The Role of Cholesterol in Chronic Lymphocytic Leukemia Development and Pathogenesis
Abstract
:1. Introduction—The Role of Cholesterol in Cancer
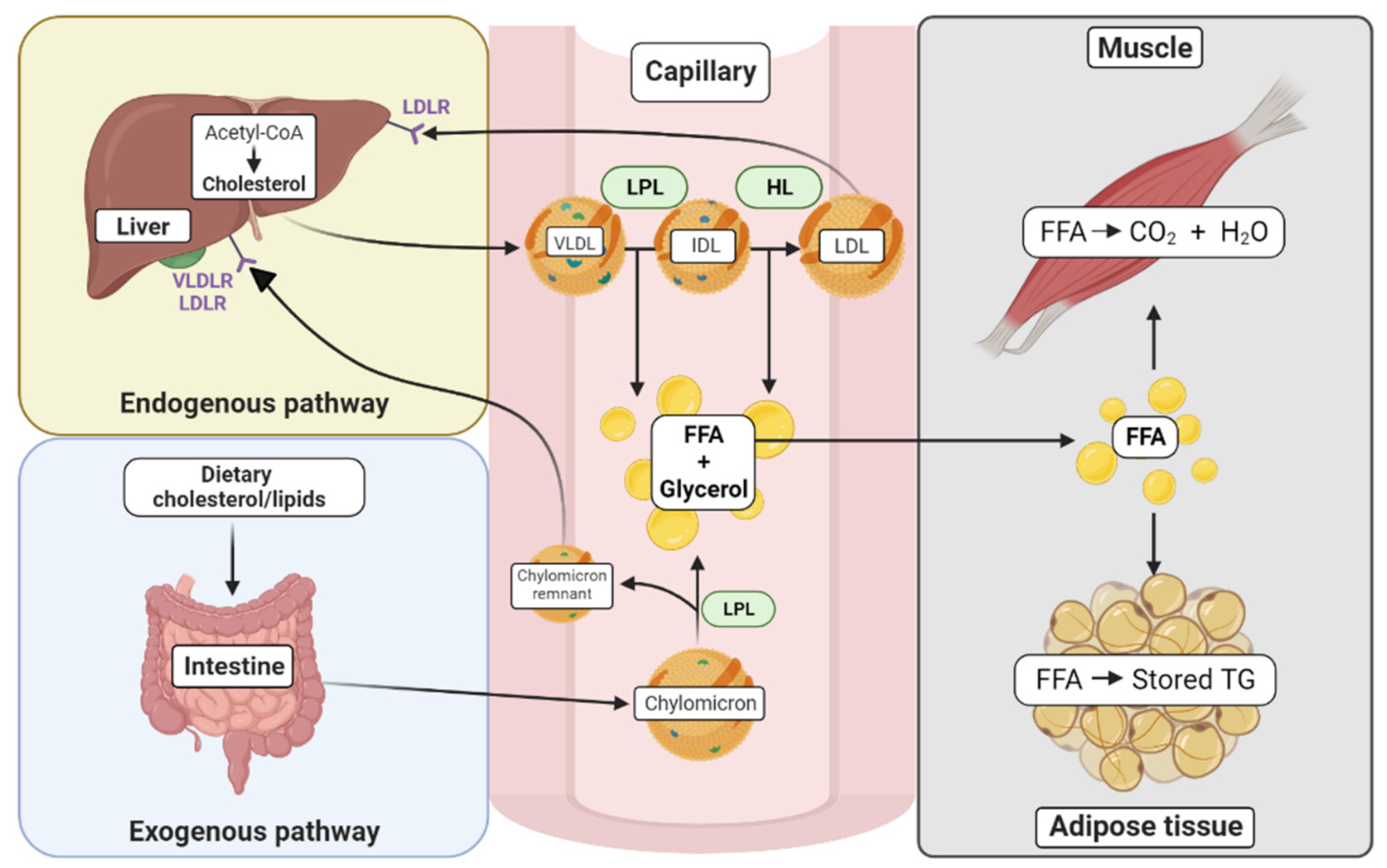
2. Cholesterol Synthesis and Exogenous Sources
3. Chronic Lymphocytic Leukemia (CLL)
4. Serum Cholesterol Levels and Prognosis in CLL
5. The Role of Intracellular Cholesterol in CLL
Intracellular Cholesterol Levels in CLL
6. Cholesterol Uptake in CLL
6.1. HDL Uptake
6.2. LDL Uptake
6.3. Chylomicron Metabolism
7. LDLs as Signaling Molecules
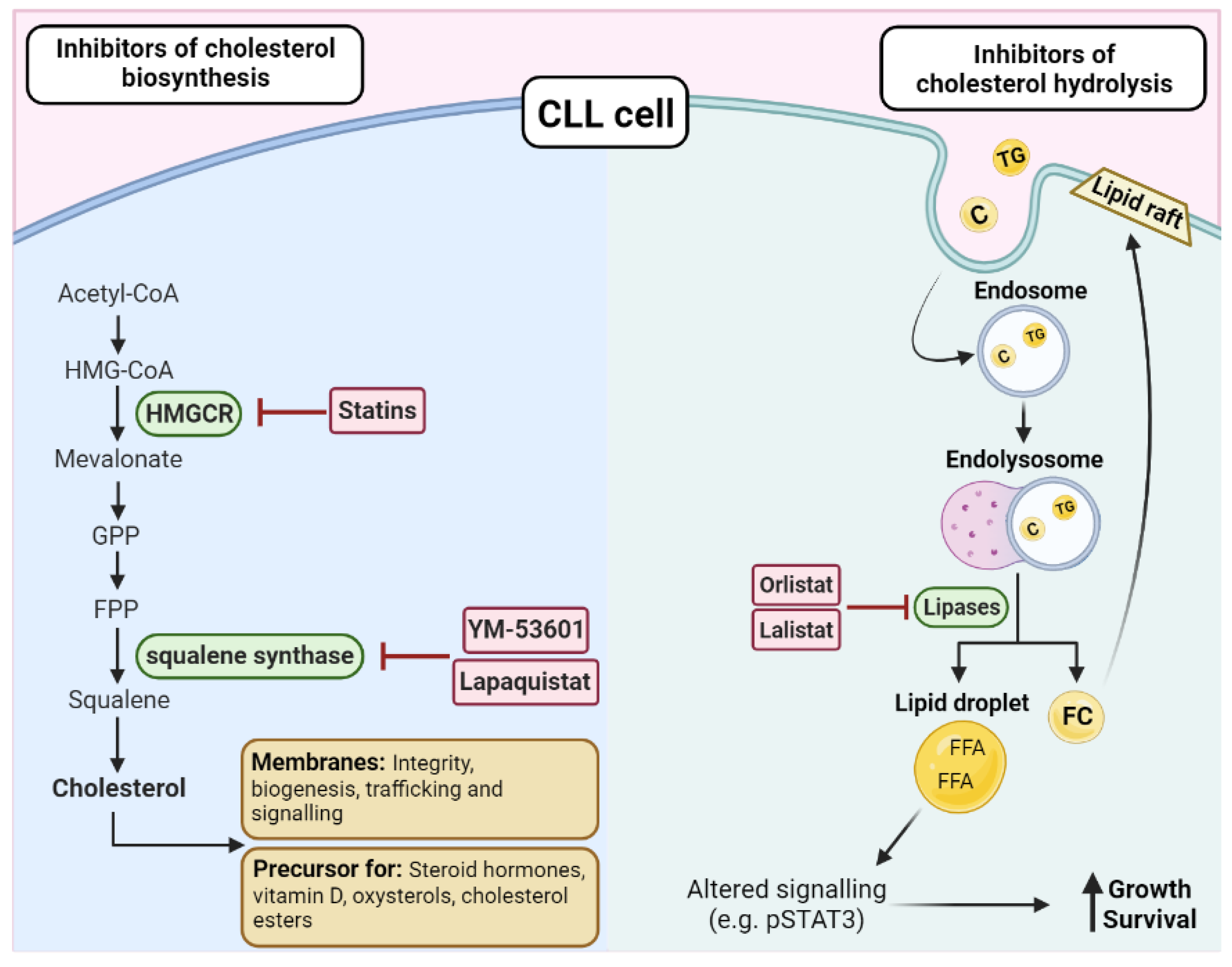
8. Endogenous Sterol Synthesis Pathways
9. The Use of Cholesterol Lowering Drugs in CLL
9.1. Statins
9.2. Other Cholesterol Lowering Drugs in CLL
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radišauskas, R.; Kuzmickienė, I.; Milinavičienė, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina (Kaunas Lith.) 2016, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Howard, L.E.; Cooperberg, M.R.; Kane, C.J.; Aronson, W.J.; Terris, M.K.; Amling, C.L.; Freedland, S.J. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 2349–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [Green Version]
- Marini, A.; Carulli, G.; Azzara, A.; Grassi, B.; Ambrogi, F. Serum cholesterol and triglycerides in hematological malignancies. Acta Haematol. 1989, 81, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.R.; Sorlie, P.D.; Feinleib, M.; McNamara, P.M.; Kannel, W.B.; Dawber, T.R. Cancer incidence by levels of cholesterol. JAMA 1981, 245, 247–252. [Google Scholar] [CrossRef]
- Murai, T. Cholesterol lowering: Role in cancer prevention and treatment. Biol. Chem. 2015, 396, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar]
- Hoffmann, P.; Roumeguère, T.; Schulman, C.; van Velthoven, R. Use of statins and outcome of BCG treatment for bladder cancer. N. Engl. J. Med. 2006, 355, 2705–2707. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.K.; Barrett-Connor, E.; Edelstein, S. Low plasma cholesterol predicts an increased risk of lung cancer in elderly women. Prev. Med. 1995, 24, 557–562. [Google Scholar] [CrossRef]
- Horton, B.J.; Sabine, J.R. Metabolic controls in precancerous liver: Defective control of cholesterol synthesis in rats fed N-2-fluorenylacetamide. Eur. J. Cancer 1971, 7, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Thurgood, L.A.; Dwyer, E.S.; Lower, K.M.; Chataway, T.K.; Kuss, B.J. Altered expression of metabolic pathways in CLL detected by unlabelled quantitative mass spectrometry analysis. Br. J. Haematol. 2019, 185, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Scarfò, L.; Ferreri, A.J.M.; Ghia, P. Chronic lymphocytic leukaemia. Crit. Rev. Oncol./Hematol. 2016, 104, 169–182. [Google Scholar] [CrossRef]
- Burger, J.A. Treatment of Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2020, 383, 460–473. [Google Scholar] [CrossRef]
- Mozessohn, L.; Earle, C.; Spaner, D.; Cheng, S.Y.; Kumar, M.; Buckstein, R. The Association of Dyslipidemia with Chronic Lymphocytic Leukemia: A Population-Based Study. JNCI J. Natl. Cancer Inst. 2016, 109, djw226. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Buckstein, R.; Spaner, D.E. A link between hypercholesterolemia and chronic lymphocytic leukemia. Leuk. Lymphoma 2016, 57, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Cucuianu, A.; Malide, D.; Petrov, L.; Patiu, M.; Vlaicu, S.; Cucuianu, M. Serum cholesterol and apoprotein B levels and serum cholinesterase activity in selected hematologic malignancies. Rom. J. Intern. Med. = Rev. Roum. De Med. Interne 1992, 30, 261–268. [Google Scholar]
- Gao, R.; Du, K.; Liang, J.; Xia, Y.; Wu, J.; Li, Y.; Pan, B.; Wang, L.; Li, J.; Xu, W. Low Serum Cholesterol Level Is a Significant Prognostic Factor That Improves CLL-IPI in Chronic Lymphocytic Leukaemia. Int. J. Mol. Sci. 2023, 24, 7396. [Google Scholar] [CrossRef]
- Sankanagoudar, S.; Singh, G.; Mahapatra, M.; Kumar, L.; Chandra, N. Cholesterol Homeostasis in Isolated Lymphocytes: A Differential Correlation Between Male Control and Chronic Lymphocytic Leukemia Subjects. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 23–30. [Google Scholar] [CrossRef]
- MacIntyre, D.A.; Jiménez, B.; Lewintre, E.J.; Martín, C.R.; Schäfer, H.; Ballesteros, C.G.; Mayans, J.R.; Spraul, M.; García-Conde, J.; Pineda-Lucena, A. Serum metabolome analysis by 1H-NMR reveals differences between chronic lymphocytic leukaemia molecular subgroups. Leukemia 2010, 24, 788–797. [Google Scholar] [CrossRef] [Green Version]
- Tomic, J.; Lichty, B.; Spaner, D.E. Aberrant interferon-signaling is associated with aggressive chronic lymphocytic leukemia. Blood 2011, 117, 2668–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, D.R.; Magura, L.A.; Warren, H.A.; Harrison, J.D.; Diehl, L.F.; Weinberg, J.B. Statin use and need for therapy in chronic lymphocytic leukemia. Leuk. Lymphoma 2010, 51, 2295–2298. [Google Scholar] [CrossRef] [PubMed]
- McCaw, L.; Shi, Y.; Wang, G.; Li, Y.J.; Spaner, D.E. Low Density Lipoproteins Amplify Cytokine-signaling in Chronic Lymphocytic Leukemia Cells. EBioMedicine 2017, 15, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litvinov, D.Y.; Savushkin, E.V.; Dergunov, A.D. Intracellular and Plasma Membrane Events in Cholesterol Transport and Homeostasis. J. Lipids 2018, 2018, 3965054. [Google Scholar] [CrossRef] [Green Version]
- Tabas, I. Free cholesterol-induced cytotoxicity a possible contributing factor to macrophage foam cell necrosis in advanced atherosclerotic lesions. Trends Cardiovasc. Med. 1997, 7, 256–263. [Google Scholar] [CrossRef]
- Warner, G.J.; Stoudt, G.; Bamberger, M.; Johnson, W.J.; Rothblat, G.H. Cell toxicity induced by inhibition of acyl coenzyme A: Cholesterol acyltransferase and accumulation of unesterified cholesterol. J. Biol. Chem. 1995, 270, 5772–5778. [Google Scholar] [CrossRef] [Green Version]
- Mulas, M.F.; Abete, C.; Pulisci, D.; Pani, A.; Massidda, B.; Dessì, S.; Mandas, A. Cholesterol esters as growth regulators of lymphocytic leukaemia cells. Cell Prolif. 2011, 44, 360–371. [Google Scholar] [CrossRef]
- Hildebrand, J.; Stryckmans, P.; Stoffyn, P. Neutral glycolipids in leukemic and nonleukemic leukocytes. J. Lipid Res. 1971, 12, 361–366. [Google Scholar] [CrossRef]
- Gottfried, E.L. Lipids of human leukocytes: Relation to celltype. J. Lipid Res. 1967, 8, 321–327. [Google Scholar] [CrossRef]
- Golomb, H.M.; Saffold, C.W.; Nathans, A.H.; Dawson, G. Phospholipid and cholesterol differences amongst leukemic cell types with special reference to hairy cell leukemia: A preliminary report. Clin. Chim. Acta 1981, 116, 311–318. [Google Scholar] [CrossRef]
- Schörghofer, D.; Kinslechner, K.; Preitschopf, A.; Schütz, B.; Röhrl, C.; Hengstschläger, M.; Stangl, H.; Mikula, M. The HDL receptor SR-BI is associated with human prostate cancer progression and plays a possible role in establishing androgen independence. Reprod. Biol. Endocrinol. RBE 2015, 13, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Yavasoglu, I.; Sargin, G.; Yilmaz, F.; Altındag, S.; Akgun, G.; Tombak, A.; Toka, B.; Dal, S.; Ozbas, H.; Cetin, G.; et al. Cholesterol Levels in Patients with Chronic Lymphocytic Leukemia. J. Natl. Med. Assoc. 2017, 109, 23–27. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.M.; Scielzo, C.; Angeloni, N.L.; Deiss-Yehiely, E.; Scarfo, L.; Ranghetti, P.; Ma, S.; Kaplan, J.; Barbaglio, F.; Gordon, L.I.; et al. Synthetic high-density lipoproteins as targeted monotherapy for chronic lymphocytic leukemia. Oncotarget 2017, 8, 11219–11227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Damiano, M.G.; Zhang, H.; Tripathy, S.; Luthi, A.J.; Rink, J.S.; Ugolkov, A.V.; Singh, A.T.; Dave, S.S.; Gordon, L.I.; et al. Biomimetic, synthetic HDL nanostructures for lymphoma. Proc. Natl. Acad. Sci. USA. 2013, 110, 2511–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, J.L.; Brown, M.S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 1977, 46, 897–930. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, K.L.; Ruan, X.Z.; Liu, B.C. Dysregulation of the Low-Density Lipoprotein Receptor Pathway Is Involved in Lipid Disorder-Mediated Organ Injury. Int. J. Biol. Sci. 2016, 12, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hughes-Fulford, M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int. J. Cancer 2001, 91, 41–45. [Google Scholar] [CrossRef]
- Juliusson, G.; Vitols, S. Impaired low-density lipoprotein receptor activity in chronic B-lymphocytic leukaemia cells. Eur. J. Haematol. 1988, 40, 18–24. [Google Scholar] [CrossRef]
- Vitols, S.; Gahrton, G.; Ost, A.; Peterson, C. Elevated low density lipoprotein receptor activity in leukemic cells with monocytic differentiation. Blood 1984, 63, 1186–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damle, R.N.; Calissano, C.; Chiorazzi, N. Chronic lymphocytic leukaemia: A disease of activated monoclonal B cells. Best Pract. Res. Clin. Haematol. 2010, 23, 33–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calissano, C.; Damle, R.N.; Marsilio, S.; Yan, X.-J.; Yancopoulos, S.; Hayes, G.; Emson, C.; Murphy, E.J.; Hellerstein, M.K.; Sison, C.; et al. Intraclonal Complexity in Chronic Lymphocytic Leukemia: Fractions Enriched in Recently Born/Divided and Older/Quiescent Cells. Mol. Med. 2011, 17, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, T.; Basheeruddin, K.; Ping, L.; Frazer, S.; Getz, G.S. Mechanism of the growth-related activation of the low density lipoprotein receptor pathway. J. Biol. Chem. 1989, 264(3), 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Seija, N.; Uriepero, A.; Souto-Padron, T.; Oliver, C.; Irigoin, V.; Guillermo, C.; Navarrete, M.A.; Inés Landoni, A.; Dighiero, G.; et al. LPL protein in Chronic Lymphocytic Leukaemia have different origins in Mutated and Unmutated patients. Advances for a new prognostic marker in CLL. Br. J. Haematol. 2018, 182, 521–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombout, A.; Verhasselt, B.; Philippé, J. Lipoprotein lipase in chronic lymphocytic leukemia: Function and prognostic implications. Eur. J. Haematol. 2016, 97, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Heintel, D.; Kienle, D.; Shehata, M.; Kröber, A.; Kroemer, E.; Schwarzinger, I.; Mitteregger, D.; Le, T.; Gleiss, A.; Mannhalter, C.; et al. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia 2005, 19, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, J.B.; Volkheimer, A.D.; Mihovilovic, M.; Jiang, N.; Chen, Y.; Bond, K.; Moore, J.O.; Gockerman, J.P.; Diehl, L.F.; de Castro, C.M.; et al. Apolipoprotein E genotype as a determinant of survival in chronic lymphocytic leukemia. Leukemia 2008, 22, 2184–2192. [Google Scholar] [CrossRef] [Green Version]
- Thurgood, L.A.; Best, O.G.; Rowland, A.; Lower, K.M.; Brooks, D.A.; Kuss, B.J. Lipid uptake in chronic lymphocytic leukemia. Exp. Hematol. 2022, 106, 58–67. [Google Scholar] [CrossRef]
- Sakashita, A.M.; Bydlowski, S.P.; Chamone, D.A.; Maranhão, R.C. Plasma kinetics of an artificial emulsion resembling chylomicrons in patients with chronic lymphocytic leukemia. Ann. Hematol. 2000, 79, 687–690. [Google Scholar] [CrossRef]
- Rozovski, U.; Grgurevic, S.; Bueso-Ramos, C.; Harris, D.M.; Li, P.; Liu, Z.; Wu, J.Y.; Jain, P.; Wierda, W.; Burger, J.; et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Mol. Cancer Res. MCR 2015, 13, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Spaner, D.E.; Lee, E.; Shi, Y.; Wen, F.; Li, Y.; Tung, S.; McCaw, L.; Wong, K.; Gary-Gouy, H.; Dalloul, A.; et al. PPAR-alpha is a therapeutic target for chronic lymphocytic leukemia. Leukemia 2013, 27, 1090–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severin, F.; Frezzato, F.; Visentin, A.; Martini, V.; Trimarco, V.; Carraro, S.; Tibaldi, E.; Brunati, A.M.; Piazza, F.; Semenzato, G.; et al. In Chronic Lymphocytic Leukemia the JAK2/STAT3 Pathway Is Constitutively Activated and Its Inhibition Leads to CLL Cell Death Unaffected by the Protective Bone Marrow Microenvironment. Cancers 2019, 11, 1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madden, E.A.; Melnykovych, G.; Fiskin, A.M. Compactin (ML-236B) reduces the content of filipin-cholesterol complexes in the plasma membrane of chronic lymphocytic leukemia cells. Exp. Cell Res. 1984, 153, 91–98. [Google Scholar] [CrossRef]
- Harwood, H.J., Jr.; Alvarez, I.M.; Noyes, W.D.; Stacpoole, P.W. In vivo regulation of human leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase: Increased enzyme protein concentration and catalytic efficiency in human leukemia and lymphoma. J. Lipid Res. 1991, 32, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Inbar, M.; Shinitzky, M. Increase of cholesterol level in the surface membrane of lymphoma cells and its inhibitory effect on ascites tumor development. Proc. Natl. Acad. Sci. USA 1974, 71, 2128–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinitzky, M.; Inbar, M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J. Mol. Biol. 1974, 85, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Deliconstantinos, G.; Daefler, S.; Krueger, G.R. Cholesterol modulation of membrane fluidity and ecto-nucleotide triphosphatase activity in human normal and CLL lymphocytes. Anticancer Res. 1987, 7, 347–352. [Google Scholar]
- Daefler, S.; Krueger, G.R.; Mödder, B.; Deliconstantinos, G. Cell membrane fluidity in chronic lymphocytic leukemia (CLL) lymphocytes and its relation to membrane receptor expression. J. Exp. Pathol. 1987, 3, 147–154. [Google Scholar]
- Inbar, M.; Shinitzky, M. Cholesterol as a bioregulator in the development and inhibition of leukemia. Proc. Natl. Acad. Sci. USA 1974, 71, 4229–4231. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.J.; Poznansky, M.J. Modulation of 3-hydroxy-3-methylglutaryl-CoA reductase by changes in microsomal cholesterol content or phospholipid composition. Proc. Natl. Acad. Sci. USA 1987, 84, 118–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feingold, K.R.; Wiley, M.H.; Moser, A.H.; Siperstein, M.D. Altered activation state of hydroxymethylglutaryl-coenzyme A reductase in liver tumors. Arch. Biochem. Biophys. 1983, 226, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.G.; Wilce, P.A. Reversible phosphorylation of 3-hydroxy-3-methylglutaryl CoA reductase in Morris hepatomas. Biochem. Biophys. Res. Commun. 1983, 114, 473–478. [Google Scholar] [CrossRef]
- Kaplan, M.R.; Simoni, R.D. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J. Cell Biol. 1985, 101, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Endo, A. The discovery and development of HMG-CoA reductase inhibitors. 1992. Atheroscler. Suppl. 2004, 5, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-L.; Qin, X. Does adherence to lipid-lowering medications improve cancer survival? A nationwide study of breast and colorectal cancer, and melanoma. Br. J. Clin. Pharmacol. 2021, 87, 1847–1858. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A. Current perspectives on statins as potential anti-cancer therapeutics: Clinical outcomes and underlying molecular mechanisms. Transl. Lung Cancer Res. 2019, 8, 692–699. [Google Scholar] [CrossRef]
- Vitols, S.; Angelin, B.; Juliusson, G. Simvastatin impairs mitogen-induced proliferation of malignant B-lymphocytes from humans—In Vitro and In Vivo studies. Lipids 1997, 32, 255–262. [Google Scholar] [CrossRef]
- Chapman-Shimshoni, D.; Yuklea, M.; Radnay, J.; Shapiro, H.; Lishner, M. Simvastatin induces apoptosis of B-CLL cells by activation of mitochondrial caspase 9. Exp. Hematol. 2003, 31, 779–783. [Google Scholar] [CrossRef]
- Pallasch, C.P.; Schwamb, J.; Königs, S.; Schulz, A.; Debey, S.; Kofler, D.; Schultze, J.L.; Hallek, M.; Ultsch, A.; Wendtner, C.M. Targeting lipid metabolism by the lipoprotein lipase inhibitor orlistat results in apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia 2008, 22, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Podhorecka, M.; Halicka, D.; Klimek, P.; Kowal, M.; Chocholska, S.; Dmoszynska, A. Simvastatin and purine analogs have a synergic effect on apoptosis of chronic lymphocytic leukemia cells. Ann. Hematol. 2010, 89, 1115–1124. [Google Scholar] [CrossRef] [Green Version]
- Żołnierczyk, J.D.; Borowiak, A.; Hikisz, P.; Cebula-Obrzut, B.; Błoński, J.Z.; Smolewski, P.; Robak, T.; Kiliańska, Z.M. Promising anti-leukemic activity of atorvastatin. Oncol. Rep. 2013, 29, 2065–2071. [Google Scholar] [CrossRef] [Green Version]
- Benakanakere, I.; Johnson, T.; Sleightholm, R.; Villeda, V.; Arya, M.; Bobba, R.; Freter, C.; Huang, C. Targeting cholesterol synthesis increases chemoimmuno-sensitivity in chronic lymphocytic leukemia cells. Exp. Hematol. Oncol. 2014, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoni, M.M.; Riganti, C.; Vitale, C.; Griggio, V.; Campia, I.; Robino, M.; Foglietta, M.; Castella, B.; Sciancalepore, P.; Buondonno, I.; et al. Simvastatin and downstream inhibitors circumvent constitutive and stromal cell-induced resistance to doxorubicin in IGHV unmutated CLL cells. Oncotarget 2015, 6, 29833–29846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Roberts, A.; Juarez, D.; Vo, T.T.; Bhatt, S.; Herzog, L.O.; Mallya, S.; Bellin, R.J.; Agarwal, S.K.; Salem, A.H.; et al. Statins enhance efficacy of venetoclax in blood cancers. Sci Transl. Med. 2018, 10, eaaq1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez, N.; Tripathi, R.; Giró, A.; Rosich, L.; López-Guerra, M.; López-Oreja, I.; Playa-Albinyana, H.; Arenas, F.; Mas, J.M.; Pérez-Galán, P.; et al. Systems biology drug screening identifies statins as enhancers of current therapies in chronic lymphocytic leukemia. Sci. Rep. 2020, 10, 22153. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Rabe, K.G.; Kay, N.E.; Zent, C.S.; Call, T.G.; Slager, S.L.; Bowen, D.A.; Schwager, S.M.; Nowakowski, G.S. Statin and non-steroidal anti-inflammatory drug use in relation to clinical outcome among patients with Rai stage 0 chronic lymphocytic leukemia. Leuk. Lymphoma 2010, 51, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.M.; Dave, V.; Srinivas, S.; Chang, V.T.-S.; McPherson, M.; Kasimis, B. Statins and survival in a cohort of veterans with chronic lymphocytic leukemia (CLL). J. Clin. Oncol. 2014, 32, 1610. [Google Scholar] [CrossRef]
- Chae, Y.K.; Trinh, L.; Jain, P.; Wang, X.; Rozovski, U.; Wierda, W.G.; Keating, M.J.; Estrov, Z. Statin and aspirin use is associated with improved outcome of FCR therapy in relapsed/refractory chronic lymphocytic leukemia. Blood 2014, 123, 1424–1426. [Google Scholar] [CrossRef] [Green Version]
- Righolt, C.H.; Zhang, G.; Ye, X.; Banerji, V.; Johnston, J.B.; Gibson, S.; Mahmud, S.M. Statin Use and Chronic Lymphocytic Leukemia Incidence: A Nested Case-Control Study in Manitoba, Canada. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1495–1501. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochem. Pharmacol. 2008, 75, 907–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuní, S.; Pérez-Aciego, P.; Pérez-Chacón, G.; Vargas, J.A.; Sánchez, A.; Martín-Saavedra, F.M.; Ballester, S.; García-Marco, J.; Jordá, J.; Durántez, A. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia 2004, 18, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Cragg, M.S. The potential effect of statins on rituximab immunotherapy. PLoS Med. 2008, 5, e77. [Google Scholar] [CrossRef]
- Winiarska, M.; Bil, J.; Wilczek, E.; Wilczynski, G.M.; Lekka, M.; Engelberts, P.J.; Mackus, W.J.; Gorska, E.; Bojarski, L.; Stoklosa, T.; et al. Statins impair antitumor effects of rituximab by inducing conformational changes of CD20. PLoS Med. 2008, 5, e64. [Google Scholar] [CrossRef] [Green Version]
- Pirmoradi, L.; Seyfizadeh, N.; Ghavami, S.; Zeki, A.A.; Shojaei, S. Targeting cholesterol metabolism in glioblastoma: A new therapeutic approach in cancer therapy. J. Investig. Med. 2019, 67, 715. [Google Scholar] [CrossRef] [Green Version]
- Segala, G.; de Medina, P.; Iuliano, L.; Zerbinati, C.; Paillasse, M.R.; Noguer, E.; Dalenc, F.; Payré, B.; Jordan, V.C.; Record, M.; et al. 5,6-Epoxy-cholesterols contribute to the anticancer pharmacology of tamoxifen in breast cancer cells. Biochem. Pharmacol. 2013, 86, 175–189. [Google Scholar] [CrossRef]
- Solomon, K.R.; Pelton, K.; Boucher, K.; Joo, J.; Tully, C.; Zurakowski, D.; Schaffner, C.P.; Kim, J.; Freeman, M.R. Ezetimibe is an inhibitor of tumor angiogenesis. Am. J. Pathol. 2009, 174, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, A.; Jia, Z.; Qiao, C.; Wang, M.; Ding, X. Cholesterol metabolism in drug-resistant cancer (Review). Int. J. Oncol. 2020, 57, 1103–1115. [Google Scholar] [CrossRef] [PubMed]


| Year | Compound(s) | Model(s) | Study Summary | Dependent Variables Assessed | Key Findings | Reference |
|---|---|---|---|---|---|---|
| 1984 | Mevastatin | Primary CLL cells | Assessed cholesterol content in the plasma membrane of CLL cells with and without mevastatin (compactin) treatment | Filipin–cholesterol complexes within the plasma membrane and cell growth | Mevastatin reduced plasma membrane cholesterol in CLL cells, even in the presence of lipid-rich serum | [54] |
| 1997 | Simvastatin, lovastatin, pravastatin | Primary CLL cells | Investigated multiple statins as inhibitors of CLL cell proliferation | Mitogen-induced thymidine uptake in presence and absence of statins | Statins inhibited thymidine uptake in a concentration-dependent manner | [68] |
| 2003 | Simvastatin | Primary CLL cells | Assessed response of primary CLL cells to simvastatin | Viability-, apoptosis-, necrosis-, apoptotic-signalling cascade | Significant reduction in CLL cell viability and a significant increase in apoptosis and necrosis | [69] |
| 2008 | Orlistat | Primary B-cells from CLL patients and healthy control donors | Examined the inhibitory effects of orlistat on primary CLL and healthy B cells | Apoptosis, viability, and lipase activity | Orlistat induced apoptosis in primary CLL cells and acted in synergy with fludarabine. Effects were reduced by BCR stimulation. | [70] |
| 2010 | Simvastatin | Primary peripheral blood and bone marrow mononuclear cells isolated from newly diagnosed, untreated CLL patients | Ex-vivo assessment of simvastatin alone and in combination with fludarabine and cladribine | Apoptosis and DNA damage | Simvastatin induced tumour-specific apoptosis when administered alone and with the purine analogues | [71] |
| 2013 | Atorvastatin | Primary CLL cells | Evaluated the effect of atorvastatin on primary CLL cells | Apoptosis, viability, expression of proteins that regulate apoptosis | Atorvastatin induced apoptosis in CLL cells, but not healthy mononuclear cells | [72] |
| 2013 | Atorvastatin | Primary CLL cells | Investigated the effect of atorvastatin on lymphocytes from CLL patient cells | Apoptosis and expression of CD5, CD38, ZAP-70, and Annexin V | Atorvastatin increased apoptosis and decreased expression of CD5 and ZAP-70 in patient CLL cells | [33] |
| 2014 | BIBB-515, YM-53601 and TAK-475 (cholesterol lowering agents) | MEC-2 CLL cell line and primary CLL cells | Explored effects of agents that target cholesterol synthesis on the efficacy of anti-CD20 chemoimmunotherapeutic regimens | Total cholesterol levels, CD20 expression, and cell viability | Agents reduced total cholesterol levels, increased CD20 expression and chemoimmuno-sensitivity to various therapeutic agents | [73] |
| 2015 | Simvastatin | Primary CLL cells | Comparison of IGHV mutated and unmutated multidrug resistant CLL cells to identify therapeutic targets | Cholesterol levels, activation of pathways (Ras/ERK1-2, RhoA/RhoA, HIF-1α/P-glycoprotein axis) | Increased activation of Ras/ERK1–2 and RhoA/RhoA kinase-signalling pathways conferred drug resistance in CLL cells, which was countered by simvastatin | [74] |
| 2017 | Lalistat | Primary CLL cells | Functional analysis of the role of low-density lipoproteins (LDLs) in CLL | CLL cell count, and assessment of lipid droplet and membrane cholesterol content, phosphorylated STAT3 and cytokine levels | LDL adminstration increased levels of cholesterol in plasma membrane, increased phosphorylation of STAT3 and CLL cell number, indicating a proliferative effect | [23] |
| 2018 | Simvastatin | Primary CLL cells, AML and DLBCL cell lines, C57BL/6N mice injected with lymphoma cells | Assessed effects of simvastatin, with and without venetoclax, on cell lines and primary cells from a variety of lymphomas and leukemias | Viability, protein geranylgeranylation, levels of pro-apoptotic PUMA protein | Statin-mediated inhibition of HMGCR enhanced antitumor effects of venetoclax (decreased protein geranylgeranylation and increased expression of PUMA) | [75] |
| 2020 | Simvastatin, lovastatin, fluvastatin, rosuvastatin | HG3 and MEC-1 CLL cells lines, Primary CLL cells | An in-silico and in-vitro approach to identify drug and drug combinations for targeting CLL cells in the tumour microenvironment | CLL cell viability, cytotoxicity, and proliferation | Simvastatin potentiated the cytotoxic effects of venetoclax and ibrutinib | [76] |
| Year | Study Summary | Subjects (n) | Results | Key Findings | Reference |
|---|---|---|---|---|---|
| 2010 | Evaluated the effects of statins on clinical outcomes and rituximab efficacy | Newly diagnosed Rai stage 0 CLL patients (n = 686) | TFT and OS were not significantly different between CLL patients who were or were not taking statins at time of diagnosis (TFT 7.9 with statins vs. 11.8 years without, p = 0.52; OS 10.1 with statins vs. 11.4 years without, p = 0.1) | Statins did not appear to impact the clinical outcomes assessed or rituximab efficacy | [77] |
| 2010 | Assessed overall survival (OS) and treatment free survival (TFS) | CLL patients taking statins at diagnosis (n = 254) | No significant difference in TFT or OS between CLL patients who were or were not taking statins at diagnosis | Administration of statins at time of diagnosis did not affect the clinical outcomes assessed | [22] |
| 2014 | Explored whether statin use could be a predictor of survival in CLL patients | CLL patients (n = 130) | Statin use was able to predict patient overall survival in a multivariate analysis (p < 0.017) | Statin use was a predictor of survival in older patients (median age = 72) with early-stage disease and an ECOG performance status of 1–2. | [78] |
| 2014 | Retrospective analysis of CLL patients treated with or without adjunct statin and/or aspirin treatment | Relapsed/refractory (R/R) CLL patients treated with FCR as a salvage therapy (n = 280) | Patients receiving both statins and aspirin (PFS—6.1 years, OS—9.2 years) vs. PFS—1.6 years, OS—3.7 years in patients not receiving statins (PFS p = 0.003; OS p = 0.05). CLL patients receiving both statins and aspirin had a 66% reduced risk of disease progression and a 60% reduced risk of death (PFS hazard ratio [HR] = 0.34, 95% confidence interval [CI] = 0.18–0.65, p < 0.001; OS HR = 0.40, 95% CI = 0.21–0.79, p = 0.008). | Administration of statins and aspirin was associated with a significant improvement in response rate and survival in R/R CLL patients treated with FCR as a salvage therapy | [79] |
| 2016 | Reviewed clinical data to establish a link between hypercholesterolaemia and CLL | CLL patients (n = 231) | Time to first treatment (TFT) was longer in CLL patients receiving statins (57.5 (IQR = 32, 77) vs. 36 (IQR = 11, 100) months, p < 0.02) | Study suggests a high incidence of hypercholesterolaemia in CLL patients, and that statins may reduce disease progression | [16] |
| 2016 | Population-based, case-control study investigating a potential association of dyslipidaemia with CLL | CLL patients > 66 years of age (n = 2124) and matched healthy controls (n = 7935) | Statin use prior to or after CLL diagnosis was associated with an improvement in OS (7.8 years, 95% CI 7.3 to 8.4 vs. 4.1 years, 95% CI 3.7 to 4.5 years, p < 0.001) | Statins and other lipid-lowering medications was associated with improved survival rates in CLL patients | [15] |
| 2019 | Nested case-control study examining the risk of CLL among statin users. Patients were grouped by chemical and pharmacodynamic properties of the medication | CLL patients > 40 years of age (n = 1385) and healthy controls matched based on gender, age and country of residence (n = 6841) | Low-potency lipophilic statins were associated with a lower risk of CLL (OR = 0.64, 95% CI 0.45–0.92), particularly in patients who more regularly received the stains (OR = 0.44, 95% CI 0.22–0.88). Administration of hydrophilic or high-potency lipophilic statins had no effect on incidence of CLL. | Administration of low-potency lipophilic statins was associated with a dose-dependent reduction in risk of CLL development | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, A.M.; Best, O.G.; Hotinski, A.K.; Kuss, B.J.; Thurgood, L.A. The Role of Cholesterol in Chronic Lymphocytic Leukemia Development and Pathogenesis. Metabolites 2023, 13, 799. https://doi.org/10.3390/metabo13070799
White AM, Best OG, Hotinski AK, Kuss BJ, Thurgood LA. The Role of Cholesterol in Chronic Lymphocytic Leukemia Development and Pathogenesis. Metabolites. 2023; 13(7):799. https://doi.org/10.3390/metabo13070799
Chicago/Turabian StyleWhite, Alana M., Oliver G. Best, Anya K. Hotinski, Bryone J. Kuss, and Lauren A. Thurgood. 2023. "The Role of Cholesterol in Chronic Lymphocytic Leukemia Development and Pathogenesis" Metabolites 13, no. 7: 799. https://doi.org/10.3390/metabo13070799





