Plasma Tsukushi Concentration Is Associated with High Levels of Insulin and FGF21 and Low Level of Total Cholesterol in a General Population without Medication
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Subjects
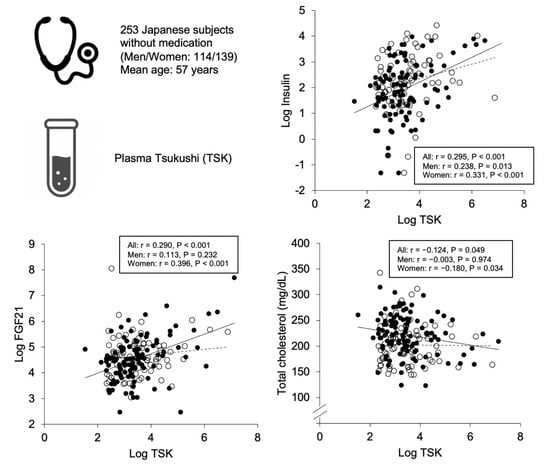
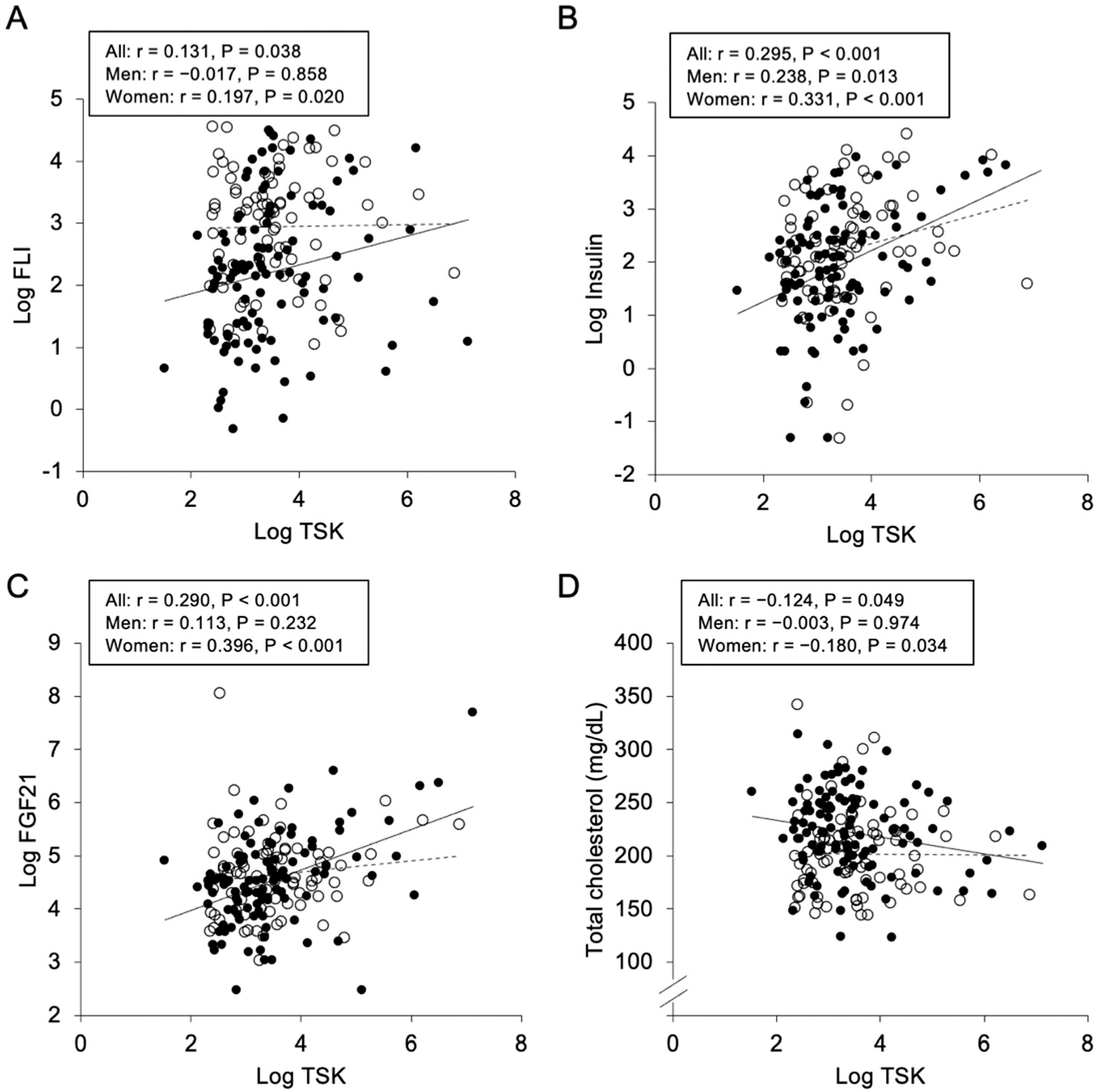
2.2. Correlations and Associations of Plasma Tsukushi with Parameters
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Measurements
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, L.; Iozzo, R.V. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, K.; Lupo, G.; Kuriyama, S.; Keynes, R.; Holt, C.E.; Harris, W.A.; Tanaka, H.; Ohnuma, S.I. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev. Cell 2004, 7, 347–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Li, Y.; Guo, C.; Zhao, Z.; Yuan, G. Novel roles of Tsukushi in signaling pathways and multiple disease processes. Biofactors 2021, 47, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.I.; Anam, M.B.; Ito, N.; Ohta, K. Involvement of Tsukushi in diverse developmental processes. J. Cell Commun. Signal. 2018, 12, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouchiroud, M.; Camire, E.; Aldow, M.; Caron, A.; Jubinville, E.; Turcotte, L.; Kaci, I.; Beaulieu, M.J.; Roy, C.; Labbe, S.M.; et al. The hepatokine Tsukushi is released in response to NAFLD and impacts cholesterol homeostasis. JCI Insight 2019, 4, e129492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Wang, Q.; Wang, S.; Zhang, J.; Liu, T.; Guo, L.; Yu, Y.; Lin, J.D. Mapping the molecular signatures of diet-induced NASH and its regulation by the hepatokine Tsukushi. Mol. Metab. 2019, 20, 128–137. [Google Scholar] [CrossRef]
- Wang, Q.; Sharma, V.P.; Shen, H.; Xiao, Y.; Zhu, Q.; Xiong, X.; Guo, L.; Jiang, L.; Ohta, K.; Li, S.; et al. The hepatokine Tsukushi gates energy expenditure via brown fat sympathetic innervation. Nat. Metab. 2019, 1, 251–260. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, P.; Cakir, I.; Mi, L.; Cone, R.D.; Lin, J.D. Deletion of the Feeding-Induced Hepatokine TSK Ameliorates the Melanocortin Obesity Syndrome. Diabetes 2021, 70, 2081–2091. [Google Scholar] [CrossRef]
- Mouchiroud, M.; Camire, E.; Aldow, M.; Caron, A.; Jubinville, E.; Turcotte, L.; Kaci, I.; Beaulieu, M.J.; Roy, C.; Labbe, S.M.; et al. The Hepatokine TSK does not affect brown fat thermogenic capacity, body weight gain, and glucose homeostasis. Mol. Metab. 2019, 30, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Leung, P.S. Fibroblast growth factor 21: A regulator of metabolic disease and health span. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E292–E302. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014, 5, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wente, W.; Efanov, A.M.; Brenner, M.; Kharitonenkov, A.; Koster, A.; Sandusky, G.E.; Sewing, S.; Treinies, I.; Zitzer, H.; Gromada, J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 2006, 55, 2470–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Pan, X.; Wu, F.; Ye, D.; Zhang, Y.; Wang, Y.; Jin, L.; Lian, Q.; Huang, Y.; Ding, H.; et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 2015, 131, 1861–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, W.S.; Xu, A.; Woo, Y.C.; Tso, A.W.; Cheung, S.C.; Fong, C.H.; Tse, H.F.; Chau, M.T.; Cheung, B.M.; Lam, K.S. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2454–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, P.; Wei, X.; Deng, Y.; Liu, W.; Guo, D.; Liu, J.; Xu, B.; Huang, C.; Huang, J.; et al. Elevated Serum Tsukushi Levels in Patients With Hyperthyroidism. Front. Endocrinol. 2020, 11, 580097. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, L.; Yan, J.; Huang, Y.; Zhang, H.; Zhang, R.; Hu, C. Tsukushi and TSKU genotype in obesity and related metabolic disorders. J. Endocrinol. Investig. 2021, 44, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Lu, P.D.; Zhang, Y.; Scheuner, D.; Kaufman, R.J.; Sonenberg, N.; Harding, H.P.; Ron, D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell Biol. 2004, 24, 10161–10168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, P.; Han, Z.; Couvillon, A.D.; Kaufman, R.J.; Exton, J.H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell Biol. 2006, 26, 3071–3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, U.; Yilmaz, E.; Ozcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Gorgun, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimura, S.; Furuhashi, M.; Mita, T.; Fuseya, T.; Watanabe, Y.; Hoshina, K.; Kokubu, N.; Inoue, K.; Yoshida, H.; Miura, T. Reduction of endoplasmic reticulum stress inhibits neointima formation after vascular injury. Sci. Rep. 2014, 4, 6943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, M.; Furuhashi, M.; Ishimura, S.; Mita, T.; Fuseya, T.; Okazaki, Y.; Yoshida, H.; Tsuchihashi, K.; Miura, T. Reduction of endoplasmic reticulum stress by 4-phenylbutyric acid prevents the development of hypoxia-induced pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1314–H1323. [Google Scholar] [CrossRef] [Green Version]
- Fruhbeck, G.; Gomez-Ambrosi, J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001, 15, 1996–2006. [Google Scholar] [CrossRef]
- Stefan, N.; Haring, H.U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 2013, 9, 144–152. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, A.H.; Bednarek, A.K.; Daniel, R.L.; Hawkins, K.A.; Laflin, K.J.; Gaddis, S.; MacLeod, M.C.; Aldaz, C.M. Effects of estrogen on global gene expression: Identification of novel targets of estrogen action. Cancer Res. 2000, 60, 5977–5983. [Google Scholar] [PubMed]
- Ishimura, S.; Furuhashi, M.; Watanabe, Y.; Hoshina, K.; Fuseya, T.; Mita, T.; Okazaki, Y.; Koyama, M.; Tanaka, M.; Akasaka, H.; et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE 2013, 8, e81318. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, M.; Matsumoto, M.; Tanaka, M.; Moniwa, N.; Murase, T.; Nakamura, T.; Ohnishi, H.; Saitoh, S.; Shimamoto, K.; Miura, T. Plasma xanthine oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ. J. 2018, 82, 1892–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, M.; Matsumoto, M.; Murase, T.; Nakamura, T.; Higashiura, Y.; Koyama, M.; Tanaka, M.; Moniwa, N.; Ohnishi, H.; Saitoh, S.; et al. Independent links between plasma xanthine oxidoreductase activity and levels of adipokines. J. Diabetes Investig. 2019, 10, 1059–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otgonsuren, M.; Estep, M.J.; Hossain, N.; Younossi, E.; Frost, S.; Henry, L.; Hunt, S.; Fang, Y.; Goodman, Z.; Younossi, Z.M. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J. Gastroenterol. Hepatol. 2014, 29, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tanaka, M.; Higashiura, Y.; Mori, K.; Hanawa, N.; Ohnishi, H.; Furuhashi, M. Prediction and validation of nonalcoholic fatty liver disease by fatty liver index in a Japanese population. Endocr. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar] [CrossRef] [Green Version]
| All (n = 253) | Male (n = 114) | Female (n = 139) | p | |
|---|---|---|---|---|
| Age (years) | 57 ± 16 | 56 ± 17 | 57 ± 16 | 0.425 |
| Body mass index | 22.6 ± 3.8 | 23.7 ± 3.6 | 21.8 ± 3.8 | <0.001 |
| Waist circumference (cm) | 82.9 ± 11.5 | 85.5 ± 10.9 | 80.7 ± 1.9 | 0.001 |
| Systolic blood pressure (mmHg) | 127 ± 20 | 130 ± 17 | 124 ± 22 | 0.019 |
| Diastolic blood pressure (mmHg) | 74 ± 11 | 76 ± 10 | 73 ± 11 | 0.024 |
| Current smoking habit | 61 (24.1) | 37 (32.5) | 24 (17.3) | 0.003 |
| Alcohol drinking habit | 103 (40.7) | 61 (53.5) | 42 (30.2) | <0.001 |
| Comorbidity | ||||
| Hypertension | 66 (26.1) | 29 (25.4) | 37 (26.6) | 0.832 |
| Diabetes mellitus | 3 (1.2) | 3 (2.6) | 0 (0) | 0.028 |
| Dyslipidemia | 115 (45.5) | 46 (40.4) | 69 (49.6) | 0.139 |
| Biochemical data | ||||
| AST (IU/L) | 22 (18–26) | 23 (20–27) | 21 (18–25) | 0.013 |
| ALT (IU/L) | 17 (14–24) | 21 (16–29) | 16 (13–20) | <0.001 |
| GGT (IU/L) | 21 (15–32) | 26 (20–39) | 17 (14–26) | <0.001 |
| FLI | 13.3 (5.3–32.4) | 24.2 (9.2–41.7) | 8.8 (3.8–20.4) | <0.001 |
| TBA (µmol/L) | 4.4 (2.1–8.4) | 5.2 (2.6–5.2) | 3.8 (2.0–7.3) | 0.042 |
| Blood urea nitrogen (mg/dL) | 15 ± 4 | 15 ± 4 | 14.6 ± 4.0 | 0.122 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.7 ± 0.1 | <0.001 |
| eGFR (mL/min/1.73 m2) | 73.3 ± 15.2 | 76.1 ± 15.7 | 70.9 ± 14.4 | 0.007 |
| Uric acid (mg/dL) | 5.2 ± 1.3 | 6.0 ± 1.1 | 4.7 ± 1.0 | <0.001 |
| Total cholesterol (mg/dL) | 213 ± 39 | 200 ± 36 | 222 ± 38 | <0.001 |
| LDL cholesterol (mg/dL) | 125 ± 34 | 117 ± 31 | 132 ± 35 | <0.001 |
| HDL cholesterol (mg/dL) | 64 ± 17 | 56 ± 16 | 69 ± 16 | <0.001 |
| Triglycerides (mg/dL) | 79 (59–114) | 90 (63–138) | 75 (54–107) | 0.003 |
| Fasting glucose (mg/dL) | 89 (85–95) | 92 (86–98) | 89 (84–93) | 0.003 |
| Hemoglobin A1c (%) | 5.4 ± 0.8 | 5.5 ± 1.1 | 5.3 ± 0.4 | 0.042 |
| Insulin (µU/mL) | 8.2 (3.8–17.6) | 9.1 (4.3–19.3) | 7.3 (3.7–15.8) | 0.098 |
| HOMA-R | 1.78 (0.88–4.02) | 1.91 (0.99–4.44) | 1.54 (0.84–3.47) | 0.045 |
| Adiponectin (µg/mL) | 7.2 (4.7–10.6) | 5.4 (3.7–8.3) | 8.9 (6.1–12.5) | <0.001 |
| FGF21 (pg/mL) | 96 (61–151) | 104 (68–158) | 92 (55–143) | 0.074 |
| BNP (pg/mL) | 14 (9–25) | 13 (7–24) | 16 (11–29) | 0.006 |
| Tsukushi (ng/mL) | 28 (18–49) | 33 (19–56) | 27 (17–45) | 0.086 |
| All (n = 253) | Male (n = 114) | Female (n = 139) | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | −0.047 | 0.459 | −0.086 | 0.361 | −0.009 | 0.920 |
| Body mass index | 0.114 | 0.069 | 0.059 | 0.536 | 0.128 | 0.133 |
| Waist circumference | 0.108 | 0.088 | 0.030 | 0.750 | 0.141 | 0.099 |
| Systolic blood pressure | 0.028 | 0.661 | −0.061 | 0.522 | 0.062 | 0.470 |
| Diastolic blood pressure | 0.100 | 0.114 | 0.032 | 0.733 | 0.131 | 0.127 |
| Biochemical data | ||||||
| Log AST | 0.014 | 0.821 | −0.053 | 0.578 | 0.051 | 0.550 |
| Log ALT | 0.089 | 0.158 | 0.001 | 0.992 | 0.143 | 0.093 |
| Log GGT | 0.067 | 0.286 | −0.089 | 0.345 | 0.156 | 0.067 |
| Log FLI | 0.131 | 0.038 | −0.017 | 0.858 | 0.197 | 0.020 |
| Log TBA | −0.038 | 0.603 | −0.116 | 0.249 | 0.031 | 0.771 |
| Blood urea nitrogen | −0.001 | 0.991 | 0.052 | 0.586 | −0.056 | 0.511 |
| Creatinine | 0.119 | 0.058 | 0.156 | 0.098 | 0.024 | 0.778 |
| eGFR | −0.032 | 0.619 | −0.116 | 0.219 | 0.014 | 0.873 |
| Uric acid | 0.049 | 0.434 | 0.060 | 0.529 | −0.026 | 0.758 |
| Total cholesterol | −0.124 | 0.049 | −0.003 | 0.974 | −0.180 | 0.034 |
| LDL cholesterol | −0.107 | 0.090 | 0.018 | 0.852 | −0.170 | 0.046 |
| HDL cholesterol | −0.043 | 0.501 | 0.135 | 0.152 | −0.125 | 0.144 |
| Log Triglycerides | 0.064 | 0.309 | −0.050 | 0.598 | 0.150 | 0.078 |
| Log Fasting glucose | 0.037 | 0.558 | −0.051 | 0.594 | 0.133 | 0.119 |
| Hemoglobin A1c | −0.040 | 0.524 | −0.094 | 0.322 | 0.024 | 0.778 |
| Log Insulin | 0.295 | <0.001 | 0.238 | 0.013 | 0.331 | <0.001 |
| Log HOMA-R | 0.293 | <0.001 | 0.223 | 0.020 | 0.338 | <0.001 |
| Log Adiponectin | 0.061 | 0.333 | 0.126 | 0.183 | 0.069 | 0.417 |
| Log FGF21 | 0.290 | <0.001 | 0.113 | 0.232 | 0.396 | <0.001 |
| Log BNP | −0.015 | 0.810 | −0.003 | 0.978 | −0.002 | 0.980 |
| Regression Coefficient | SE | Standardized Regression Coefficient (β) | p | |
|---|---|---|---|---|
| Age | −0.001 | 0.003 | −0.027 | 0.671 |
| Sex (Male) | −0.024 | 0.062 | −0.027 | 0.697 |
| Log FLI | 0.033 | 0.057 | 0.041 | 0.561 |
| Total cholesterol | −0.004 | 0.002 | −0.159 | 0.020 |
| Log Insulin | 0.229 | 0.050 | 0.285 | <0.001 |
| Log FGF21 | 0.228 | 0.076 | 0.197 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuhashi, M.; Higashiura, Y.; Sakai, A.; Koyama, M.; Tanaka, M.; Saitoh, S.; Shimamoto, K.; Ohnishi, H. Plasma Tsukushi Concentration Is Associated with High Levels of Insulin and FGF21 and Low Level of Total Cholesterol in a General Population without Medication. Metabolites 2022, 12, 237. https://doi.org/10.3390/metabo12030237
Furuhashi M, Higashiura Y, Sakai A, Koyama M, Tanaka M, Saitoh S, Shimamoto K, Ohnishi H. Plasma Tsukushi Concentration Is Associated with High Levels of Insulin and FGF21 and Low Level of Total Cholesterol in a General Population without Medication. Metabolites. 2022; 12(3):237. https://doi.org/10.3390/metabo12030237
Chicago/Turabian StyleFuruhashi, Masato, Yukimura Higashiura, Akiko Sakai, Masayuki Koyama, Marenao Tanaka, Shigeyuki Saitoh, Kazuaki Shimamoto, and Hirofumi Ohnishi. 2022. "Plasma Tsukushi Concentration Is Associated with High Levels of Insulin and FGF21 and Low Level of Total Cholesterol in a General Population without Medication" Metabolites 12, no. 3: 237. https://doi.org/10.3390/metabo12030237