Bogacheva A, Zharkova M, Ivashkin V. Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis. Metabolites. 2023; 13(7):859. https://doi.org/10.3390/metabo13070859
Chicago/Turabian StyleCao, Xinlu, Oksana Zolnikova, Roman Maslennikov, Maria Reshetova, Elena Poluektova, Arina Bogacheva, Maria Zharkova, and Vladimir Ivashkin. 2023. "Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis" Metabolites 13, no. 7: 859. https://doi.org/10.3390/metabo13070859