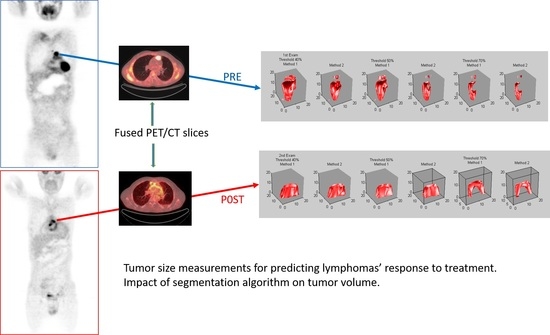
Tumor Size Measurements for Predicting Hodgkin’s and Non-Hodgkin’s Lymphoma Response to Treatment
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics and Measurements
- (a)
- Expert’s PET measurements: Metabolic tumor maximum diameter (MTDmax) and SUVmax from a single transverse PET image, metabolic tumor volume (MTV) from all slices where tumor was present, pre and post treatment.
- (b)
- Computer aided detection (CAD) algorithm PET measurements: MTDmax, metabolic tumor maximum area (MTAmax) from a single transverse PET image, MTV from all slices where tumor was present, pre and post treatment.
- (c)
- CAD CT measurements: Maximum tumor diameter (Dmax), tumor maximum area (Amax) from the single transverse CT image that corresponded to the single PET slice used in the previous measurements, tumor volume (TV) from all CT slices where tumor was present, pre and post treatment.
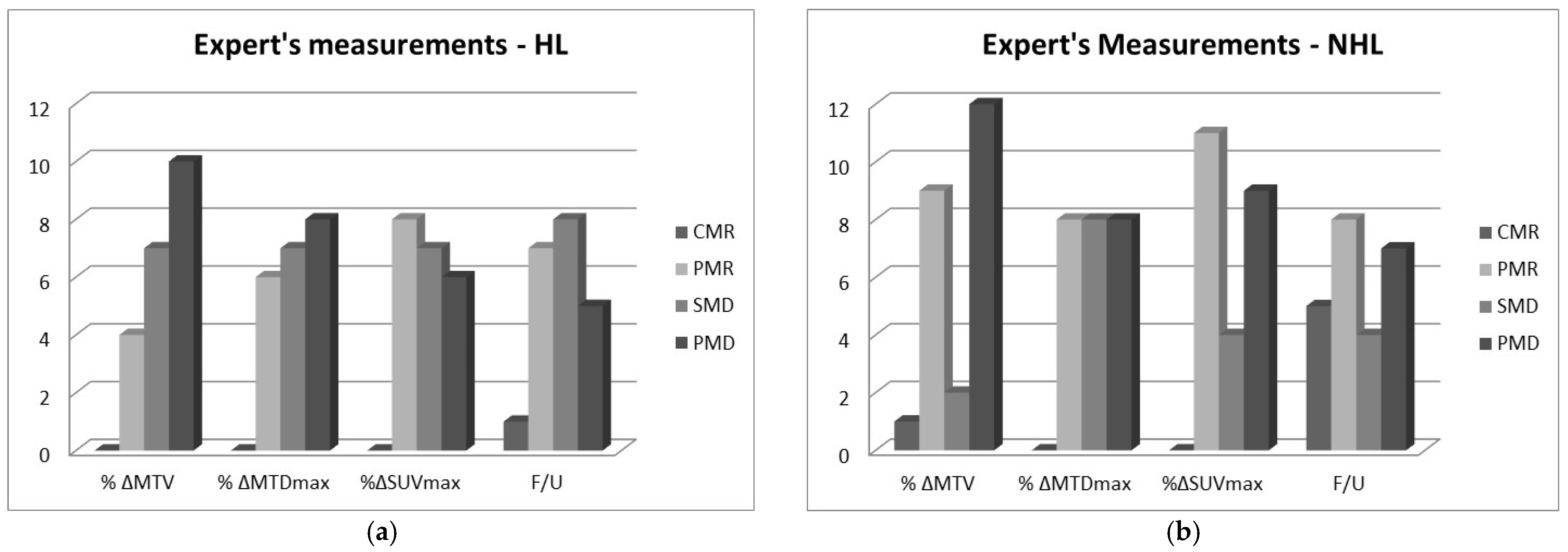
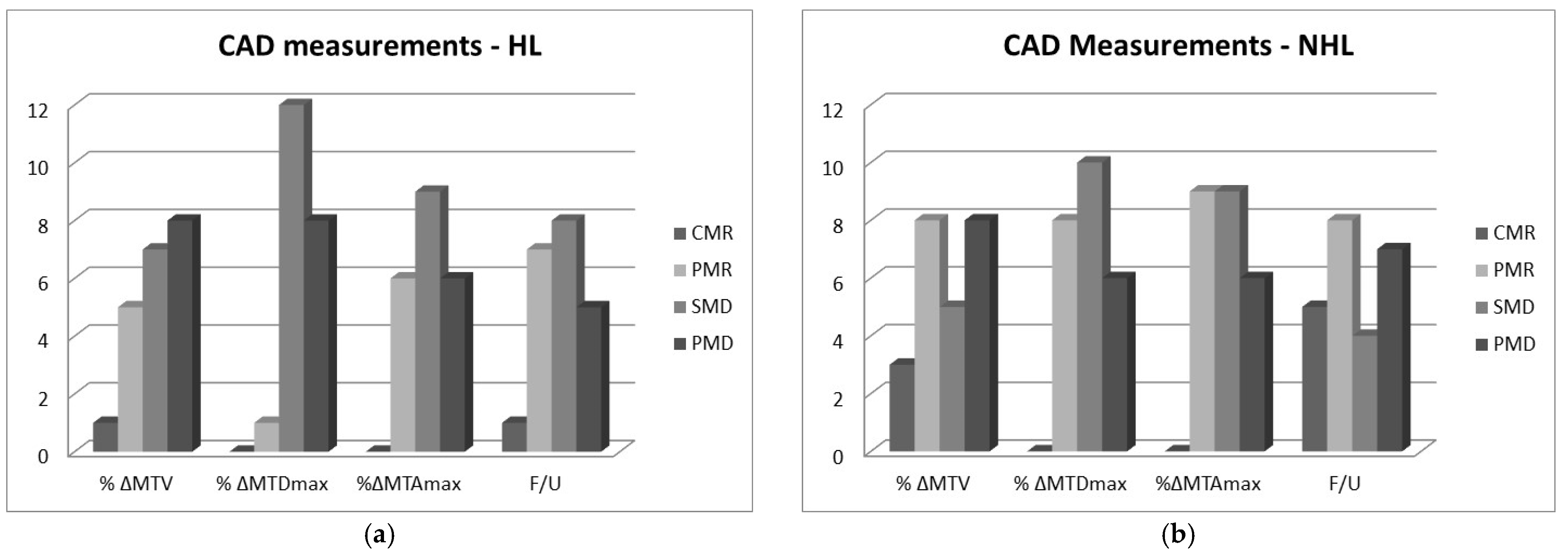
2.2. RECIL Classification of Changes in Measured Variables
- i.
- Complete metabolic response (CMR): Complete disappearance of the lesion; area of tumor is indistinguishable from surrounding tissue (−100% change).
- ii.
- Partial metabolic response (PMR): At least 30% reduction in metabolic tumor size post treatment, i.e., −100% < Δ ≤ −30%.
- iii.
- Stable metabolic disease (SMD): Less than 20% increase or less than 30% decrease in tumor size and no new lesions, i.e., −30% < Δ < 20%.
- iv.
- Progressive metabolic disease (PMD): At least 20% increase in tumor or appearance of new lesions (Δ ≥ 20%).
2.3. Differences between Expert and CAD
2.4. Weighted Kappa Measurements of Agreement
3. Discussion
- How good therapy response predictors are PET standard metabolic measurements of MTDmax and SUVmax of lymphomas? To answer this, the MTDmax and SUVmax of 24 NHL and 21 HL patients were compared to the clinical outcome 6 months post treatment. Results showed that SUVmax had the highest agreement with the clinical outcome post treatment while the expert’s MTDmax measurements had moderate agreement. The CIs for both metrics were relatively wide due to the small sample size of our study, but the relative significance is not affected, even at the lower limit [15]. The CAD MTDmax measurements differed from the expert’s measurements and had poor correlation with the clinical follow-up. Differences for the larger size masses were often more than 100%, and this was puzzling considering that an expert also evaluated the CAD algorithm’s segmentation performance and deemed it acceptable. It should be noted, however, that the expert’s MTDmax and SUVmax values used in our analysis were recorded from the clinical diagnostic report, and the expert who did the formal clinical interpretation was different from the expert who participated in our segmentation process. It is well documented in several studies that a large margin is applied during standard clinical measurements while interobserver variability is high [1]. Finally, SUVmax was also measured by our algorithm, but these values were not reported here because they did not differ from the expert’s as they were both based on similar mathematical definitions [16].
- How good are MTV measurements for the prognosis of the disease, and how do they compare to the standard measurements of MTDmax and SUVmax? Results showed that the expert’s MTV manual measurements have a fair agreement with the clinical follow-up. CAD MTV measurements showed a moderate agreement as is also indicated in other similar reports [17,18,19]. CAD’s better performance may be explained by the fact that CAD MTV values were based on more consistent ROI contours while the expert’s MTV values were based on rough elliptical contours around the tumor area in the various slices. CAD measurements were reproducible and faster compared to the expert and can be highly accurate, particularly when semi-automated, i.e., when initiated by an expert.
- Is the MTAmax of any value? This parameter is rarely used or measured in studies of metabolic tumor size measurements. It showed fair agreement with the clinical outcome and its value was not considered significant.
- Are there differences between HL and NHL cases? It seems that both the expert and the CAD performed better on the NHL than the HL masses. The NHL cases had masses with larger diameters and volumes than the HL but there was no indication, given our relatively small sample size, that the accuracy of measurements depended on the size.
- How does the PET segmentation algorithm’s parameters affect measurements? The adaptive thresholding segmentation is a key element in our CAD approach, and the selection of a threshold impacts the final result. The 50% threshold was considered the optimum threshold for our algorithm. The selection was determined by a receiver operating characteristic (ROC) study, which was performed with five threshold values (30%, 40%, 50%, 60%, and 70%) on a subset of images where the masses where outlined by an expert and these outlines were considered “ground truth” [20]. The ROC analysis showed that a threshold of 50% yielded the best agreement, with the ground truth followed by the thresholds of 40% and 70%. To test it further, all three thresholds were used for the metabolic size measurements. Comparisons with the clinical outcome were conducted for all three sets of measurements. The 50% threshold yielded the best results and these are reported here.
4. Materials and Methods
4.1. Patients and Data Coding
- (a)
- Patients should have one mass, non-operable, that underwent similar clinical treatment that included chemotherapy and radiation therapy.
- (b)
- All patients should have at least two PET/CT examinations, one before (baseline) and one after treatment.
- (c)
- All patients should have a clinical follow up 6 months after the end of their treatment and be classified according to the RECIL as in remission (positive response to treatment) with either complete or partial response with the tumor reduced in size or in relapse (negative response to treatment) with either no change in the tumor size or increase in size or appearance of new lesions.
4.2. 18F-FDG-PET/CT Imaging
4.3. Metabolic Parameter Measurements
- (1)
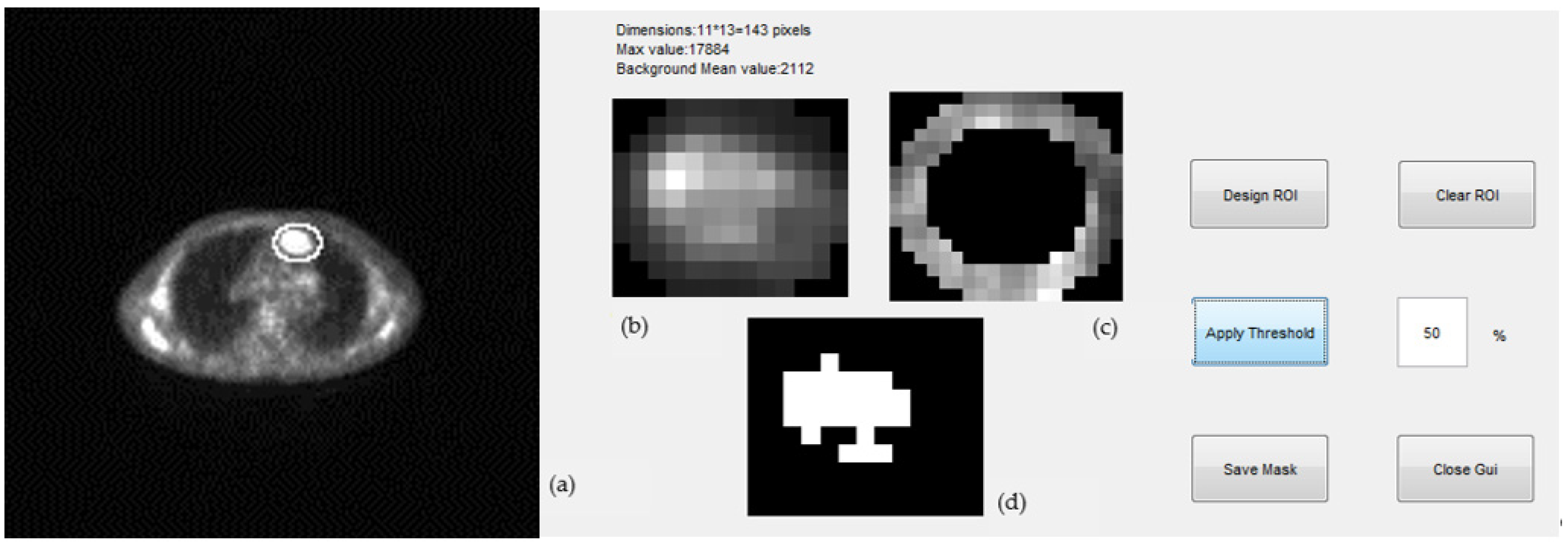
- Expert selected the PET and corresponding CT slice of a scan where tumor appeared at maximum diameter; we will refer to this as the “central” slice.
- (2)
- (3)
- A background ring was defined automatically on the border of the elliptical region drawn by the expert. The ring was 3 pixels wide and its mean pixel value was used for the estimation of the threshold of the segmentation (Figure 5c). Pixels with values greater than a selected percentage of the mean background value were considered as part of the tumor, otherwise they were rejected. A non uniform region was finally defined for a given threshold as the tumor ROI and was used for the estimation of the tumor maximum diameter (mm) and tumor maximum area (mm2) ((Figure 5d).
- (4)
- The “central” slice elliptical contour of the expert was automatically projected by the algorithm to slices above and below where tumor appeared. This number ranged from 5–20 slices per case depending on the tumor size.
- (5)
- Same ROI segmentation process of step (3) was applied to all slices and for three different thresholds, 40%, 50%, and 70%.
- (6)
- The MTV was estimated by adding all 2D ROIs and using the voxel dimensions.
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinahan, P.E.; Doot, R.K.; Wanner-Roybal, M.; Bidaut, L.M.; Armato III, S.G.; Meyer, C.R.; McLennan, G. PET/CT Assessment of Response to Therapy: Tumor Change Measurement, Truth Data, and Error. Transl. Oncol. 2009, 2, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, W.A. Assessing Tumor Response to Therapy. J. Nucl. Med. 2009, 50, 1S–10S. [Google Scholar] [PubMed] [Green Version]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Schwartz, L.H.; Zucca, L.; Lister, T.A.; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar]
- Al Tabaa, Y.; Bailly, C.; Kanoun, S. FSG-PET/CT in Lymphoma: Where Do We Go Now? Cancers 2021, 13, 5222. [Google Scholar]
- Kallergi, M.; Botsivali, M.; Politis, N.; Menychtas, D.; Georgakopoulos, A.; Chatziioannou, S. A pilot study of the prognostic significance of metabolic tumor size measurements in PET/CT imaging of lymphomas. In Proceedings of the SPIE 9417, Medical Imaging 2015: Biomedical Applications in Molecular, Structural, and Functional Imaging; SPIE: Bellingham, WA, USA, 2015; Volume 941710. [Google Scholar] [CrossRef]
- Cheson, B.D. PET/CT in Lymphoma: Current Overview and Future Directions. Semin. Nucl. Med. 2018, 48, 76–81. [Google Scholar] [CrossRef]
- McCarten, K.M.; Nadel, H.R.; Shulkin, B.L.; Cho, S.Y. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr. Radiol. 2019, 49, 1545–1564. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Chauvie, S. Metabolic Tumor Volume Metrics in Lymphoma. Semin. Nucl. Med. 2018, 48, 50–66. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Baba, S.; Endo, M.; Setsu, N.; Iida, K.; Fukushi, J.I.; Kawaguchi, K.; Okada, S.; Bekki, H.; Nakashima, Y.; et al. Metabolic Tumor Volume by 18F-FDG PET/CT Can Predict the Clinical Outcome of Primary Malignant Spine/Spinal Tumors. BioMed Res. Intern. 2017, 2017, 8132676. [Google Scholar] [CrossRef] [Green Version]
- Rezai, P.; Pisaneschi, M.J.; Feng, C.; Yaghmai, V. A Radiologist’s Guide to Treatment Response Criteria in Oncologic Imaging: Functional, Molecular, and Disease-Specific Imaging Biomarker. Am. J. Roentgenol. 2013, 201, 246–256. [Google Scholar]
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Seshan, V.E.; et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Yi, Q.L. How do I interpret a confidence interval? Transfusion 2016, 56, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Manabe, O.; Magota, K.; Furuya, S.; Shiga, T.; Kudo, K. A Preliminary Study to Use SUVmax of FDG PET-CT as an Identifier of Lesion for Artificial Intelligence. Front. Med. 2021, 8, 647562. [Google Scholar] [CrossRef]
- Ferrari, A.; Miceli, R.; Meazza, C.; Casanova, M.; Favini, F.; Morosi, C.; Trecate, G.; Luksch, R.; Cefalo, G.; Mariani, L.; et al. Comparison of the Prognostic Value of Assessing Tumor Diameter Versus Tumor Volume at Diagnosis or in Response to Initial Chemotherapy o Rhabodmyosarcoma. J. Clin. Oncol. 2010, 28, 1322–1328. [Google Scholar] [CrossRef]
- Zhao, B.; Oxnard, G.R.; Moskowitz, C.S.; Kris, M.G.; Pao, W.; Guo, P.; Rusch, V.M.; Ladanyi, M.; Rizvi, N.A.; Schwartz, L.H. A Pilot Study of Volume Measurement as a Method of Tumor Response Evaluation to Aid Biomarker Development. Clin. Cancer Res. 2010, 16, 4647–4653. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.R.; Grigsby, P.W. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 353–359. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Gatos, I.; Tsantis, S.; Karamesini, M.; Spiliopoulos, S.; Karnabatidis, D.; Hazle, J.D.; Kagadis, G.C. Focal liver lesions segmentation and classification in nonenhanced T2-weighted MRI. Med. Phys. 2017, 44, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Kallergi, M.; Clarke, L.P.; Jain, V.K.; Clark, R.A. Markov random field for tumor detection in digital mammography. IEEE Trans. Med. Imaging 1995, 14, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Bouron, C.; Mathie, C.; Seegers, V.; Morel, O.; Jézéquel, P.; Lasla, H.; Sher, A.; Lacoeuille, F.; Patsouris, A.; Testard, A.; et al. Prognostic Value of Metabolic, Volumetric and Textural Parameters of Baseline [18F]FDG PET/CT in Early Triple-Negative Breast Cancer. Cancers 2022, 14, 637. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, Y.; Chen, Z.; Li, J.; Sang, S.; Deng, S. Radiomic Features of 18F-FDG PET in Hodgkin Lymphoma Are Predictive of Outcomes. Contrast Media Mol. Imaging 2021, 2021, 6347404. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Bading, J.; Conti, P.S. Tumor Quantification in Clinical Positron Emission Tomography. Theranostics 2013, 3, 787–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallamini, A.; Barrington, S.F.; Biggi, A.; Chauvie, S.; Kostakoglu, L.; Gregianin, M.; Brice, P.; Bolis, S.; Salvi, F.; Hutchings, M.; et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica 2014, 99. [Google Scholar] [CrossRef] [PubMed]


| HL Parameter | Expert | CAD | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| MTDmax (mm) | 18 (9) | 16 (9) | 10 (3) | 10 (2) |
| MTAmax (mm2) | 1181 (597) | 1060 (278) | ||
| MTV (mm3) | 8557 (9718) | 12,485 (14,370) | 7507 (4216) | 8845 (6410) |
| SUVmax | 7 (4) | 6 (3) | ||
| NHL Parameter | Expert | CAD | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| MTDmax (mm) | 28 (22) | 26 (23) | 11 (6) | 11 (7) |
| MTAmax (mm2) | 1768 (2283) | 2070 (3403) | ||
| MTV (mm3) | 52,884 (188,602) | 17,688 (22,625) | 50,621 (149,033) | 19,167 (27,691) |
| SUVmax | 10 (8) | 8 (6) | ||
| NHL Parameter | HL | NHL | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| MTDmax (mm) | 20 (8) | 24 (10) | 34 (37) | 26 (24) |
| MTAmax (mm2) | 167 (130) | 235 (161) | 740 (1410) | 478 (987) |
| MTV (mm3) | 9284 (10,876) | 15,034 (15,348) | 160,370 (402,619) | 22,943 (53,945) |
| Pair | Weighted k-Value | Agreement | 95% CI |
|---|---|---|---|
| Expert % change in MTDmax—Clinical F/U | 0.47 | Moderate | (0.25,0.70) |
| CAD %change in MTDmax—Clinical F/U | 0.18 | Slight | (−0.19,0.54) |
| CAD %change in MTAmax—Clinical F/U | 0.34 | Fair | (0.09,0.60) |
| CAD % change in MTV—Clinical F/U | 0.52 | Moderate | (0.30,0.70) |
| Parameter | NHL | HL | p-Value |
|---|---|---|---|
| Age (yr) | 48.5 ± 17.7 | 43.5 ± 19.2 | 0.38 |
| Weight (kg) | 77.0 ± 11.9 | 78.7 ± 5.9 | 0.49 |
| Height (cm) | 171.5 ± 7.1 | 169.9 ± 8.9 | 0.51 |
| Gender (Male/Female) | 16/8 | 8/13 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallergi, M.; Georgakopoulos, A.; Lyra, V.; Chatziioannou, S. Tumor Size Measurements for Predicting Hodgkin’s and Non-Hodgkin’s Lymphoma Response to Treatment. Metabolites 2022, 12, 285. https://doi.org/10.3390/metabo12040285
Kallergi M, Georgakopoulos A, Lyra V, Chatziioannou S. Tumor Size Measurements for Predicting Hodgkin’s and Non-Hodgkin’s Lymphoma Response to Treatment. Metabolites. 2022; 12(4):285. https://doi.org/10.3390/metabo12040285
Chicago/Turabian StyleKallergi, Maria, Alexandros Georgakopoulos, Vassiliki Lyra, and Sofia Chatziioannou. 2022. "Tumor Size Measurements for Predicting Hodgkin’s and Non-Hodgkin’s Lymphoma Response to Treatment" Metabolites 12, no. 4: 285. https://doi.org/10.3390/metabo12040285