Inflammation Control and Immunotherapeutic Strategies in Comprehensive Cancer Treatment
Abstract
:Highlights
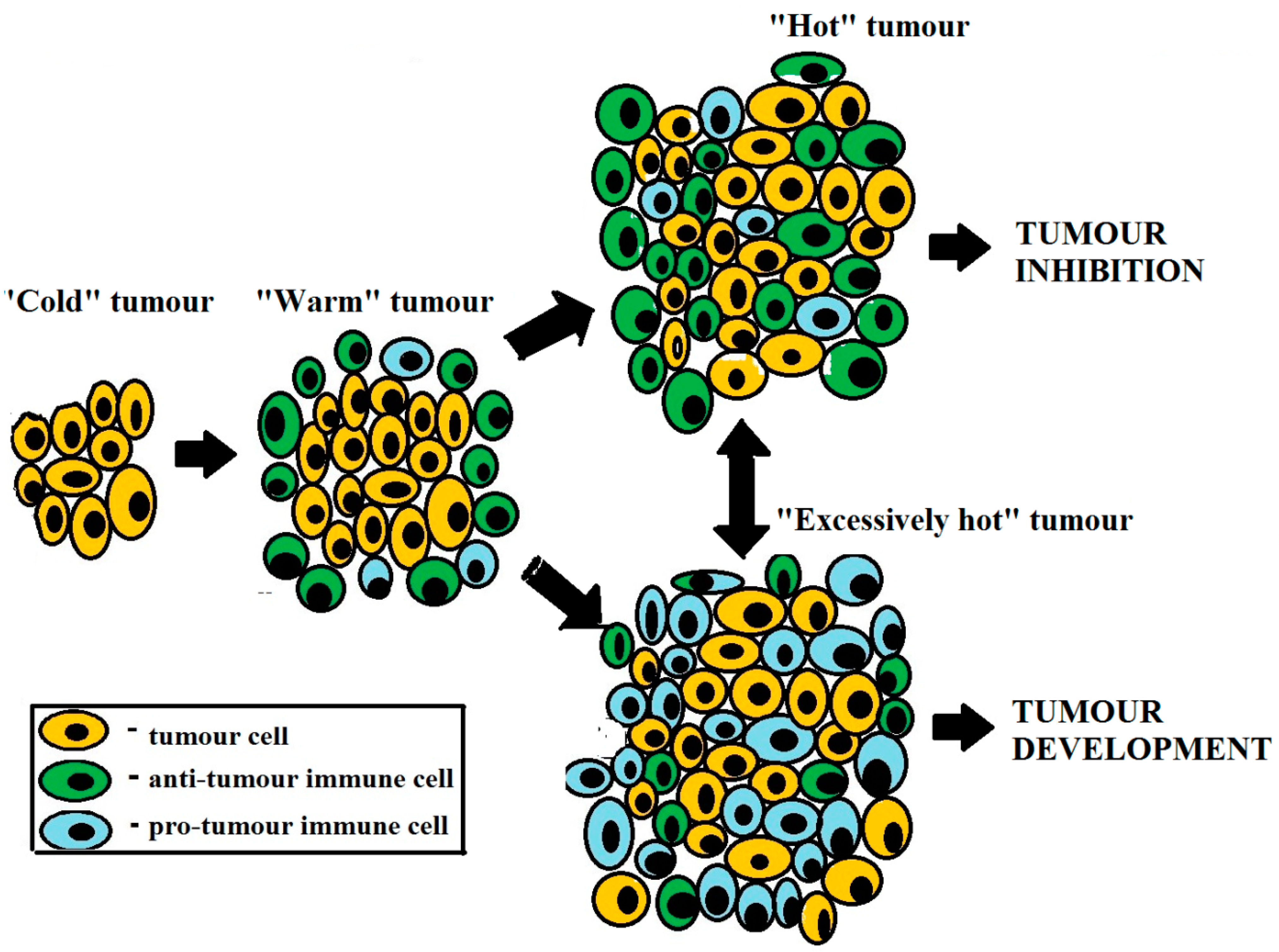
- Tumor progression and regression are determined by immunological properties of the tumor microenvironment.
- Tumor is capable of generating tumor-protective inflammation.
- Immunotherapy should upregulate tumor-inhibiting immunity and/or downregulate tumor-promoting immunity.
- Anti-cancer therapy for advanced disease should ensure long-term tumor cell/mass dormancy, rather than tumor elimination.
- C-reactive protein, lactate dehydrogenase, and neutrophil-to-lymphocyte ratio are important prognostic markers for cancer development.
Abstract
1. Introduction
2. The Role of Inflammation in Tumor Development
3. Boosting Anti-Tumor Immunity
4. Suppressing Pro-Tumor Immunity
5. Role of Immunotherapy in Multimodal Multipurpose Treatment of Cancer
6. Prognostic Effectivity Assessment of Combined Anti-Tumor Therapy Protocols
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dvorak, H.F. Tumors: Wounds That Do Not Heal—Redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Bergers, G. Tumors vs. Chronic Wounds: An Immune Cell’s Perspective. Front. Immunol. 2019, 10, 2178. [Google Scholar] [CrossRef] [Green Version]
- Seledtsov, V.I.; Goncharov, A.; Seledtsova, G. Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum. Vaccines Immunother. 2015, 11, 851–869. [Google Scholar] [CrossRef] [Green Version]
- Seledtsov, V.I.; von Delwig, A. Clinically feasible and prospective immunotherapeutic interventions in multidirectional comprehensive treatment of cancer. Expert Opin. Biol. Ther. 2021, 21, 323–342. [Google Scholar] [CrossRef]
- Seledtsov, V.; Seledtsova, G. A balance between tissue-destructive and tissue-protective immunities: A role of toll-like receptors in regulation of adaptive immunity. Immunobiology 2012, 217, 430–435. [Google Scholar] [CrossRef]
- Darcy, P.K.; Neeson, P.; Yong, C.S.; Kershaw, M.H. Manipulating immune cells for adoptive immunotherapy of cancer. Curr. Opin. Immunol. 2014, 27, 46–52. [Google Scholar] [CrossRef]
- Tanel, A.; Fonseca, S.G.; Yassine-Diab, B.; Bordi, R.; Zeidan, J.; Shi, Y.; Benne, C.; Sékaly, R.-P. Cellular and molecular mechanisms of memory T-cell survival. Expert Rev. Vaccines 2009, 8, 299–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front. Cell Dev. Biol. 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.; Theodorescu, D. Determinants of Resistance to Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 1594. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Wang, C.; Wang, B.; Yang, J.; Wang, Y.; Luo, F.; Xu, J.; Zhao, C.; Liu, R.; Chu, Y. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: Hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflammation 2018, 15, 1–13. [Google Scholar] [CrossRef]
- Liang, C.; Jiang, E.; Yao, J.; Wang, M.; Chen, S.; Zhou, Z.; Zhai, W.; Ma, Q.; Feng, S.; Han, M. Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology 2018, 23, 44–49. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.L.; Francescangeli, F.; La Torre, F.; Zeuner, A. Stem Cell Plasticity and Dormancy in the Development of Cancer Therapy Resistance. Front. Oncol. 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Seledtsov, V.; Goncharov, A.; Seledtsova, G. Multiple-purpose immunotherapy for cancer. Biomed. Pharmacother. 2015, 76, 24–29. [Google Scholar] [CrossRef]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900.e10. [Google Scholar] [CrossRef]
- Trujillo, J.A.; Sweis, R.F.; Bao, R.; Luke, J.J. T Cell–Inflamed versus Non-T Cell–Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol. Res. 2018, 6, 990–1000. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Tie, Y.; Tu, C.; Wei, X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020, 10, 199–223. [Google Scholar] [CrossRef]
- Chiarella, P.; Vermeulen, M.; Montagna, D.R.; Vallecorsa, P.; Strazza, A.R.; Meiss, R.P.; Bustuoabad, O.D.; Ruggiero, R.A.; Prehn, R.T. Improvement of Antitumor Therapies Based on Vaccines and Immune-Checkpoint Inhibitors by Counteracting Tumor-Immunostimulation. Front. Oncol. 2018, 8, 6. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs induce an-ti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993.e10. [Google Scholar] [CrossRef]
- Chiang, C.L.-L.; Kandalaft, L.E.; Coukos, G. Adjuvants for Enhancing the Immunogenicity of Whole Tumor Cell Vaccines. Int. Rev. Immunol. 2011, 30, 150–182. [Google Scholar] [CrossRef]
- Müller, E.; Speth, M.; Christopoulos, P.F.; Lunde, A.; Avdagic, A.; Øynebråten, I.; Corthay, A. Both Type I and Type II Interferons Can Activate Antitumor M1 Macrophages When Combined With TLR Stimulation. Front. Immunol. 2018, 9, 2520. [Google Scholar] [CrossRef] [Green Version]
- Perfilyeva, Y.; Ostapchuk, Y.O.; Abdolla, N.; Tleulieva, R.; Krasnoshtanov, V.C.; Belyaev, N.N. Exogenous Melatonin Up-Regulates Expression of CD62L by Lymphocytes in Aged Mice under Inflammatory and Non-Inflammatory Conditions. Immunol. Investig. 2019, 48, 632–643. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Shishkov, A.A.; Seledtsova, G.V. Xenovaccinotherapy for Cancer, Current Cancer Treatment—Novel Beyond Conventional Approaches, Öner Özdemir (Ed.), ISBN: 978-953-307-397-2, InTech, 2011; pp.416–428. Available online: http://www.intechopen.com/articles/show/title/xenovaccinotherapy-for-cancer (accessed on 3 May 2022).
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Mittica, G.; Capellero, S.; Genta, S.; Cagnazzo, C.; Aglietta, M.; Sangiolo, D.; Valabrega, G. Adoptive immunotherapy against ovarian cancer. J. Ovarian Res. 2016, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J.B.A.G. Adoptive cellular therapies: The current landscape. Virchows Arch. 2019, 474, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Seledtsov, V.I.; Malashchenko, V.; Gazatova, N.D.; Meniailo, M.E.; Morozova, E.M.; Seledtsova, G.V. Directs effects of granulocyte-macrophage colony stimulating factor (GM-CSF) on adaptive immunogenesis. Hum. Vaccines Immunother. 2019, 15, 2903–2909. [Google Scholar] [CrossRef]
- Maharaj, D.; Vianna, P.G.; Ward, W.; Messina, A.J.; Rayborn, T.; Gouvea, J.V.; Hammer, R.D.; Cui, Z. Young donor white blood cell immunotherapy induces extensive tumor necrosis in advanced-stage solid tumors. Heliyon 2017, 3, e00438. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.-H.; Kwack, K.; Choi, S.-W. Natural Killer Cell Therapy: A New Treatment Paradigm for Solid Tumors. Cancers 2019, 11, 1534. [Google Scholar] [CrossRef] [Green Version]
- Seledtsov, V.I.; Seledtsova, G.V.; Avdeev, E.V.; Samarin, D.M.; Kozlov, V.A. Induction of mixed allogeneic chimerism for leukemia. Leuk. Res. 1997, 21, 907–909. [Google Scholar] [CrossRef]
- Takeuchi, A.; Eto, M.; Yamada, H.; Tatsugami, K.; Naito, S.; Yoshikai, Y. A reduction of recipient regulatory T cells by cyclophosphamide contributes to an anti-tumor effect of nonmyeloablative allogeneic stem cell transplantation in mice. Int. J. Cancer 2012, 130, 365–376. [Google Scholar] [CrossRef]
- Şahin, U.; Demirer, T. Graft-versus-cancereffect and innovative approaches in the treatment of refractory solid tumors. Turk. J. Med Sci. 2020, 50, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, S.; Tawara, I.; Shimizu, J.; Sakaguchi, S.; Fujita, T.; Nakayama, E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999, 59, 3128–3133. [Google Scholar] [PubMed]
- Dunne, A.; Marshall, N.A.; Mills, K.H. TLR based therapeutics. Curr. Opin. Pharmacol. 2011, 11, 404–411. [Google Scholar] [CrossRef]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef] [Green Version]
- Orillion, A.; Hashimoto, A.; Damayanti, N.; Shen, L.; Adelaiye-Ogala, R.; Arisa, S.; Chintala, S.; Ordentlich, P.; Kao, C.; Elzey, B.; et al. Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 5187–5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashraheel, S.S.; Domling, A.; Goda, S.K. Update on targeted cancer therapies, single or in combination, and their fine tuning for precision medicine. Biomed. Pharmacother. 2020, 125, 110009. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Seledtsova, G.V. Attaining threshold antibody cytotoxicity for selective tumor cell destruction: An opinion article. Oncotarget 2018, 9, 35790–35794. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The Role of Hyperthermia in the Multidisciplinary Treatment of Malignant Tumors. Integr. Cancer Ther. 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Seledtsov, V.I.; von Delwig, A.A. Oxygen therapy in traditional and immunotherapeutic treatment protocols of cancer patients: Current reality and future prospects. Expert Rev. Anticancer. Ther. 2022, 22, 575–581. [Google Scholar] [CrossRef]
- Müller, L.; Tunger, A.; Plesca, I.; Wehner, R.; Temme, A.; Westphal, D.; Meier, F.; Bachmann, M.; Schmitz, M. Bidirectional Crosstalk Between Cancer Stem Cells and Immune Cell Subsets. Front. Immunol. 2020, 11, 140. [Google Scholar] [CrossRef]
- Wong, K.K.; Hassan, R.; Yaacob, N.S. Hypomethylating Agents and Immunotherapy: Therapeutic Synergism in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Front. Oncol. 2021, 11, 624742. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Avdeev, I.V.; Morenkov, A.V.; Seledtsova, G.V.; Kozlov, V.A. Antiproliferative Effect of Bone Marrow Cells on Leukemic Cells. Immunobiology 1995, 192, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.; Avdeev, I.; Seledtsova, G.; Samarin, D.; Prokopenko, I.; Kozlov, V. Bone marrow cells as cytostatic effectors responsible for suppressing leukemia growth in vitro. Biomed. Pharmacother. 1995, 49, 293–299. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Taraban, V.Y.; Seledtsova, G.V.; Samarin, D.M.; Avdeev, I.V.; Senyukov, V.V.; Kozlov, V.A. Tumor Growth Inhibitory and Natural Suppressor Activities of Murine Bone Marrow Cells: A Comparative Study. Cell. Immunol. 1997, 182, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Senyukov, V.V.; Seledtsov, V.; Poveshchenko, O.; Taraban, V.Y.; Kozlov, V. Soluble factor and cell-cell interaction in cytostasis induced by bone marrow cells. Bull. Exp. Biol. Med. 2000, 129, 559–561. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar]
- Qi, F.; Xu, Y.; Zheng, Y.; Li, X.; Gao, Y. Pre-treatment Glasgow prognostic score and modified Glasgow prognostic score may be potential prognostic biomarkers in urological cancers: A systematic review and meta-analysis. Ann. Transl. Med. 2019, 7, 531. [Google Scholar] [CrossRef]

| Anti-Tumor Activity | Pro-Tumor Activity |
|---|---|
| Cells | |
| N1 neutrophil; M1 macrophage; natural killer (NK) cell; NKT (natural killer T) cell; γ/δ T cell; classical mature dendritic cell (DC); Th (T helper) 1 cell; Th17 cell; cytotoxic T lymphocyte (CTL); B cell. | N2 neutrophil; M2 macrophage; myeloid-derived suppressor cell (MDSC); tolerogenic immature DC; Th2 cell; Th17 cell; regulatory T cell (Treg); regulatory B cell (Breg). |
| Soluble factors | |
| Tumor necrosis factor (TNF); IFN (interferon)-γ; type I IFNs; IL (interleukin)-2; IL-7; IL-15; IL-18; granulocyte-macrophage colony-stimulating factor (GM-CSF); reactive oxygen species (ROS); proteases, extracellular adenosine triphosphate (eATP); phospholipases. | Transforming growth factor (TGF-β); IL-2; IL4; IL6; IL10; GM-CSF; granulocyte colony-stimulating factor (G-CSF); vascular endothelial growth factor VEGF); indoleamine 2,3-dioxygenase-1 (IDO-1); proteases; prostaglandin E (PGE); ROSs; phospholipases; histamine; adenosine (ADO). |
| Effects | |
| Inhibition of neovascularization, downregulation of pro-tumor immunity, tumor inhibition. | Stimulation of neovascularization, downregulation of anti-tumor immunity, tumor promotion. |
| Disease Stage | Possible Treatments | Results |
|---|---|---|
| Local tumor (I–II stage) | Surgery; immunotherapy; hyperthermia; oxygen therapy. | Recovery |
| Advanced tumor (III–IV stage) | Surgery; immunotherapy; hyperthermia; oxygen therapy; radiotherapy; chemotherapy. | Tumor growth inhibition; prolongation of patient’s life |
| Parameter | Normal | Increased |
|---|---|---|
| C-reactive protein (CRP) | 0 | 1 |
| Lactate dehydrogenase (LDH) | 0 | 1 |
| Neutrophil-to-lymphocyte ratio (NLR) | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seledtsov, V.I.; Darinskas, A.; Von Delwig, A.; Seledtsova, G.V. Inflammation Control and Immunotherapeutic Strategies in Comprehensive Cancer Treatment. Metabolites 2023, 13, 123. https://doi.org/10.3390/metabo13010123
Seledtsov VI, Darinskas A, Von Delwig A, Seledtsova GV. Inflammation Control and Immunotherapeutic Strategies in Comprehensive Cancer Treatment. Metabolites. 2023; 13(1):123. https://doi.org/10.3390/metabo13010123
Chicago/Turabian StyleSeledtsov, Victor Ivanovich, Adas Darinskas, Alexei Von Delwig, and Galina Victorovna Seledtsova. 2023. "Inflammation Control and Immunotherapeutic Strategies in Comprehensive Cancer Treatment" Metabolites 13, no. 1: 123. https://doi.org/10.3390/metabo13010123





