Dietary Egg White Hydrolysate Prevents Male Reproductive Dysfunction after Long-Term Exposure to Aluminum in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of EWH
2.2. Animal Treatment
2.3. Sperm Quality Analysis
2.3.1. Daily Sperm Production per Testis, Sperm Number and Transit Time in Epididymis
2.3.2. Sperm Morphology
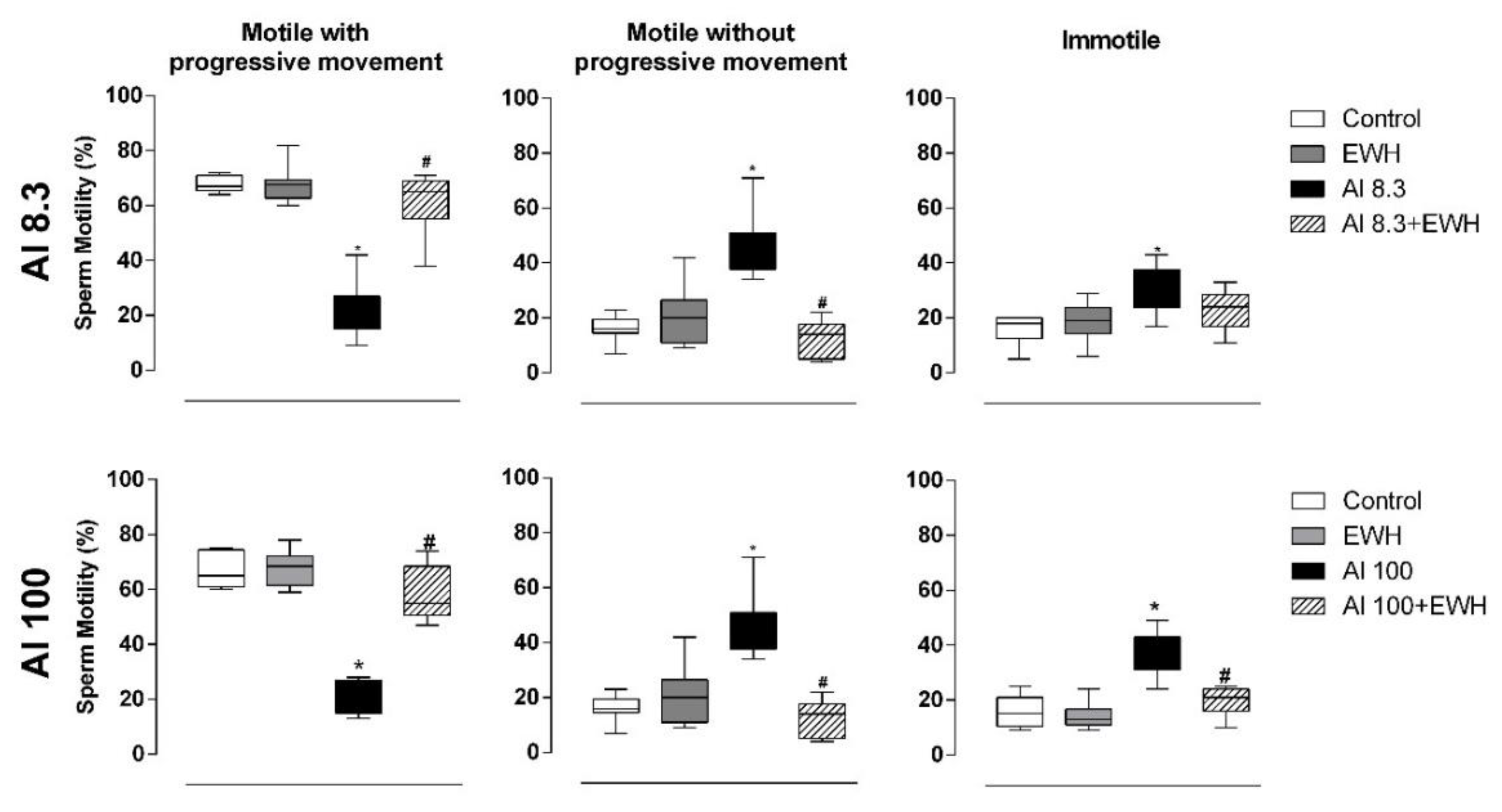
2.3.3. Sperm Motility
2.4. Biochemical Assay
2.4.1. Reactive Species Levels
2.4.2. Lipid Peroxidation
2.4.3. Ferric Reducing/Antioxidant Power (FRAP) Assay
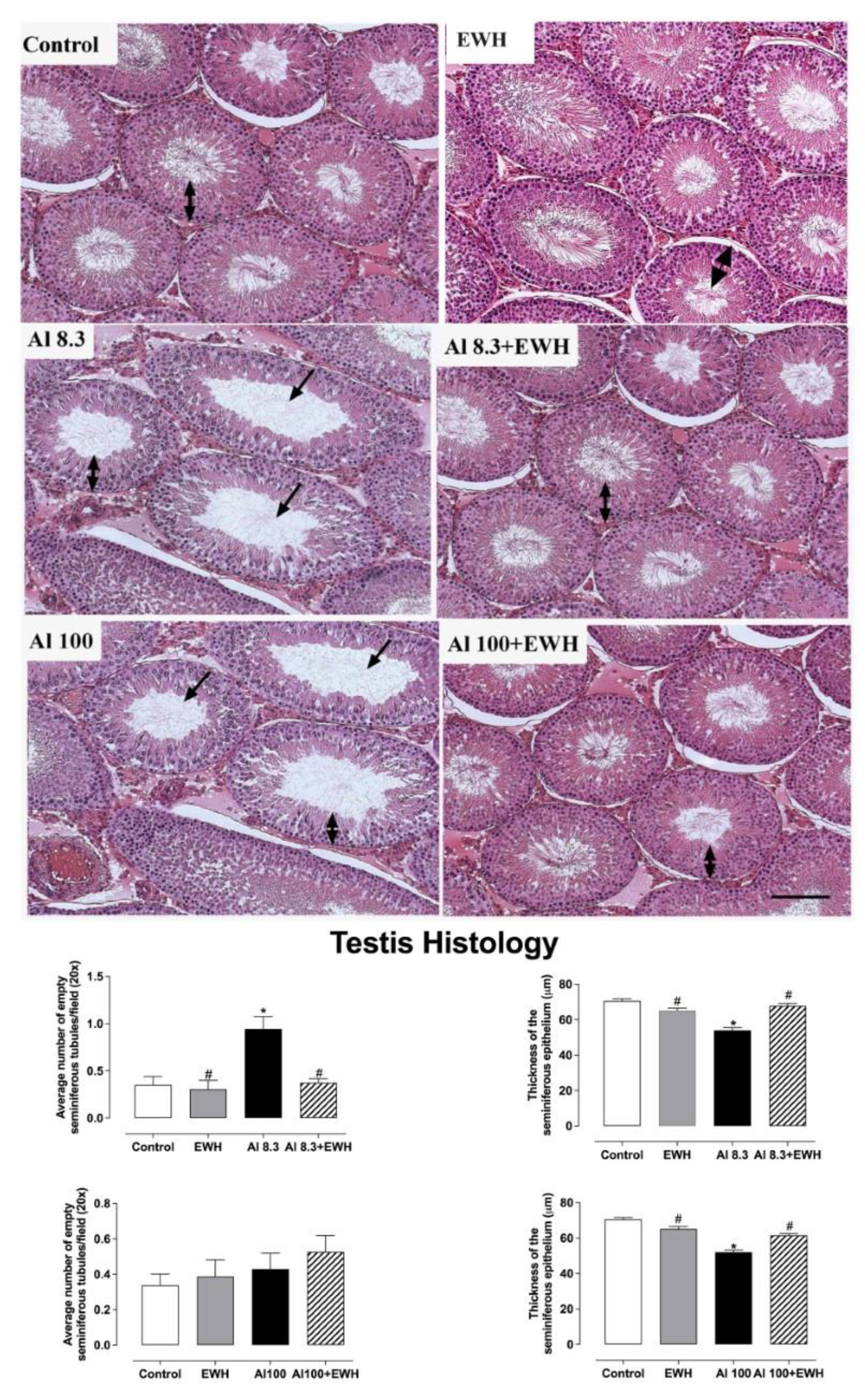
2.5. Testis and Epididymis Histology
2.6. Testis Immunohistochemistry
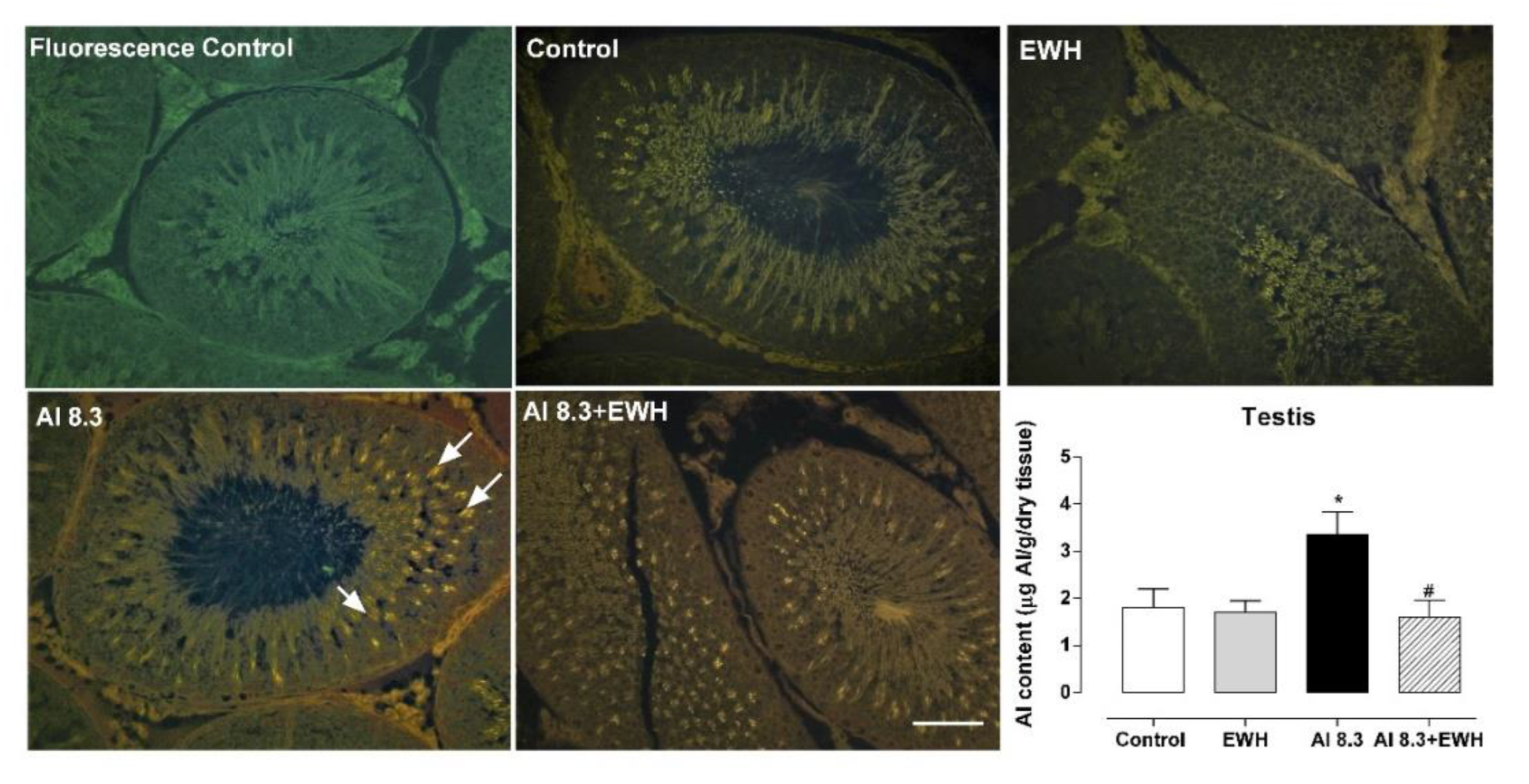
2.7. Aluminum Content in Testis and Epididymis
2.8. Lumogallion Staining
2.9. Statistical Analysis
3. Results
3.1. Body and Organs Mass, Daily Sperm Production, Sperm Number, Morphology, and Motility
3.2. Reactive Species, Lipid Peroxidation, and Total Antioxidant Capacity
3.3. Testis and Epididymis Histology
3.4. Testis Immunohistochemistry
3.5. Aluminum Content and Lumogallion Staining in Testis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Exley, C. Human exposure to aluminium. Environ. Sci. Process. Impacts 2013, 10, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Elucidating aluminium’s exposome. Curr. Inorg. Chem. 2012, 2, 3–7. [Google Scholar] [CrossRef]
- Kindgren, E.; Guerrero-Bosagna, C.; Ludvigsson, J. Heavy metals in fish and its association with autoimmunity in the development of juvenile idiopathic arthritis: A prospective birth cohort study. Pediatr. Rheumatol. Online J. 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Röllin, H.B.; Channa, K.; Olutola, B.; Nogueira, C.; Odland, J.Ø. In Utero Exposure to Aluminium and Other Neurotoxic Elements in Urban Coastal South African Women at Delivery: An Emerging Concern. Int. J. Environ. Res. Public Health 2020, 17, 1724. [Google Scholar] [CrossRef]
- Rebelo, F.M.; Caldas, E.D. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants. Environ. Res. 2016, 151, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Caito, S.; Farina, M.; da Rocha, J.B.T.; Aschner, M.; Carvalho, C. Biomarkers of mercury toxicity: Past, present and future trends. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Fekete, V.; Vandevijvere, S.; Bolle, F.; Van Loco, J. Estimation of dietary aluminum exposure of the Belgian adult population: Evaluation of contribution of food and kitchenware. Food Chem. Toxicol. 2013, 55, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 2015, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Crépeaux, G.; Eidi, H.; David, M.O.; Baba-Amer, Y.; Tzavara, E.; Giros, B.; Authier, F.-J.; Exley, C.; Shaw, C.A.; Cadusseau, J.; et al. Non-linear dose-response of aluminium hydroxide adjuvant particles: Selective low dose neurotoxicity. Toxicology 2017, 375, 48–57. [Google Scholar] [CrossRef]
- Martinez, C.S.; Alterman, C.D.; Peçanha, F.M.; Vassallo, D.V.; Mello-Carpes, P.B.; Miguel, M.; Wiggers, G.A. Aluminum Exposure at Human Dietary Levels for 60 Days Reaches a Threshold Sufficient to Promote Memory Impairment in Rats. Neurotox. Res. 2017, 31, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.D.; D’Haese, P.C.; Pires, C.; Lamberts, L.V.; Simoes, J.; De Broe, M.E. Lowdose (5 mg/kg) desferrioxamine treatment in acutely aluminium-intoxicated haemodialysis patients using two drug administration schedules. Nephrol. Dial. Transplant. 1996, 11, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Olsen, L.; Lind, L. Circulating levels of metals are related to carotid atherosclerosis in elderly. Sci. Total Environ. 2012, 416, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.P.; Mold, M.; Mery, L.; Cottier, M.; Exley, C. Aluminum content of human semen: Implications for semen quality. Reprod. Toxicol. 2014, 50, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.K.; Aouizerate, J.; Cadusseau, J.; Yara, S.; Authier, F.J. Aluminum adjuvants of vaccines injected into the muscle: Normal fate, pathology and associated disease. Morphologie 2016, 100, 85–94. [Google Scholar] [CrossRef]
- Exley, C. The pro-oxidant activity of aluminum. Free Radic. Biol. Med. 2004, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Aluminum Should Now Be Considered a Primary Etiological Factor in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 1, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wakode, S.; Sharma, A.; Nair, N.; Dhobi, M.; Wani, M.A.; Pottoo, F.H. Effect of environmental toxicants on neuronal functions. Environ. Sci. Pollut. Res. 2020, 27, 44906–44921. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Raymick, J.; Sarkar, S. Role of metals in Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. Br. Med. J. 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Frizza, F.; Balercia, G.; Barbonetti, A.; Behre, H.M.; Calogero, A.E.; Cremers, J.F.; Francavilla, F.; Isidori, A.M.; Kliesch, S.; et al. The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: Clinical, seminal and biochemical characteristics. Andrology 2020, 8, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.S.; Escobar, A.G.; Uranga-Ocio, J.A.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Miguel, M.; Wiggers, G.A. Aluminum exposure for 60days at human dietary levels impairs spermatogenesis and sperm quality in rats. Reprod. Toxicol. 2017, 73, 128–141. [Google Scholar] [CrossRef]
- Yokel, R.A. Aluminum reproductive toxicity: A summary and interpretation of scientific reports. Crit. Rev. Toxicol. 2020, 50, 551–593. [Google Scholar] [CrossRef]
- Miguel, M.; Recio, I.; Gomez-Ruiz, J.Á.; Ramos, M.; Lopez-Fandino, R. Angiotensin I-converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Rimón, M.; Gonzalez, C.; Uranga, J.A.; Lopez-Miranda, V.; Fandino, R.L.; Miguel, M. Pepsin Egg White Hydrolysate Ameliorates Obesity-Related Oxidative Stress, Inflammation and Steatosis in Zucker Fatty Rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef] [PubMed]
- Júnior, J.E.G.P.; Martinez, C.S.; Moraes, P.Z.; Stasiaki, J.E.; Trost, M.E.; Vassallo, D.V.; Junior, F.B.; Peçanha, F.M.; Cibin, F.W.S.; Miguel, M.; et al. Egg white hydrolysate prevents reproductive impairments induced by cadmium in rats. J. Funct Foods 2020, 67, 103823. [Google Scholar]
- Martinez, C.S.; Alterman, C.D.C.; Vera, G.; Márquez, A.; Uranga, J.A.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Mello-Carpes, P.B.; Miguel, M.; et al. Egg White Hydrolysate as a functional food ingredient to prevent cognitive dysfunction in rats following long-term exposure to aluminum. Sci. Rep. 2019, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.S.; Piagette, J.T.; Escobar, A.G.; Martín, Á.; Palacios, R.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Alonso, M.J.; Salaices, M.; et al. Egg White Hydrolysate: A new putative agent to prevent vascular dysfunction in rats following long-term exposure to aluminum. Food Chem. Toxicol. 2019, 133, 110799. [Google Scholar] [CrossRef]
- Miguel, M.; Dávalos, A.; Manso, M.A.; de la Peña, G.; Lasunción, M.A.; López-Fandiño, R. Transepithelial transport across Caco-2 cell monolayers of antihypertensive egg-derived peptides. PepT1-mediated flux of Tyr-Pro-Ile. Mol. Nutr. Food Res. 2008, 12, 1507–1513. [Google Scholar] [CrossRef]
- Miguel, M.; Lopez-Fandino, R.; Ramos, M.; Aleixandre, A. Long-Term Intake of Egg White Hydrolysate Attenuates the Development of Hypertension in Spontaneously Hypertensive Rats. Life Sci. 2006, 78, 2960e–2966e. [Google Scholar] [CrossRef]
- Prakash, A.; Kumar, A. Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin. Pharmacol. Toxicol. 2009, 2, 98–104. [Google Scholar] [CrossRef]
- Robb, G.W.; Amman, R.P.; Killian, G.J. Daily sperm production and epididymal sperm reserves of puberal and adult rats. J. Reprod. Fertil. 1978, 54, 103–107. [Google Scholar] [CrossRef]
- Filler, R. Methods for evaluation of rats epididymal sperm morphology. In Male Reproductive Toxicology; Chapin, R.E., Heindel, J.H., Eds.; Academic Press: Cambridge, MA, USA, 1993; pp. 334–343. [Google Scholar]
- Loetchutinat, C.; Kothan, S.; Dechsupa, S.; Meesungnoen, J.; Jay-Gerin, J.; Mankhetkorn, S. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Rad. Phys. Chem. 2005, 72, 323–331. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘‘Antioxidant Power’’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- House, E.; Esiri, M.; Forster, G.; Ince, P.G.; Exley, C. Aluminium, iron and copper in human brain tissues donated to the medical research council’s cognitive function and ageing study. Metallomics 2012, 4, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. The identification of aluminum in human brain tissue using lumogallion and fluorescence microscopy. J. Alzheimer’s Dis. 2016, 54, 1333–1338. [Google Scholar] [CrossRef]
- Mold, M.; Eriksson, H.; Siesjö, P.; Darabi, A.; Shardlow, E.; Exley, C. Unequivocal identification of intracellular aluminium adjuvant in a monocytic THP-1 cell line. Sci. Rep. 2014, 4, 6287. [Google Scholar] [CrossRef]
- Dávalos, A.; Miguel, M.; Bartolomé, B.; Lopez-Fadiño, R. Antioxidant Activity of Peptides Derived From Egg White Proteins By Enzymatic Hydrolysis. J. Food Prot. 2004, 9, 1939–1944. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; González, C.; Vera, G.; Uranga, J.A.; López-Fandiño, R.; López-Miranda, V.; Miguel, M. Pepsin egg white hydrolysate improves glucose metabolism complications related to metabolic syndrome in zucker fatty rats. Nutrients 2018, 10, 441. [Google Scholar] [CrossRef]
- Lewis, R.C.; Meeker, J.D.; Basu, N.; Gauthier, A.M.; Cantoral, A.; Mercado-García, A.; Peterson, K.E.; Téllez-Rojo, M.M.; Watkins, D.J. Urinary metal concentrations among mothers and children in a Mexico City birth cohort study. Int. J. Hyg. Environ. Health 2018, 4, 609–615. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zhang, W.; Su, W.; Zhou, A.; Xu, S.; Li, Y.; Chen, D. Aluminum Exposure and Gestational Diabetes Mellitus: Associations and Potential Mediation by n-6 Polyunsaturated Fatty Acids. Environ. Sci. Technol. 2020, 8, 5031–5040. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.K.S.; Li, Y.; Shaw, C.A. Is exposure to aluminium adjuvants associated with social impairments in mice? A pilot study. J. Inorg. Biochem. 2018, 181, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Giugliano, L.; Sarno, L.; Landolfi, A.; Richards, S.; Symes, S.; Colucci, A.; Maruotti, G.; Adair, D.; Guida, M.; et al. Serum metallome in pregnant women and the relationship with congenital malformations of the central nervous system: A case-control study. BMC Pregnancy Childbirth 2019, 19, 471. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, R.D. International disparities in access to infertility services. Rev. Fertil. Steril. 2006, 4, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Barazani, Y.; Katz, B.F.; Nagler, H.M.; Stember, D.S. Lifestyle, environment, and male reproductive health. Urol. Clin. N. Am. 2013, 1, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Corona, G.; Vitale, P.; Maseroli, E.; Rossi, M.; Fino, M.G.; Maggi, M. Current smoking is associated with lower seminal vesicles and ejaculate volume, despite higher testosterone levels, in male subjects of infertile couples. Hum. Reprod. 2015, 3, 590–602. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Radwan, P.; Jakubowski, L.; Hawuła, W.; Ulańska, A.; Hanke, W. Lifestyle factors and sperm aneuploidy. Reprod. Biol. 2014, 3, 190–199. [Google Scholar] [CrossRef]
- Hovatta, O.; Venäläinen, E.R.; Kuusimäki, L.; Heikkilä, J.; Hirvi, T.; Reima, I. Aluminium, lead and cadmium concentrations in seminal plasma and spermatozoa, and semen quality in Finnish men. Hum. Reprod. Oxf. Eng. 1998, 13, 115–119. [Google Scholar] [CrossRef][Green Version]
- Dawson, E.B.; Ritter, S.; Harris, W.A.; Evans, D.R.; Powell, L.C. Comparison of sperm viability with seminal plasma metal levels. Biol. Trace Elem. Res. 1998, 64, 215–219. [Google Scholar] [CrossRef]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 2, 215–223. [Google Scholar]
- Guo, C.H.; Lu, Y.F.; Hsu, G.S.W. The influence of aluminum exposure on male reproduction and offspring in mice. Environ. Toxicol. Pharmacol. 2005, 20, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Sun, H.; Fu, Y.; Wang, J.; Song, M.; Li, M.; Li, Y.F.; Miao, L.G. Effects of sub-chronic aluminum chloride on spermatogenesis and testicular enzymatic activity in male rats. Life Sci. 2014, 1, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, C.; Jia, L.; Zhu, Y.; Zhao, H.; Shao, B.; Wang, N.; Zhang, Z.; Li, Y. Effects of aluminum exposure on serum sex hormones and androgen receptor expression in male rats. Biol. Trace Elem. Res. 2011, 144, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Ige, S.F.; Akhigbe, R.E. The role of Allium cepa on aluminum-induced reproductive dysfunction in experimental male rat models. J. Hum. Reprod. Sci. 2012, 2, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.R. Functional impairment in aged rats chronically exposed to human range dietary aluminum equivalents. Neurotoxicology 2009, 2, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.S.; MinakshI, N.; Nihal, A. Dose translation from animal to human studies revisited. FASEB J. 2008, 3, 659–661. [Google Scholar]
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. Review. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Rizzetti, D.A.; Martinez, C.S.; Escobar, A.G.; da Silva, T.M.; Uranga-Ocio, J.A.; Peçanha, F.M.; Vassalo, D.V.; Miguel, M.; Wiggers, G.A. Egg white-derived peptides prevent male reproductive dysfunction induced by mercury in rats. Food Chem. Toxicol. 2017, 100, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.S.; Uranga-Ocio, J.A.; Peçanha, F.M.; Vassalo, D.V.; Miguel, M.; Wiggers, G.A. Egg White Hydrolysate as a new bioactive food ingrediente in the prevention of gastrointestinal effects induced by aluminum exposure in rats. Acad. J. Health Sci. 2022, 3, 76–83. [Google Scholar]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein Protection of Cadmium Toxicity. Toxicol. Appl. Pharmacol. 2009, 3, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Chickpea chelating peptides inhibit copper-mediated lipid peroxidation. J. Sci. Food Agric. 2014, 94, 3181–3188. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E. Chelation: Harnessing and enhancing heavy metal detoxification- a review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Kim, M.; Sato, K. Functional proteins and peptides of hen’s egg origin. Bioact. Food Pept. Health Dis. 2013, 115–144. [Google Scholar] [CrossRef]
- Pantoja Munoz, L.; Purchase, D.; Jones, H.; Raab, A.; Urgast, D.; Feldmann, J.; Garelick, H. The mechanisms of detoxification of As(III), dimethylarsenic acid (DMA) and As(V) in the microalga Chlorella vulgaris. Aquat. Toxicol. 2016, 175, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Strain, J.J. Nutritional factors may modify the toxic action of methyl mercury in fish-eating populations. J. Nutr. 2009, 133, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Rizzetti, D.A.; Corrales, P.; Uranga-Ocio, J.A.; Medina-Gómez, G.; Peçanha, F.M.; Vassallo, D.V.; Miguel, M.; Wiggers, G.A. Potential benefits of egg white hydrolysate in the prevention of Hg-induced dysfunction in adipose tissue. Food Funct. 2022, 13, 5996–6007. [Google Scholar] [CrossRef]
- Exley, C. Why industry propaganda and political interference cannot disguise the inevitable role played by human exposure to aluminum in neurodegenerative diseases, including Alzheimer’s disease. Front. Neurol. 2014, 27, 5–212. [Google Scholar] [CrossRef] [PubMed]
- Davenward, S.; Bentham, P.; Wright, J.; Crome, P.; Job, D.; Polwart, A.; Exley, C. Silicon-Rich Mineral Water as a Non-Invasive Test of the ‘Aluminum Hypothesis’ in Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, B.; Adelakun, S.; Iteire, K. Sulforaphane response on aluminum-induced oxidative stress, alterations in sperm characterization and testicular histomorphometry in Wistar rats. Int. J. Reprod. Biomed. 2020, 8, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Aghashahi, M.; Momeni, H.R.; Darbandi, N. Impact of aluminium toxicity on vital human sperm parameters-Protective effects of silymarin. Andrologia 2020, 5210, e13742. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhang, H.; Wang, K.; Li, X. Selenium-Rich Yeast Mitigates Aluminum-Mediated Testicular Toxicity by Blocking Oxidative Stress, Inhibiting NO Production, and Disturbing Ionic Homeostasis. Biol. Trace Elem. Res. 2020, 1, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Odo, R.I.; Uchendu, C.N.; Okeke, S.E. Protective effects of Citrullus lanatus seed ethanol extract on aluminum chloride-induced testosterone, testicular and hematological changes in an experimental male rat model. Vet. Res. Forum. Int. Q. J. 2021, 1, 7–13. [Google Scholar]
- Güvenç, M.; Cellat, M.; Gökçek, İ.; Arkalı, G.; Uyar, A.; Tekeli, İ.O.; Yavaş, İ. Tyrosol prevents AlCl3 induced male reproductive damage by suppressing apoptosis and activating the Nrf-2/HO-1 pathway. Andrologia 2020, 52, e13499. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, E.; Golkar, A.; Roshanaei, K.; Alani, B. Aluminium-Induced Oxidative Stress, Apoptosis and Alterations in Testicular Tissue and Sperm Quality in Wistar Rats: Ameliorative Effects of Curcumin. Int. J. Fertil. Steril. 2017, 3, 166–175. [Google Scholar]



| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control (n = 8) | Al 8.3 (n = 8) | EWH (n = 8) | Al 8.3 + EWH (n = 8) | |
| Initial body mass (g) | 346 ± 12.05 | 330 ± 8.77 | 348 ± 7.30 | 328 ± 10.65 |
| Final body mass (g) | 460 ± 15.70 | 436 ± 6.44 | 453 ± 12.47 | 454 ± 7.03 |
| Testis (g) | 1.82 ± 0.04 | 1.75 ± 0.04 | 1.77 ± 0.03 | 1.80 ± 0.04 |
| Testis (g/100 g) | 0.40 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 |
| Epididymis (mg) | 565 ± 25.57 | 498 ± 10.44 | 559 ± 17.46 | 547 ± 21.06 |
| Epididymis (mg/100 g) | 125 ± 7.81 | 114 ± 3.78 | 124 ± 6.90 | 119 ± 4.85 |
| Ventral prostate (mg) | 445 ± 10.17 | 390 ± 8.55 | 444 ± 35.97 | 361 ± 30.25 |
| Ventral prostate (mg/100 g) | 94.24 ± 3.50 | 89.74 ± 3.21 | 95.19 ± 9.02 | 80.19 ± 7.78 |
| Full seminal vesicle (g) | 1.51 ± 0.11 | 1.45 ± 0.10 | 1.48 ± 0.08 | 1.30 ± 0.07 |
| Full seminal vesicle (g/100 g) | 0.34 ± 0.02 | 0.32 ± 0.02 | 0.32 ± 0.01 | 0.30 ± 0.01 |
| Empty seminal vesicle (g) | 0.66 ± 0.07 | 0.65 ± 0.08 | 0.65 ± 0.05 | 0.62 ± 0.08 |
| Empty seminal vesicle (g/100 g) | 0.15 ± 0.01 | 0.14 ± 0.02 | 0.14 ± 0.01 | 0.13 ± 0.02 |
| Vesicular secretion (g) | 0.85 ± 0.11 | 0.80 ± 0.07 | 0.83 ± 0.06 | 0.69 ± 0.09 |
| Vas deferens (mg) | 87.50 ± 6.33 | 120 ± 19.02 | 140 ± 19.02 | 113 ± 24.25 |
| Vas deferens (mg/100 g) | 20.35 ± 1.68 | 27.99 ± 4.05 | 32.26 ± 4.07 | 25.34 ± 5.21 |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control (n = 8) | Al 100 (n = 8) | EWH (n = 8) | Al 100 + EWH (n = 8) | |
| Initial body mass (g) | 365 ± 10.32 | 409 ± 9.57 | 385 ± 14.71 | 398 ± 8.64 |
| Final body mass (g) | 437 ± 7.81 | 452 ± 8.81 | 415 ± 11.73 | 445 ± 10.43 |
| Testis (g) | 1.69 ± 0.03 | 1.80 ± 0.04 | 1.71 ± 0.03 | 1.72 ± 0.04 |
| Testis (g/100 g) | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.01 |
| Epididymis (mg) | 483 ± 22.13 | 502 ± 36.92 | 468 ± 13.30 | 511 ± 16.01 |
| Epididymis (mg/100 g) | 111 ± 6.08 | 110 ± 9.02 | 110.2 ± 4.69 | 117 ± 7.35 |
| Ventral prostate (mg) | 408 ± 28.66 | 312 ± 35.24 | 475 ± 30.77 | 440 ± 26.74 |
| Ventral prostate (mg/100 g) | 96.37 ± 8.81 | 64.44 ± 5.71 * | 119 ± 6.01 | 101 ± 7.63 # |
| Full seminal vesicle (g) | 1.33 ± 0.10 | 1.33 ± 0.10 | 1.53 ± 0.06 | 1.53 ± 0.14 |
| Full seminal vesicle (g/100 g) | 0.31 ± 0.03 | 0.31 ± 0.02 | 0.38 ± 0.01 | 0.36 ± 0.04 |
| Empty seminal vesicle (g) | 0.58 ± 0.08 | 0.53 ± 0.06 | 0.70 ± 0.07 | 0.72 ± 0.09 |
| Empty seminal vesicle (g/100 g) | 0.14 ± 0.02 | 0.12 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.02 |
| Vesicular secretion (g) | 0.71 ± 0.09 | 0.80 ± 0.08 | 0.83 ± 0.07 | 0.81 ± 0.02 |
| Vas deferens (mg) | 96.75 ± 5.96 | 83.50 ± 1.19 | 90.60 ± 5.10 | 106 ± 10.77 |
| Vas deferens (mg/100 g) | 21.96 ± 1.36 | 17.68 ± 0.76 | 22.80 ± 1.13 | 23.60 ± 2.32 |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Sperm count | Control (n = 8) | Al 8.3 (n = 8) | EWH (n = 8) | Al 8.3 + EWH (n = 8) |
| Testis | ||||
| Sperm number (×106) | 140 ± 6.12 | 70.4 ± 3.35 * | 139 ± 6.61 # | 130 ± 4 # |
| Sperm number (×106/g) | 101 ± 2.15 | 48.97 ± 3.55 * | 97.51 ± 5.87 # | 93.38 ± 4.53 # |
| DSP (×106/testis/day) | 22.93 ± 1 | 11.54 ± 0.55 * | 22.86 ± 1.23 # | 21.37 ± 0.65 # |
| DSPr (×106/testis/day/g) | 16.59 ± 0.35 | 8.02 ± 0.58 * | 16.27 ± 1.04 # | 15.29 ± 0.74 # |
| Epididymis | ||||
| Caput/Corpus | ||||
| Sperm number (×106) | 122 ± 5.73 | 93.17 ± 6.93 * | 134 ± 5.84 # | 127 ± 5.53 # |
| Sperm number (×106/g) | 364 ± 17.68 | 270 ± 17.33 * | 400 ± 22 # | 379 ± 12.38 # |
| Sperm transit time (days) | 5.39 ± 0.34 | 8.19 ± 0.70 * | 5.75 ± 0.16 # | 5.95 ± 0.25 # |
| Cauda | ||||
| Sperm number (×106) | 169 ± 5.45 | 113 ± 5.04 * | 163 ± 8.31 # | 167 ± 10.57 # |
| Sperm number (×106/g) | 845 ± 24.95 | 610 ± 25.83 * | 825 ± 48.83 # | 831 ± 44.90 # |
| Sperm transit time (days) | 7.44 ± 0.33 | 10.14 ± 0.91 * | 7.01 ± 0.30 # | 7.90 ± 0.61 |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Sperm count | Control (n = 8) | Al 100 (n = 8) | EWH (n = 8) | Al 100 + EWH (n = 8) |
| Testis | ||||
| Sperm number (×106) | 128 ± 4.69 | 62.3 ± 6.75 * | 135 ± 6.15 # | 114 ± 5.26 # |
| Sperm number (×106/g) | 94.17 ± 6.84 | 42.59 ± 3.93 * | 94.44 ± 6.28 # | 84.56 ± 3.07 # |
| DSP (×106/testis/day) | 21.06 ± 0.76 | 10.21 ± 1.10 * | 21.80 ± 1.02 # | 18.73 ± 0.86 # |
| DSPr (×106/testis/day/g) | 15.44 ± 1.12 | 6.98 ± 0.64 * | 15.51 ± 1.02 # | 13.86 ± 0.50 # |
| Epididymis | ||||
| Caput/Corpus | ||||
| Sperm number (×106) | 132 ± 5.58 | 98.9 ± 4.94 * | 140 ± 6.15 # | 131 ± 5.24 # |
| Sperm number (×106/g) | 410 ± 16.44 | 271 ± 19.72 * | 379 ± 21.40 # | 392 ± 14.30 # |
| Sperm transit time (days) | 6.32 ± 0.38 | 10.57 ± 1.3 * | 6.23 ± 0.3 # | 7.04 ± 0.36 # |
| Cauda | ||||
| Sperm number (×106) | 190 ± 8.02 | 114 ± 8.93 * | 172 ± 6.83 # | 160 ± 9.53 # |
| Sperm number (×106/g) | 905 ± 31.82 | 589 ± 18.63 * | 855 ± 40.68 # | 847 ± 18.71 # |
| Sperm transit time (days) | 8.66 ± 0.31 | 11.52 ± 0.64 * | 7.73 ± 0.26 # | 8.59 ± 0.49 # |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control (n = 8) | Al 8.3 (n = 8) | EWH (n = 8) | Al 8.3 + EWH (n = 8) | |
| Normal | 96 (94–97) | 76 (67–80) * | 92.5 (91.2–95.7) # | 92 (89.5–93.2) |
| Head Abnormalities | ||||
| Amorphous | 0 (0–0.5) | 9 (2–19) * | 1 (0.2–2.7) | 1 (0–3) |
| Banana Head | 0 (0–1) | 4 (1–6) * | 0.5 (0–1) | 2 (0.7–2.2) |
| Detached Head | 0 (0–1) | 2 (0–6) | 0 (0–1) | 0.5 (0–6) |
| Total of Head Abnormalities | 2 (0–3) | 15 (9–26) * | 3 (1.2–3.7) # | 3.5 (2–5.2) |
| Tail Abnormalities | ||||
| Bent Tail | 0 (0–0.5) | 5 (2–6) * | 0 (0–1) # | 0.5 (0–2) |
| Broken Tail | 1 (0.0–2.5) | 0 (0–1) | 0 (0–0) | 0 (0–0.2) |
| Total of Tail Abnormalities | 3 (1–4.5) | 9 (6–13) * | 4.5 (0.7–6) | 4 (2.7–6.2) |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control (n = 8) | Al100 (n = 8) | EWH (n = 8) | AL100 + EWH (n = 8) | |
| Normal | 96 (94–97) | 76 (67–80) * | 92.5 (91.2–95.7) # | 92 (89.5–93.2) * |
| Head Abnormalities | ||||
| Amorphous | 0 (0–0.5) | 9 (2–19) * | 1 (0.2–2.7) | 0 (0–3) |
| Banana Head | 0 (0–1) | 4 (1–6) * | 0.5 (0–1) | 2 (0.7–2.2) |
| Detached Head | 0 (0–1) | 2 (0–6) | 0 (0–1) | 0.5 (0–1) |
| Total of Head Abnormalities | 2 (0–3) | 15 (9–26) * | 3 (1.2–3.7) # | 3.5 (2–5.2) |
| Tail Abnormalities | ||||
| Bent Tail | 1 (0–3) | 4 (2–5) * | 4.5 (0.2–5.7) | 2.5 (1–4.5) # |
| Broken Tail | 1 (0–2.5) | 0 (0–1) | 0 (0–0) | 0 (0–0.2) |
| Total of Tail Abnormalities | 3 (1–4.5) | 9 (6–13) * | 4.5 (0.7–6) | 4 (2.7–6.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, C.S.; Uranga-Ocio, J.A.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Miguel-Castro, M.; Wiggers, G.A. Dietary Egg White Hydrolysate Prevents Male Reproductive Dysfunction after Long-Term Exposure to Aluminum in Rats. Metabolites 2022, 12, 1188. https://doi.org/10.3390/metabo12121188
Martinez CS, Uranga-Ocio JA, Peçanha FM, Vassallo DV, Exley C, Miguel-Castro M, Wiggers GA. Dietary Egg White Hydrolysate Prevents Male Reproductive Dysfunction after Long-Term Exposure to Aluminum in Rats. Metabolites. 2022; 12(12):1188. https://doi.org/10.3390/metabo12121188
Chicago/Turabian StyleMartinez, Caroline Silveira, Jose Antonio Uranga-Ocio, Franck Maciel Peçanha, Dalton Valentim Vassallo, Christopher Exley, Marta Miguel-Castro, and Giulia Alessandra Wiggers. 2022. "Dietary Egg White Hydrolysate Prevents Male Reproductive Dysfunction after Long-Term Exposure to Aluminum in Rats" Metabolites 12, no. 12: 1188. https://doi.org/10.3390/metabo12121188
APA StyleMartinez, C. S., Uranga-Ocio, J. A., Peçanha, F. M., Vassallo, D. V., Exley, C., Miguel-Castro, M., & Wiggers, G. A. (2022). Dietary Egg White Hydrolysate Prevents Male Reproductive Dysfunction after Long-Term Exposure to Aluminum in Rats. Metabolites, 12(12), 1188. https://doi.org/10.3390/metabo12121188












