A GABA Receptor Modulator and Semiochemical Compounds Evidenced Using Volatolomics as Candidate Markers of Chronic Exposure to Fipronil in Apis mellifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Honeybees
2.2. Insecticide Exposure and Artificial Rearing
2.3. Volatolomic Analysis
2.4. Data Treatment
2.4.1. Consumption and Survival Monitoring
2.4.2. Volatolome Analysis
3. Results
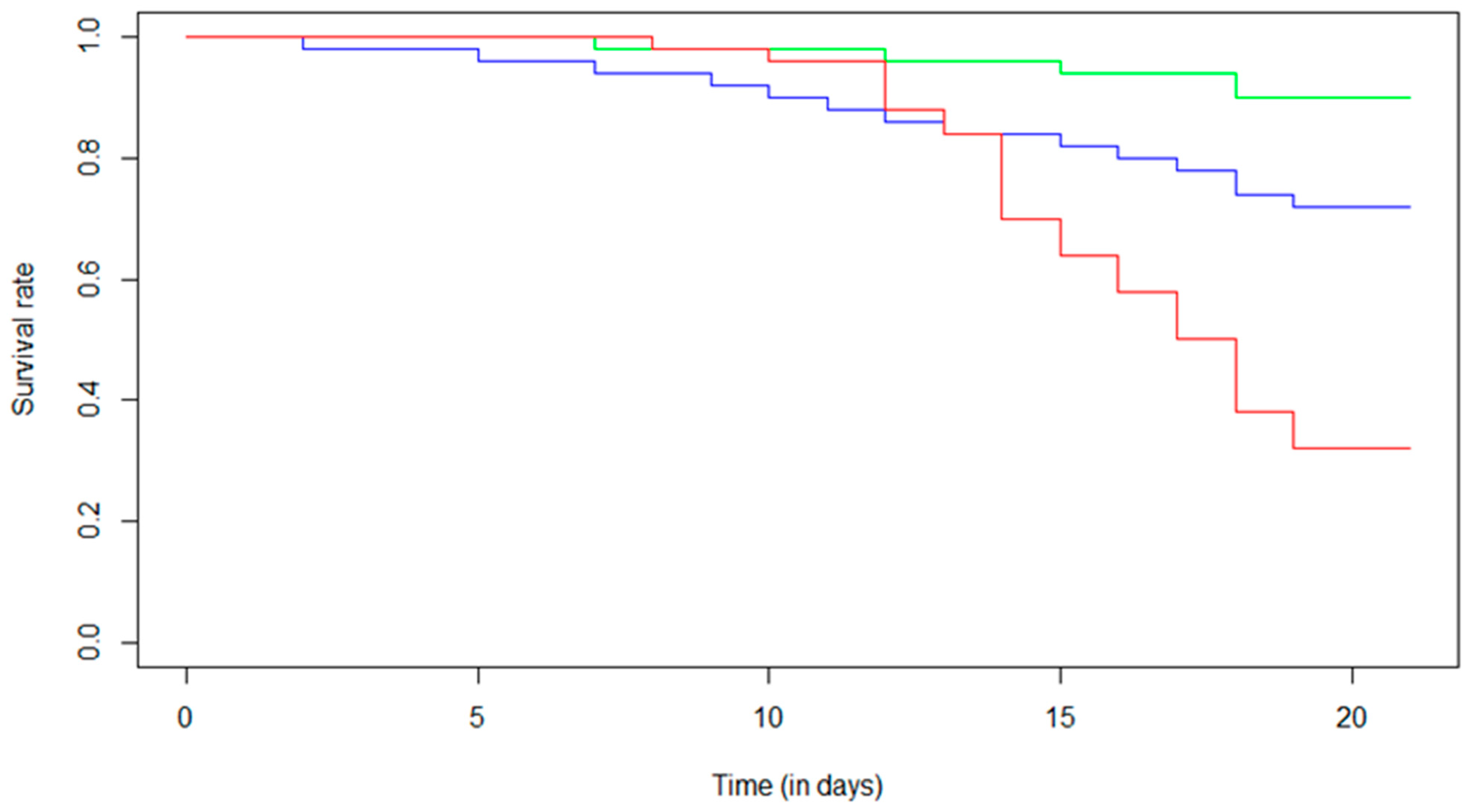
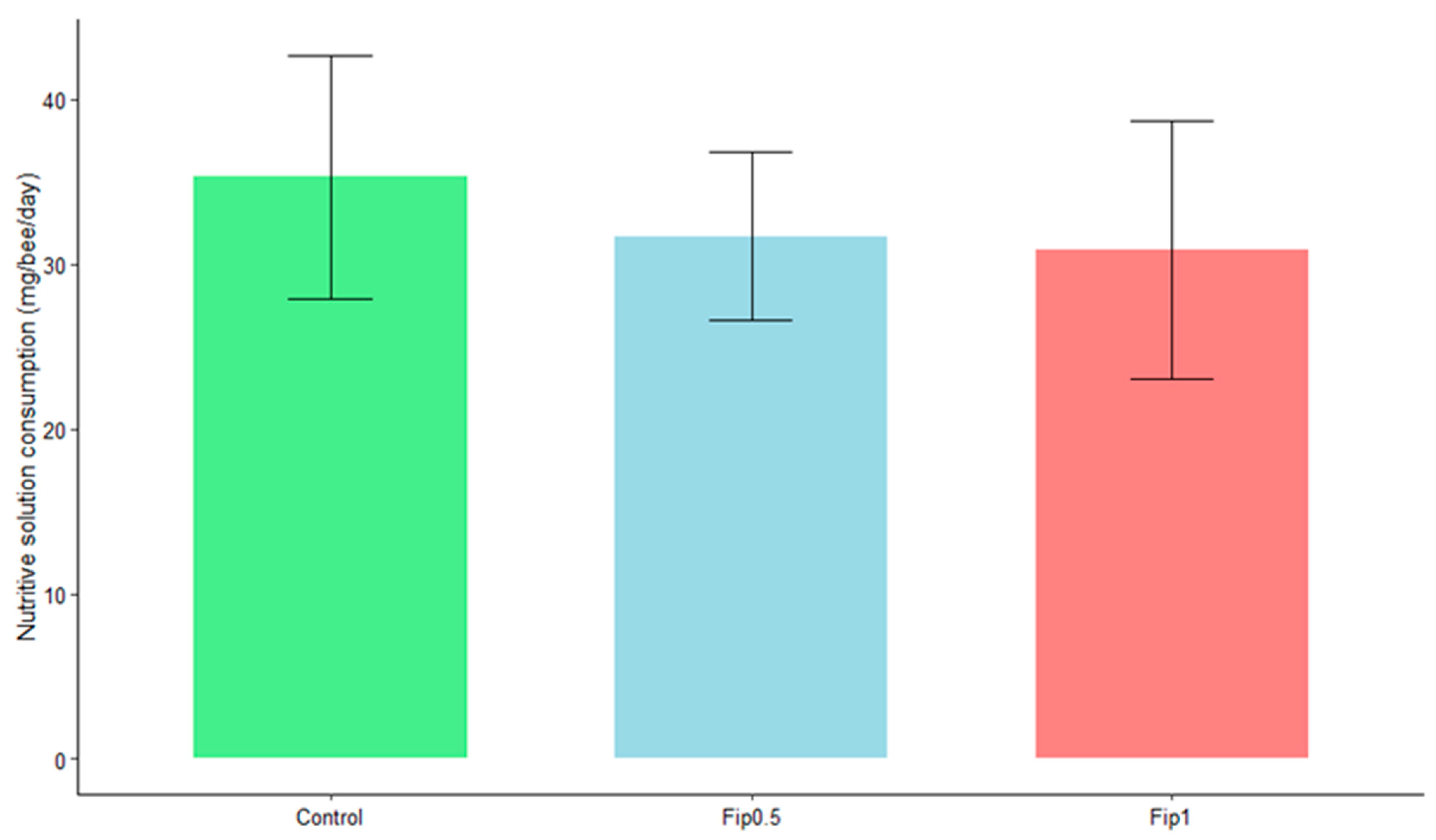
3.1. Effect of Fipronil Exposure on Survival and Sucrose Consumption
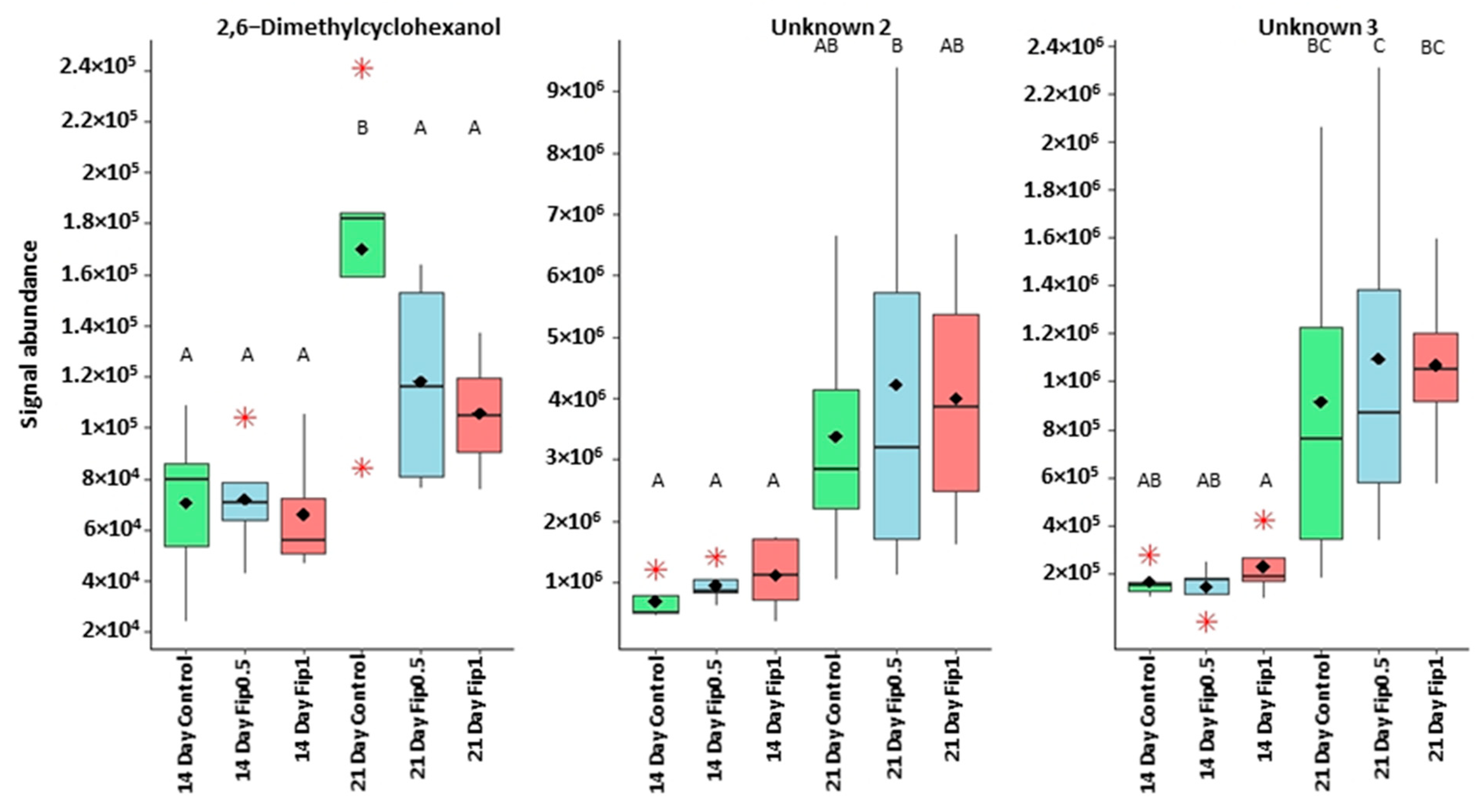
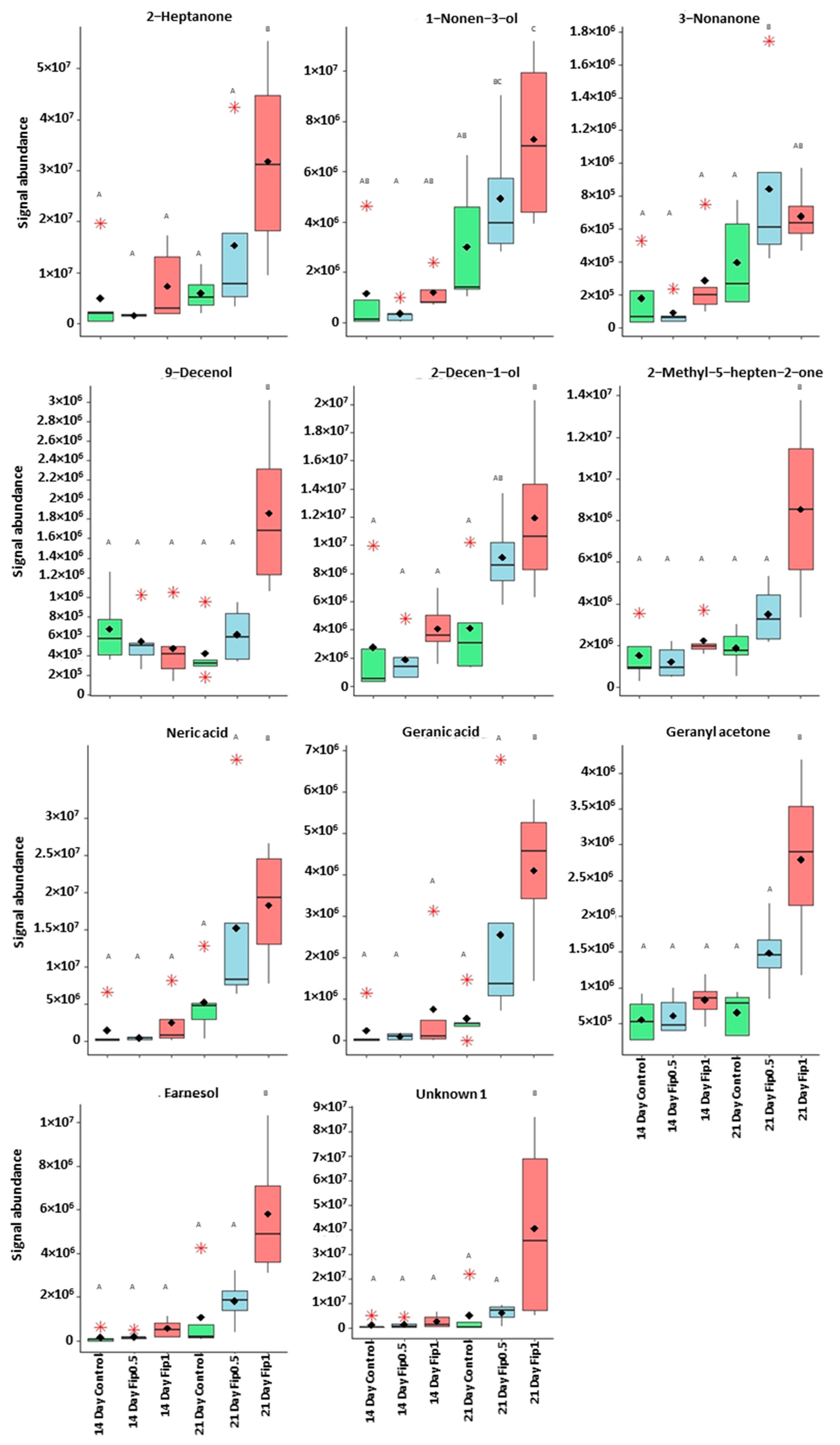
3.2. Volatolome Analysis of A. mellifera Exposed to Fipronil
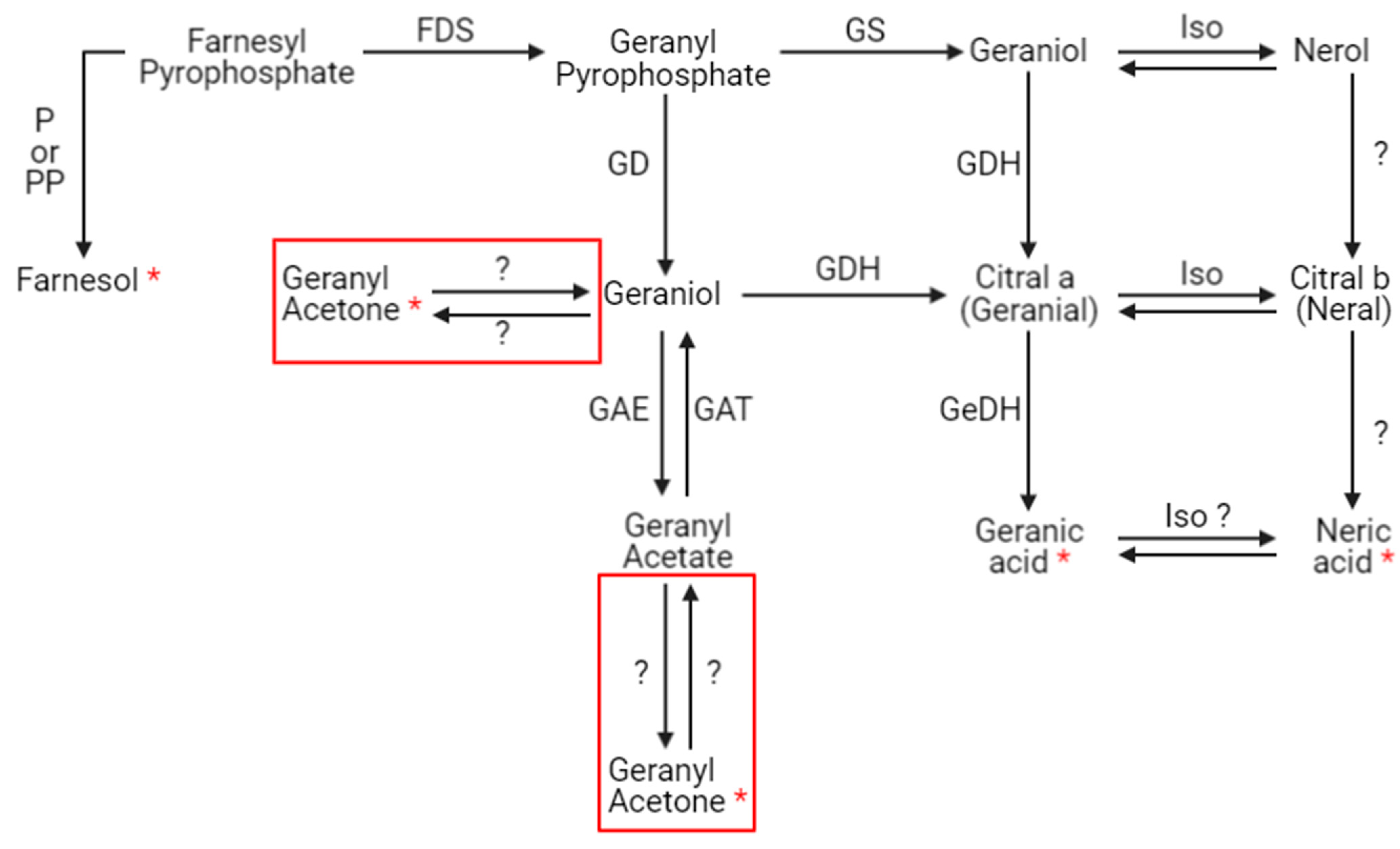
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamadeh, H.K.; Amin, R.P.; Paules, R.S.; Afshari, C.A. An Overview of Toxicogenomics. Curr. Issues Mol. Biol. 2002, 4, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Hayward, S.A.L. Application of Functional “Omics” in Environmental Stress Physiology: Insights, Limitations, and Future Challenges. Curr. Opin. Insect Sci. 2014, 4, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Brown, R.J.; Browne, M.A.; Dissanayake, A.; Lowe, D.; Jones, M.B.; Depledge, M.H. A Multibiomarker Approach to Environmental Assessment. Environ. Sci. Technol. 2004, 38, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Daston, G.P.; Degitz, S.J.; Denslow, N.D.; Hoke, R.A.; Kennedy, S.W.; Miracle, A.L.; Perkins, E.J.; Snape, J.; Tillitt, D.E.; et al. Toxicogenomics in Regulatory Ecotoxicology. Environ. Sci. Technol. 2006, 40, 4055–4065. [Google Scholar] [CrossRef] [Green Version]
- Poynton, H.C.; Varshavsky, J.R.; Chang, B.; Cavigiolio, G.; Chan, S.; Holman, P.S.; Loguinov, A.V.; Bauer, D.J.; Komachi, K.; Theil, E.C.; et al. Daphnia Magna Ecotoxicogenomics Provides Mechanistic Insights into Metal Toxicity. Environ. Sci. Technol. 2007, 41, 1044–1050. [Google Scholar] [CrossRef]
- Bijlsma, R.; Loeschcke, V. Environmental Stress, Adaptation and Evolution: An Overview. J. Evol. Biol. 2005, 18, 744–749. [Google Scholar] [CrossRef]
- Hidalgo, K.; Ratel, J.; Mercier, F.; Gauriat, B.; Bouchard, P.; Engel, E. Volatolomics in Bacterial Ecotoxicology, a Novel Method for Detecting Signatures of Pesticide Exposure? Front. Microbiol. 2019, 9, 3113. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid Volatolomics and Disease Detection. Angew. Chem. Int. Ed. 2015, 54, 11036–11048. [Google Scholar] [CrossRef] [PubMed]
- Giannoukos, S.; Agapiou, A.; Brkić, B.; Taylor, S. Volatolomics: A Broad Area of Experimentation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1105, 136–147. [Google Scholar] [CrossRef]
- Ratel, J.; Mercier, F.; Angénieux, M.; Kondjoyan, N.; Abouelkaram, S.; Blinet, P.; Travel, A.; Royer, E.; Baéza-Campone, E.; Cariou, R.; et al. Identification by Volatolomics of Hydrocarbon, Oxygenated, Sulfur and Aromatic Markers of Livestock Exposure to α-Hexabromocyclododecane. Food Chem. 2021, 374, 131504. [Google Scholar] [CrossRef]
- Berge, P.; Ratel, J.; Fournier, A.; Jondreville, C.; Feidt, C.; Roudaut, B.; Le Bizec, B.; Engel, E. Use of Volatile Compound Metabolic Signatures in Poultry Liver to Back-Trace Dietary Exposure to Rapidly Metabolized Xenobiotics. Environ. Sci. Technol. 2011, 45, 6584–6591. [Google Scholar] [CrossRef] [PubMed]
- Ratel, J.; Planche, C.; Mercier, F.; Blinet, P.; Kondjoyan, N.; Marchand, P.; Fournier, A.; Travel, A.; Jondreville, C.; Engel, E. Liver Volatolomics to Reveal Poultry Exposure to Γ-Hexabromocyclododecane (HBCD). Chemosphere 2017, 189, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, J.; Bouveresse, D.J.-R.; Abouelkaram, S.; Baéza, E.; Jondreville, C.; Travel, A.; Ratel, J.; Engel, E.; Rutledge, D.N. Comparison of Common Components Analysis with Principal Components Analysis and Independent Components Analysis: Application to SPME-GC-MS Volatolomic Signatures. Talanta 2018, 178, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Defois, C.; Ratel, J.; Denis, S.; Batut, B.; Beugnot, R.; Peyretaillade, E.; Engel, E.; Peyret, P. Environmental Pollutant Benzo[a]Pyrene Impacts the Volatile Metabolome and Transcriptome of the Human Gut Microbiota. Front. Microbiol. 2017, 8, 1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defois, C.; Ratel, J.; Garrait, G.; Denis, S.; Le Goff, O.; Talvas, J.; Mosoni, P.; Engel, E.; Peyret, P. Food Chemicals Disrupt Human Gut Microbiota Activity and Impact Intestinal Homeostasis As Revealed By In Vitro Systems. Sci. Rep. 2018, 8, 11006. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.; Larik, F.A.; Channar, P.A. Recent Synthetic Approaches to Fipronil, a Super-Effective and Safe Pesticide. Res. Chem. Intermed. 2016, 42, 6805–6813. [Google Scholar] [CrossRef]
- Agence française de sécurité sanitaire des aliments and Agence française de sécurité sanitaire environnementale (report), Evaluation Des Risques Pour La Santé Humaine Liés à Une Exposition Au Fipronil—Synthèse et Conclusion Du Rapport Du Groupe de Travail: L’ Exposition Au Fipronil Pourrait-Elle Entraîner Un Risque Pour l’ Homme ? 2005, 1–11. Available online: https://www.anses.fr/fr/system/files/RCCP-Ra-Fipronil.pdf (accessed on 15 December 2022).
- Bonnard, N.; Jagot, D.; Falcy, M.; Angeli, K.; Fastier, A.M.-O.R. Fiche Toxicologique N° 286: Fipronil. Institut National de Recherche et de Sécurité, 2012, 1–9. Available online: https://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_286 (accessed on 15 December 2022).
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Mcfield, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, D.; Morrissey, C.; Mineau, P. A Review of the Direct and Indirect Effects of Neonicotinoids and Fipronil on Vertebrate Wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [Green Version]
- vanEngelsdorp, D.; Meixner, M.D. A Historical Review of Managed Honey Bee Populations in Europe and the United States and the Factors That May Affect Them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Johnson, R.M. Honey Bee Toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef]
- Es-Salah, Z. Analyse Électrophysiologique, Pharmacologique et Moléculaire de Facteurs Modulant Les Effets d’ Un Insecticide, Le Fipronil, Sur Des Récepteurs Gabaergiques d’ Insectes; Université d’Angers: Angers, France, 2008. [Google Scholar]
- Casida, J.E.; Durkin, K.A. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Kairo, G.; Provost, B.; Tchamitchian, S.; Abdelkader, F.B.; Bonnet, M.; Cousin, M.; Sénéchal, J.; Benet, P.; Kretzschmar, A.; Belzunces, L.P.; et al. Drone Exposure to the Systemic Insecticide Fipronil Indirectly Impairs Queen Reproductive Potential. Sci. Rep. 2016, 6, 31904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kairo, G.; Biron, D.G.; Abdelkader, F.B.; Bonnet, M.; Tchamitchian, S.; Cousin, M.; Dussaubat, C.; Benoit, B.; Kretzschmar, A.; Belzunces, L.P.; et al. Nosema Ceranae, Fipronil and Their Combination Compromise Honey Bee Reproduction via Changes in Male Physiology. Sci. Rep. 2017, 7, 8556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaluski, R.; Kadri, S.M.; Alonso, D.P.; Martins Ribolla, P.E.; de Oliveira Orsi, R. Fipronil Promotes Motor and Behavioral Changes in Honey Bees (Apis Mellifera) and Affects the Development of Colonies Exposed to Sublethal Doses. Environ. Toxicol. Chem. 2015, 34, 1062–1069. [Google Scholar] [CrossRef]
- Colin, M.E.; Bonmatin, J.M.; Moineau, I.; Gaimon, C.; Brun, S.; Vermandere, J.P. A Method to Quantify and Analyze the Foraging Activity of Honey Bees: Relevance to the Sublethal Effects Induced by Systemic Insecticides. Arch. Environ. Contam. Toxicol. 2004, 47, 387–395. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome Analyses of the Honeybee Response to Nosema Ceranae and Insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef]
- Rouzé, R.; Moné, A.; Delbac, F.; Belzunces, L.; Blot, N. The Honeybee Gut Microbiota Is Altered after Chronic Exposure to Different Families of Insecticides and Infection by Nosema Ceranae. Microbes Environ. 2019, 34, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.L.; Texier, C.; Biron, D.G.; Blot, N.; Alaoui, H.; et al. Exposure to Sublethal Doses of Fipronil and Thiacloprid Highly Increases Mortality of Honeybees Previously Infected by Nosema Ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef] [Green Version]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-Insecticide Interactions: A Case Study of Nosema Ceranae and Fipronil Synergy on Honeybee. Sci. Rep. 2012, 2, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, L.; Roussel, M.; Pereira, B.; Delbac, F.; Diogon, M. Disruption of Oxidative Balance in the Gut of the Western Honeybee Apis Mellifera Exposed to the Intracellular Parasite Nosema Ceranae and to the Insecticide Fipronil. Microb. Biotechnol. 2017, 10, 1702–1717. [Google Scholar] [CrossRef]
- Chowdhury, L.; Croft, C.J.; Goel, S.; Zaman, N.; Tai, A.C.S.; Walch, E.M.; Smith, K.; Page, A.; Shea, K.M.; Hall, C.D.; et al. Differential Potency of 2,6-Dimethylcyclohexanol Isomers for Positive Modulation of GABAA Receptor Currents. J. Pharmacol. Exp. Ther. 2016, 357, 570–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T. Insect Repellent. US Patent US 20180064109A1, 8 March 2018. [Google Scholar]
- Hefetz, A.; Batra, S.W.T. Geranyl acetate and 2-decen-1-ol in the cephalic secretion of the solitary wasp Sceliphron caementarium (Sphecidae; Hymenoptera). Experientia 1979, 35, 1138–1139. [Google Scholar] [CrossRef]
- Collins, A.M.; Blum, M.S. Alarm Responses Caused by Newly Identified Compounds Dereived from the Honeybee Sting. J. Chem. Ecol. 1983, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Rochat, D.; Ramirez-Lucas, P.; Malosse, C.; Aldana, R.; Kakul, T.; Morin, J.P. Role of Solid-Phase Microextraction in the Identification of Highly Volatile Pheromones of Two Rhinoceros Beetles Scapanes Australis and Strategus Aloeus (Coleoptera, Scarabaeidae, Dynastinae). J. Chromatogr. A 2000, 885, 433–444. [Google Scholar] [CrossRef]
- Schorkopf, D.L.P.; Hrncir, M.; Mateus, S.; Zucchi, R.; Schmidt, V.M.; Barth, F.G. Mandibular Gland Secretions of Meliponine Worker Bees: Further Evidence for Their Role in Interspecific and Intraspecific Defence and Aggression and against Their Role in Food Source Signalling. J. Exp. Biol. 2009, 212, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Blum, M.S.; Brand, J.M. Social Insect Pheromones: Their Chemistry and Function. Integr. Comp. Biol. 1972, 12, 553–576. [Google Scholar] [CrossRef] [Green Version]
- Crewe, R.M.; Blum, M.S. Alarm Pheromones in the Genus Myrmica (Hymenoptera: Formicidae)—Their Composition, and Species Specificity. Z. Vgl. Physiol. 1970, 70, 363–373. [Google Scholar] [CrossRef]
- Tengö, J.; Bergströ, G.; Borg-Karlson, B.; Groth, I.; Francke, W. Volatile Compounds from Cephalic Secretions of Females in Two Cleptoparasite Bee Genera, Epeolus (Hym., Anthophoridae) and Coelioxys (Hym., Megachilidae). Z. Fur Naturforsch. Sect. C J. Biosci. 1982, 37, 376–380. [Google Scholar] [CrossRef]
- Pickett, J.A.; Williams, I.H.; Martin, A.P.; Smith, M.C. Nasonov Pheromon of the Honey Bee, Apis Mellifera L. (Hymenoptera, Apidae). Part I. Chemical Characterization J.A. J. Chem. Ecol. 1980, 6, 425–434. [Google Scholar] [CrossRef]
- Pickett, J.A.; Williams, I.H.; Smith, M.C.; Martin, A.P. Nasonov Pheromon of the Honey Bee, Apis Mellifera L. (Hymenoptera, Apidae). Part III. Regulation of Pheromone Composition and Production. J. Chem. Ecol. 1981, 7, 543–554. [Google Scholar] [CrossRef]
- Williams, I.H.; Pickett, J.A.; Martin, A.P. Nasonov Pheromon of the Honey Bee, Apis Mellifera L. (Hymenoptera, Apidae). Part II. Bioassay of the Components Using Foragers. J. Chem. Ecol. 1981, 7, 225–237. [Google Scholar] [CrossRef]
- Wolken, W.A.M.; Ten Have, R.; Van Der Werf, M.J. Amino Acid-Catalyzed Conversion of Citral: Cis-Trans Isomerization and Its Conversion into 6-Methyl-5-Hepten-2-One and Acetaldehyde. J. Agric. Food Chem. 2000, 48, 5401–5405. [Google Scholar] [CrossRef] [PubMed]
- Sowden, R.J.; Yasmin, S.; Rees, N.H.; Bell, S.G.; Wong, L.L. Biotransformation of the Sesquiterpene (+)-Valencene by Cytochrome P450cam and P450BM-3. Org. Biomol. Chem. 2005, 3, 57–64. [Google Scholar] [CrossRef]
- Ganjewala, D.; Luthra, R. Essential Oil Biosynthesis and Regulation in the Genus Cymbopogon. Nat. Prod. Commun. 2010, 5, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüddeke, F.; Wülfing, A.; Timke, M.; Germer, F.; Weber, J.; Dikfidan, A.; Rahnfeld, T.; Linder, D.; Meyerdierks, A.; Harder, J. Geraniol and Geranial Dehydrogenases Induced in Anaerobic Monoterpene Degradation by Castellaniella Defragrans. Appl. Environ. Microbiol. 2012, 78, 2128–2136. [Google Scholar] [CrossRef] [PubMed]


| Factor | Df | F-Value | p-Value |
|---|---|---|---|
| Concentration | 2 | 6.24 | 0.005 |
| Treatment duration | 1 | 25.89 | 0.01 |
| Concentration × Treatment duration | 2 | 8.16 | 0.002 |
| Putative Identity | m/z | RT | RI NIST | RI Calculated | Proposed Function |
|---|---|---|---|---|---|
| 2,6dimethylcyclohexanol | 71 | 40.8 | 1112 | 1133 | Positive modulator of GABA receptors |
| 1-nonen-3-ol 9-decenol | 55 | 38.5 | 1078 | 1079 | Repellent agent |
| 55 | 44.5 | 1256 | 1261 | ||
| 2-decen-1-ol 2-heptanone 3-nonanone | 57 | 45.0 | 1285 | 1285 | Pheromones and alarm pheromones |
| 43 | 27.0 | 891 | 898 | ||
| 43 | 38.9 | 1087 | 1087 | ||
| Farnesol | 69 | 54.1 | 1713 | NA | Compounds related to the Nasonov gland |
| Geranyl acetone | 69 | 48.0 | 1460 | 1455 | |
| Neric acid | 69 | 45.5 | 1340 | 1312 | |
| Geranic acid | 69 | 46.1 | 1345 | 1345 | |
| 6-methyl-5-hepten-2-one | 43 | 32.0 | 986 | 985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, V.; Hidalgo, K.; Diogon, M.; Mercier, F.; Angénieux, M.; Ratel, J.; Delbac, F.; Engel, E.; Bouchard, P. A GABA Receptor Modulator and Semiochemical Compounds Evidenced Using Volatolomics as Candidate Markers of Chronic Exposure to Fipronil in Apis mellifera. Metabolites 2023, 13, 185. https://doi.org/10.3390/metabo13020185
Fernandes V, Hidalgo K, Diogon M, Mercier F, Angénieux M, Ratel J, Delbac F, Engel E, Bouchard P. A GABA Receptor Modulator and Semiochemical Compounds Evidenced Using Volatolomics as Candidate Markers of Chronic Exposure to Fipronil in Apis mellifera. Metabolites. 2023; 13(2):185. https://doi.org/10.3390/metabo13020185
Chicago/Turabian StyleFernandes, Vincent, Kevin Hidalgo, Marie Diogon, Frédéric Mercier, Magaly Angénieux, Jérémy Ratel, Frédéric Delbac, Erwan Engel, and Philippe Bouchard. 2023. "A GABA Receptor Modulator and Semiochemical Compounds Evidenced Using Volatolomics as Candidate Markers of Chronic Exposure to Fipronil in Apis mellifera" Metabolites 13, no. 2: 185. https://doi.org/10.3390/metabo13020185





