Untargeted Metabolomics Pilot Study Using UHPLC-qTOF MS Profile in Sows’ Urine Reveals Metabolites of Bladder Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Histological Examination
2.3. Chemicals and Reagents
2.4. Sample Preparation
2.5. UHPLC-qTOF MS/MS Analysis
2.6. Data Analysis
2.7. MS-DIAL
3. Results
3.1. UHPLC-qTOF MS/MS Untargeted Profile
3.2. Identification of Different Metabolites
3.3. Prediction and Diagnostic Performance Test
3.4. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almond, G. An Assessment of Urinary Tract Infections in Sows. Available online: https://www.pork.org (accessed on 23 September 2022).
- Boma, M.H.; Bilkei, G. Gross pathological findings in sows of different parity, culled due to recurring swine urogenital disease (SUGD) in Kenya. Onderstepoort J. Vet. Res. 2006, 73, 139–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glock, X.T.; Bilkei, G. The effect of postparturient urogenital diseases on the lifetime reproductive performance of sows. Can. Vet. J. 2005, 46, 1103–1107. [Google Scholar] [PubMed]
- Cernat, M.; Skampardonis, V.; Papadopoulos, G.A.; Kroustallas, F.; Chalvatzi, S.; Petridou, E.; Psychas, V.; Marouda, C.; Fortomaris, P.; Leontides, L. Urinary tract infections in culled sows from Greek herds: Prevalence and associations between findings of histopathology, bacteriology and urinalysis. Porc. Health Manag. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Bellino, C.; Gianella, P.; Grattarola, C.; Miniscalco, B.; Tursi, M.; Dondo, A.; D’Angelo, A.; Cagnasso, A. Urinary tract infections in sows in Italy: Accuracy of urinalysis and urine culture against histological findings. Vet. Rec. 2013, 172, 183. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, M.; Wendt, M.; Kaup, F.J.; Drommer, W. Clinical, and light and electron microscopical findings in sows with cystitis. Vet. Rec. 1995, 137, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Biksi, I.; Takács, N.; Vetési, F.; Fodor, L.; Szenci, O.; Fenyö, E. Association between endometritis and urocystitis in culled sows. Acta Vet. Hung. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Rainville, P.; Theodoridis, G.; Plumb, R.; Wilson, I. Advances in liquid chromatography coupled to mass spectrometry for metabolic phenotyping. TrAC Trends Anal. Chem. 2014, 61, 181–191. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Plumb, R.S.; Wilson, I.D. Current practice of liquid chromatography-mass spectrometry in metabolomics and metabonomics. J. Pharm. Biomed. Anal. 2014, 87, 12–25. [Google Scholar] [CrossRef]
- Theodoridis, G.; Gika, H.; Wilson, I. LC-MS-based methodology for global metabolite profiling in metabonomics/metabolomics. TrAC Trends Anal. Chem. 2008, 27, 251–260. [Google Scholar] [CrossRef]
- Theodoridis, G.; Gika, H.G.; Wilson, I.D. Mass spectrometry-based holistic analytical approaches for metabolite profiling in systems biology studies. Mass Spectrom. Rev. 2011, 30, 884–906. [Google Scholar] [CrossRef]
- Sanz-Fernandez, M.V.; Torres-Rovira, L.; Pesantez-Pacheco, J.L.; Vazquez-Gomez, M.; Garcia-Contreras, C.; Astiz, S.; Gonzalez-Bulnes, A. A Cross-Sectional Study of Obesity Effects on the Metabolomic Profile of a Leptin-Resistant Swine Model. Metabolites 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Gao, J.; Zhao, Y.; He, M.; Ke, S.; Wu, J.; Zhou, Y.; Fu, H.; Yang, H.; Chen, C.; et al. Dramatic Remodeling of the Gut Microbiome Around Parturition and Its Relationship With Host Serum Metabolic Changes in Sows. Front. Microbiol. 2019, 10, 2123. [Google Scholar] [CrossRef] [PubMed]
- Bin, P.; Azad, M.A.K.; Liu, G.; Zhu, D.; Kim, S.W.; Yin, Y. Effects of different levels of methionine on sow health and plasma metabolomics during late gestation. Food Funct. 2018, 9, 4979–4988. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Fang, J.; Liu, G.; Veeramuthu, D.; Naif Abdullah, A.D.; Yin, Y. The impact of different levels of cysteine on the plasma metabolomics and intestinal microflora of sows from late pregnancy to lactation. Food Funct. 2019, 10, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Azad, M.A.K.; Fang, J.; Zhou, X.; Xu, K.; Yin, Y.; Liu, G. Impact of sulfur-containing amino acids on the plasma metabolomics and intestinal microflora of the sow in late pregnancy. Food Funct. 2019, 10, 5910–5921. [Google Scholar] [CrossRef]
- Hu, L.; Che, L.; Wu, C.; Curtasu, M.V.; Wu, F.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Li, J.; et al. Metabolomic Profiling Reveals the Difference on Reproductive Performance between High and Low Lactational Weight Loss Sows. Metabolites 2019, 9, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiefer, Z.E.; Koester, L.R.; Showman, L.; Studer, J.M.; Chipman, A.L.; Keating, A.F.; Schmitz-Esser, S.; Ross, J.W. Vaginal microbiome and serum metabolite differences in late gestation commercial sows at risk for pelvic organ prolapse. Sci. Rep. 2021, 11, 6189. [Google Scholar] [CrossRef]
- Fu, H.; He, M.; Wu, J.; Zhou, Y.; Ke, S.; Chen, Z.; Liu, Q.; Liu, M.; Jiang, H.; Huang, L.; et al. Deep Investigating the Changes of Gut Microbiome and Its Correlation With the Shifts of Host Serum Metabolome Around Parturition in Sows. Front. Microbiol. 2021, 12, 729039. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Ke, S.; Huang, X.; Fang, S.; He, M.; Fu, H.; Chen, C.; Huang, L. Gut and Vagina Microbiota Associated With Estrus Return of Weaning Sows and Its Correlation With the Changes in Serum Metabolites. Front. Microbiol. 2021, 12, 690091. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Liu, G.; Bin, P.; Ding, S.; Kong, X.; Guan, G.; Yin, Y. Sulfur-containing amino acid supplementation to gilts from late pregnancy to lactation altered offspring’s intestinal microbiota and plasma metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 1227–1242. [Google Scholar] [CrossRef]
- He, J.; Guo, H.; Zheng, W.; Xue, Y.; Zhao, R.; Yao, W. Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Picone, G.; Balzani, A.; Capozzi, F.; Bertocchi, M.; Salvarani, C.; Bosi, P.; Edwards, S.; Trevisi, P. Investigation of the Defatted Colostrum (1)H-NMR Metabolomics Profile of Gilts and Multiparous Sows and Its Relationship with Litter Performance. Animals 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picone, G.; Zappaterra, M.; Luise, D.; Trimigno, A.; Capozzi, F.; Motta, V.; Davoli, R.; Nanni Costa, L.; Bosi, P.; Trevisi, P. Metabolomics characterization of colostrum in three sow breeds and its influences on piglets’ survival and litter growth rates. J. Anim. Sci. Biotechnol. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Xiong, X.; Yin, J.; Zou, L.; Wang, K.; Shao, Y.; Yin, Y. Dietary Lysozyme Alters Sow’s Gut Microbiota, Serum Immunity and Milk Metabolite Profile. Front. Microbiol. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Liu, Y.; Long, J.; Zhou, X.; Zeng, S.; Li, T.; Yin, Y. Maternal serine supply from late pregnancy to lactation improves offspring performance through modulation of metabolic pathways. Food Funct. 2020, 11, 8089–8098. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhai, Z.; Ni, X.; Wang, H.; Ji, Y.; Tang, T.; Ren, W.; Long, H.; Deng, B.; Deng, J.; et al. Metabolomic Profiles Reveal Potential Factors that Correlate with Lactation Performance in Sow Milk. Sci. Rep. 2018, 8, 10712. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Jiang, F.; Zhang, J.; Xu, Q.; Chen, D.; Yu, B.; Mao, X.; Yu, J.; Luo, Y.; He, J. Amniotic fluid metabolomics and biochemistry analysis provides novel insights into the diet-regulated foetal growth in a pig model. Sci. Rep. 2017, 7, 44782. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Li, Y.; Chen, D.; Yu, B.; Luo, Y.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; Zheng, P.; et al. Daidzein supplementation enhances embryo survival by improving hormones, antioxidant capacity, and metabolic profiles of amniotic fluid in sows. Food Funct. 2020, 11, 10588–10600. [Google Scholar] [CrossRef]
- Malgarin, C.M.; MacPhee, D.J.; Harding, J.C.S. Fetal Metabolomic Alterations Following Porcine Reproductive and Respiratory Syndrome Virus Infection. Front. Mol. Biosci. 2020, 7, 559688. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Lu, M.; Yang, X.; Xue, Y.; Yao, W. A controlled heat stress during late gestation affects thermoregulation, productive performance, and metabolite profiles of primiparous sow. J. Therm. Biol. 2019, 81, 33–40. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, B.; Cai, S.; Zeng, X.; Ye, Q.; Mao, X.; Zhang, S.; Zeng, X.; Ye, C.; Qiao, S. Metabolic disorder of amino acids, fatty acids and purines reflects the decreases in oocyte quality and potential in sows. J. Proteom. 2019, 200, 134–143. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Hedemann, M.S.; Poulsen, H.D.; Corrent, E.; van Milgen, J.; Nørgaard, J.V. Nontargeted LC-MS Metabolomics Approach for Metabolic Profiling of Plasma and Urine from Pigs Fed Branched Chain Amino Acids for Maximum Growth Performance. J. Proteome Res. 2016, 15, 4195–4207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Trimigno, A.; Stanstrup, J.; Khakimov, B.; Viereck, N.; Engelsen, S.B.; Sangild, P.T.; Dragsted, L.O. Antibiotic Treatment Preventing Necrotising Enterocolitis Alters Urinary and Plasma Metabolomes in Preterm Pigs. J. Proteome Res. 2017, 16, 3547–3557. [Google Scholar] [CrossRef]
- Zabek, A.; Paslawski, R.; Paslawska, U.; Wojtowicz, W.; Drozdz, K.; Polakof, S.; Podhorska, M.; Dziegiel, P.; Mlynarz, P.; Szuba, A. The influence of different diets on metabolism and atherosclerosis processes-A porcine model: Blood serum, urine and tissues 1H NMR metabolomics targeted analysis. PLoS ONE 2017, 12, e0184798. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Pink, M.; Kersch, C.; Rettenmeier, A.W.; Schmitz-Spanke, S. Benzo[a]pyrene mediated time- and dose-dependent alteration in cellular metabolism of primary pig bladder cells with emphasis on proline cycling. Arch. Toxicol. 2019, 93, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Stastny, K.; Putecova, K.; Leva, L.; Franek, M.; Dvorak, P.; Faldyna, M. Profiling of Metabolomic Changes in Plasma and Urine of Pigs Caused by Illegal Administration of Testosterone Esters. Metabolites 2020, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Liesenfeld, S.; Steliopoulos, P.; Wenig, S.; Gottstein, V.; Hamscher, G. Comprehensive LC-HRMS metabolomics analyses for the estimation of environmental inputs of altrenogest in pig breeding. Chemosphere 2022, 287, 132353. [Google Scholar] [CrossRef]
- Gika, H.G.; Macpherson, E.; Theodoridis, G.A.; Wilson, I.D. Evaluation of the repeatability of ultra-performance liquid chromatography-TOF-MS for global metabolic profiling of human urine samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 299–305. [Google Scholar] [CrossRef]
- Gika, H.G.; Zisi, C.; Theodoridis, G.; Wilson, I.D. Protocol for quality control in metabolic profiling of biological fluids by U(H)PLC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1008, 15–25. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Wingate, J.E.; Wilson, I.D. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: Application to human urine. J. Proteome Res. 2007, 6, 3291–3303. [Google Scholar] [CrossRef]
- Lewis, M.R.; Pearce, J.T.; Spagou, K.; Green, M.; Dona, A.C.; Yuen, A.H.; David, M.; Berry, D.J.; Chappell, K.; Horneffer-van der Sluis, V.; et al. Development and Application of Ultra-Performance Liquid Chromatography-TOF MS for Precision Large Scale Urinary Metabolic Phenotyping. Anal. Chem. 2016, 88, 9004–9013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kind, T.; Tsugawa, H.; Cajka, T.; Ma, Y.; Lai, Z.; Mehta, S.S.; Wohlgemuth, G.; Barupal, D.K.; Showalter, M.R.; Arita, M.; et al. Identification of small molecules using accurate mass MS/MS search. Mass Spectrom. Rev. 2018, 37, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.B.; Watson, J.R.; Cohen, D.M.; Kline, D.; Schwaderer, A.L.; Spencer, J.D. Novel urine biomarkers to distinguish UTI from culture-negative pyuria. Pediatr. Nephrol. 2022, 37, 385–391. [Google Scholar] [CrossRef]
- Abid, M.S.R.; Qiu, H.; Tripp, B.A.; de Lima Leite, A.; Roth, H.E.; Adamec, J.; Powers, R.; Checco, J.W. Peptidomics analysis reveals changes in small urinary peptides in patients with interstitial cystitis/bladder pain syndrome. Sci. Rep. 2022, 12, 8289. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Kuo, H.C. Usefulness of Urinary Biomarkers for Assessing Bladder Condition and Histopathology in Patients with Interstitial Cystitis/Bladder Pain Syndrome. Int. J. Mol. Sci. 2022, 23, 12044. [Google Scholar] [CrossRef]
- Yu, W.R.; Jiang, Y.H.; Jhang, J.F.; Kuo, H.C. Use of Urinary Cytokine and Chemokine Levels for Identifying Bladder Conditions and Predicting Treatment Outcomes in Patients with Interstitial Cystitis/Bladder Pain Syndrome. Biomedicines 2022, 10, 1149. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Ho, H.C.; Chiou, D.Y.; Kuo, H.C. Urine Oxidative Stress Biomarkers as Novel Biomarkers in Interstitial Cystitis/Bladder Pain Syndrome. Biomedicines 2022, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Lu, J.H.; Chuang, S.M.; Chueh, K.S.; Juan, T.J.; Liu, Y.C.; Juan, Y.S. Urinary Biomarkers in Interstitial Cystitis/Bladder Pain Syndrome and Its Impact on Therapeutic Outcome. Diagnostics 2021, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.H.; Lee, K.W.; Kim, W.B.; Choi, H.W.; Moon, J.E.; Moon, A.; Kim, Y.H. β-Defensin 2, an Antimicrobial Peptide, as a Novel Biomarker for Ulcerative Interstitial Cystitis; Can β-Defensin 2 Suspect the Dysbiosis of Urine Microbiota? Diagnostics 2021, 11, 2082. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.L.; Shaw, T.; Berecz, Z.; Su, Y.; Zupkas, P.; Argade, S. Role of urinary cations in the aetiology of bladder symptoms and interstitial cystitis. BJU Int. 2014, 114, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, H.; Lu, Q.; Liu, X.; Fan, Y.; Zhu, J.; Sun, B.; Zhao, J.; Dong, X.; Li, L. Suppression of adenosine A(2a) receptors alleviates bladder overactivity and hyperalgesia in cyclophosphamide-induced cystitis by inhibiting TRPV1. Biochem. Pharmacol. 2021, 183, 114340. [Google Scholar] [CrossRef]
- Uysal, E.; Yılmaz, H.R.; Ugan, Y.; Altuntas, A.; Dogru, A.; Kutlucan, A.; Tunc, S.E. Protective Effects of Caffeic Acid Phenethyl Ester on Cyclophosphamide-Induced Hemorrhagic Cystitis in Rats. J. Biochem. Mol. Toxicol. 2015, 29, 559–563. [Google Scholar] [CrossRef]
- Schneidewind, L.; Neumann, T.; Schmidt, C.A.; Krüger, W. Comparison of intravenous or intravesical cidofovir in the treatment of BK polyomavirus-associated hemorrhagic cystitis following adult allogeneic stem cell transplantation-A systematic review. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2018, 20, e12914. [Google Scholar] [CrossRef]
- Foster, J.H.; Cheng, W.S.; Nguyen, N.Y.; Krance, R.; Martinez, C. Intravesicular cidofovir for BK hemorrhagic cystitis in pediatric patients after hematopoietic stem cell transplant. Pediatr. Transplant. 2018, 22, e13141. [Google Scholar] [CrossRef]
- David, B.T.; Sampath, S.; Dong, W.; Heiman, A.; Rella, C.E.; Elkabes, S.; Heary, R.F. A toll-like receptor 9 antagonist improves bladder function and white matter sparing in spinal cord injury. J. Neurotrauma 2014, 31, 1800–1806. [Google Scholar] [CrossRef]
- Nomiya, M.; Sagawa, K.; Yazaki, J.; Takahashi, N.; Kushida, N.; Haga, N.; Aikawa, K.; Matsui, T.; Oka, M.; Fukui, T.; et al. Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourol. Urodyn. 2012, 31, 185–189. [Google Scholar] [CrossRef]
- Kim, J.; De Hoedt, A.; Wiggins, E.; Haywood, K.; Jin, P.; Greenwood, B.; Narain, N.R.; Tolstikov, V.; Bussberg, V.; Barbour, K.E.; et al. Diagnostic Utility of Serum and Urinary Metabolite Analysis in Patients with Interstitial Cystitis/Painful Bladder Syndrome. Urology 2021, 157, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Coelho, M.C.; Ozcan, K.; Pinto, C.A.; Teixeira, J.A.; Saraiva, J.A.; Pintado, M. Emergent Technologies for the Extraction of Antioxidants from Prickly Pear Peel and Their Antimicrobial Activity. Foods 2021, 10, 570. [Google Scholar] [CrossRef]
- Palmieri, A.; Petrini, M. Tryptophol and derivatives: Natural occurrence and applications to the synthesis of bioactive compounds. Nat. Prod. Rep. 2019, 36, 490–530. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.; Würtz, P.; Singh-Manoux, A.; Shipley, M.J.; Haapakoski, R.; Lehto, M.; Desrumaux, C.; Kähönen, M.; Lehtimäki, T.; Mikkilä, V.; et al. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: Analysis of two cohort studies. Sci. Rep. 2018, 8, 8620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boretti, F.S.; Burla, B.; Deuel, J.; Gao, L.; Wenk, M.R.; Liesegang, A.; Sieber-Ruckstuhl, N.S. Serum lipidome analysis of healthy beagle dogs receiving different diets. Metab. Off. J. Metab. Soc. 2019, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Trimigno, A.; Aru, V.; Rasmussen, M.A.; Khakimov, B.; Engelsen, S.B. Influence of Age, Sex, and Diet on the Human Fecal Metabolome Investigated by (1)H NMR Spectroscopy. J. Proteome Res. 2021, 20, 3642–3653. [Google Scholar] [CrossRef]
- Su, X.; Yu, J.; Wang, N.; Zhao, S.; Han, W.; Chen, D.; Li, L.; Li, L. High-Coverage Metabolome Analysis Reveals Significant Diet Effects of Autoclaved and Irradiated Feed on Mouse Fecal and Urine Metabolomics. Mol. Nutr. Food Res. 2021, 65, e2100110. [Google Scholar] [CrossRef] [PubMed]

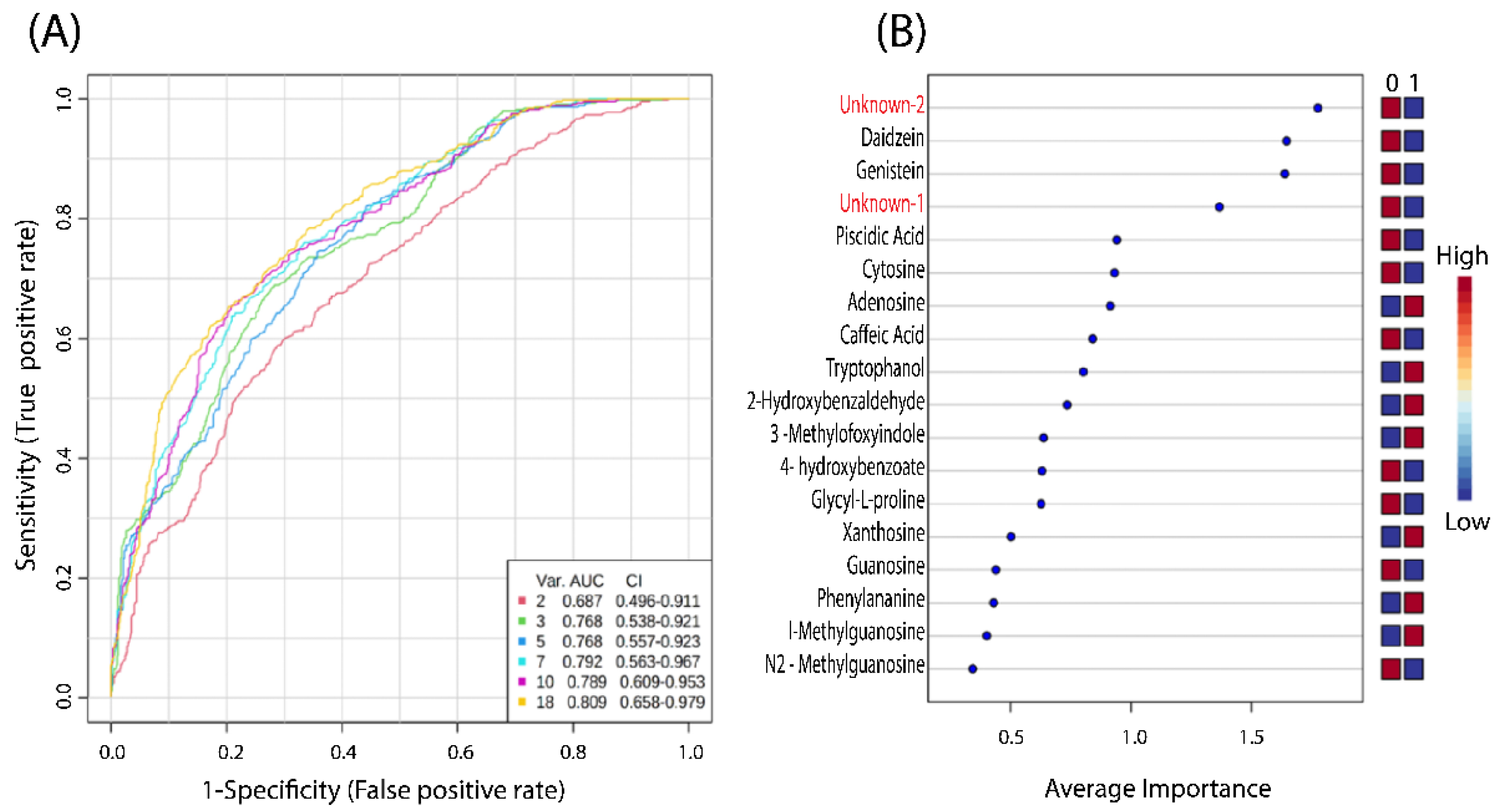
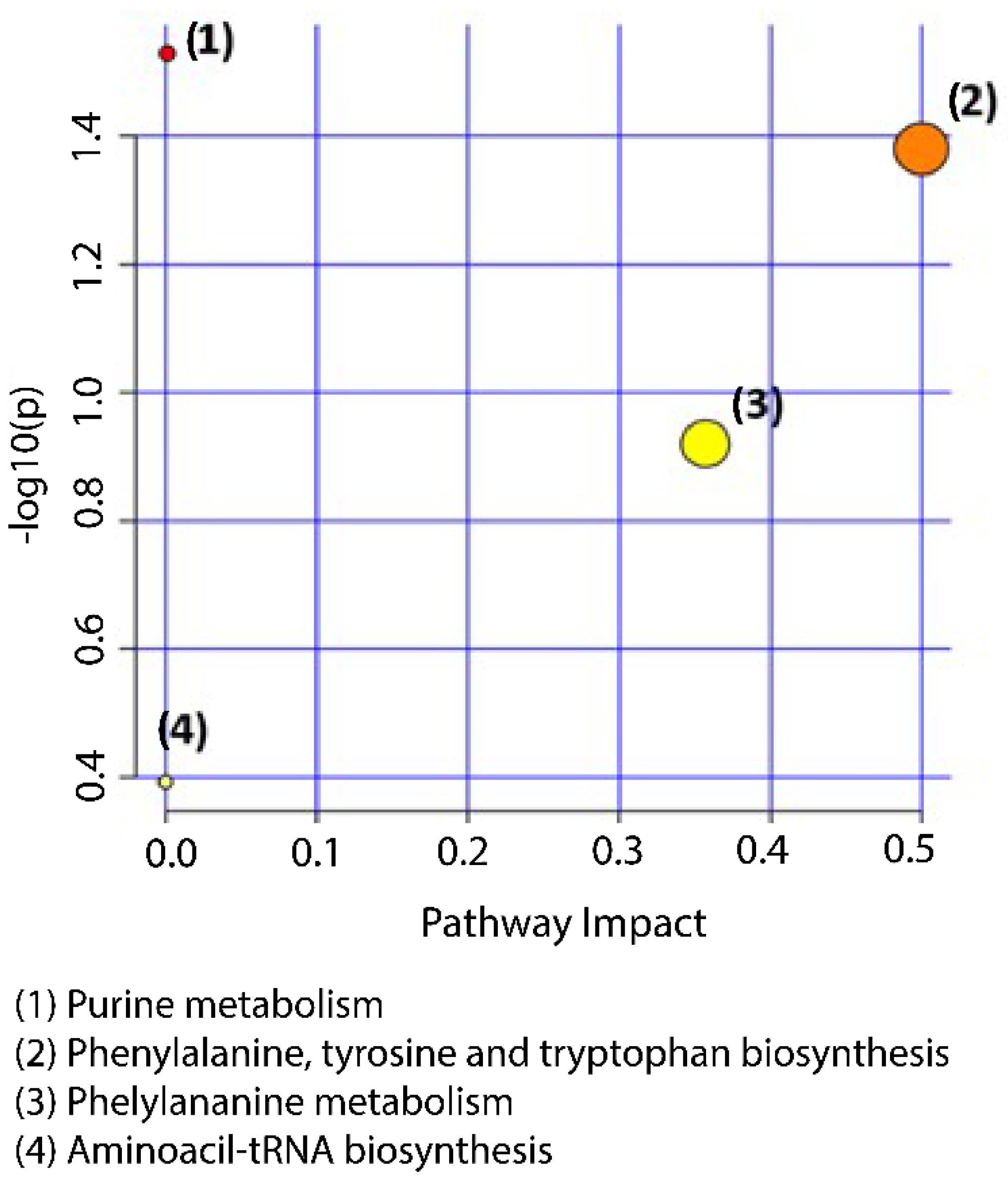
| N | MS MODE | Average RT (min) | Average Mz | Metabolite Name a | HMDB ID | Fold Change (FC) b | p Value c | RSD% (QCs) d |
|---|---|---|---|---|---|---|---|---|
| 1 | POS | 0.7 | 173.0912 | Glycyl-L-proline | HMDB0000721 | 0.4 | 0.022 | 21 |
| 2 | NEG | 1.2 | 282.0838 | Guanosine | HMDB0000133 | 0.7 | 0.011 | 7.2 |
| 3 | POS | 1.3 | 268.1036 | Adenosine | HMDB0000050 | 0.6 | 0.037 | 9.1 |
| 4 | NEG | 1.4 | 283.0678 | Xanthosine | HMDB0000299 | 0.6 | 0.017 | 8.1 |
| 5 | NEG | 1.8 | 296.0996 | N2-Methylguanosine | HMDB0005862 | 0.7 | 0.010 | 2.6 |
| 6 | POS | 1.8 | 166.0717 | L-Phenylalanine | HMDB0000159 | 0.7 | 0.014 | 11 |
| 7 | POS | 1.8 | 298.1141 | 1-Methylguanosine | HMDB0001563 | 0.7 | 0.014 | 11 |
| 8 | NEG | 1.9 | 284.088 | Unknown-1 | 0.6 | 0.003 | 7.2 | |
| 9 | POS | 1.9 | 112.05 | Cytosine | HMDB0000630 | 0.6 | 0.007 | 11 |
| 10 | NEG | 2.0 | 255.05 | Piscidic Acid | HMDB0030809 | 2.0 | 0.034 | 4.6 |
| 11 | POS | 3.0 | 181.049 | Caffeic acid | HMDB0001964 | 1.8 | 0.039 | 7.6 |
| 12 | POS | 3.0 | 148.075 | 3-Methyloxyindole | HMDB0004186 | 0.6 | 0.015 | 9.3 |
| 13 | POS | 3.0 | 144.0801 | Tryptophanol | HMDB0003447 | 2.0 | 0.014 | 9.9 |
| 14 | NEG | 4.6 | 121.0293 | 2-Hydroxybenzaldehyde | HMDB0034170 | 1.8 | 0.031 | 4.5 |
| 15 | NEG | 5.0 | 137.0241 | 4-hydroxybenzoate | HMDB0000500 | 2.2 | 0.035 | 5.4 |
| 16 | POS | 5.9 | 255.0652 | Daidzein | HMDB0003312 | 4.3 | 0.045 | 9.2 |
| 17 | POS | 6.9 | 271.0598 | Genistein | HMDB0003217 | 4.6 | 0.048 | 11 |
| 18 | NEG | 8.1 | 193.0344 | Unknown-2 | 0.2 | 0.010 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pousinis, P.; Virgiliou, C.; Mouskeftara, T.; Chalvatzi, S.; Kroustallas, F.; Panteris, E.; Papadopoulos, G.A.; Fortomaris, P.; Cernat, M.; Leontides, L.; et al. Untargeted Metabolomics Pilot Study Using UHPLC-qTOF MS Profile in Sows’ Urine Reveals Metabolites of Bladder Inflammation. Metabolites 2022, 12, 1186. https://doi.org/10.3390/metabo12121186
Pousinis P, Virgiliou C, Mouskeftara T, Chalvatzi S, Kroustallas F, Panteris E, Papadopoulos GA, Fortomaris P, Cernat M, Leontides L, et al. Untargeted Metabolomics Pilot Study Using UHPLC-qTOF MS Profile in Sows’ Urine Reveals Metabolites of Bladder Inflammation. Metabolites. 2022; 12(12):1186. https://doi.org/10.3390/metabo12121186
Chicago/Turabian StylePousinis, Petros, Christina Virgiliou, Thomai Mouskeftara, Sofia Chalvatzi, Fotios Kroustallas, Eleftherios Panteris, Georgios A. Papadopoulos, Paschalis Fortomaris, Michaela Cernat, Leonidas Leontides, and et al. 2022. "Untargeted Metabolomics Pilot Study Using UHPLC-qTOF MS Profile in Sows’ Urine Reveals Metabolites of Bladder Inflammation" Metabolites 12, no. 12: 1186. https://doi.org/10.3390/metabo12121186






