Opposing Effects of Ceanothus velutinus Phytochemistry on Herbivore Communities at Multiple Scales
Abstract
:1. Introduction
2. Results
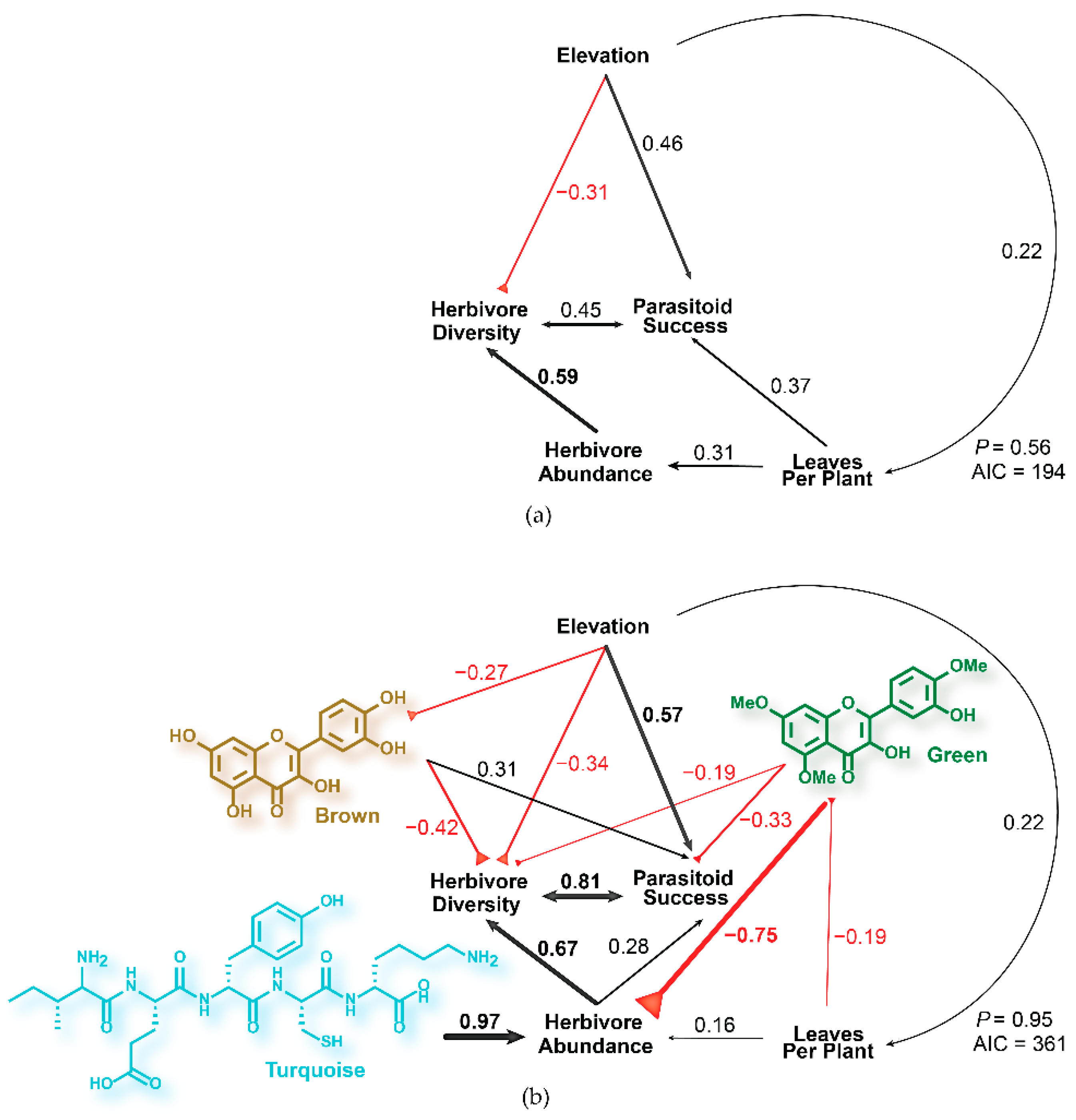
2.1. Modeling Tritrophic Interactions in C. velutinus Communities of Dog Valley and Mt. Rose
2.2. Chemically Mediated Ecological Interactions in C. velutinus Communities
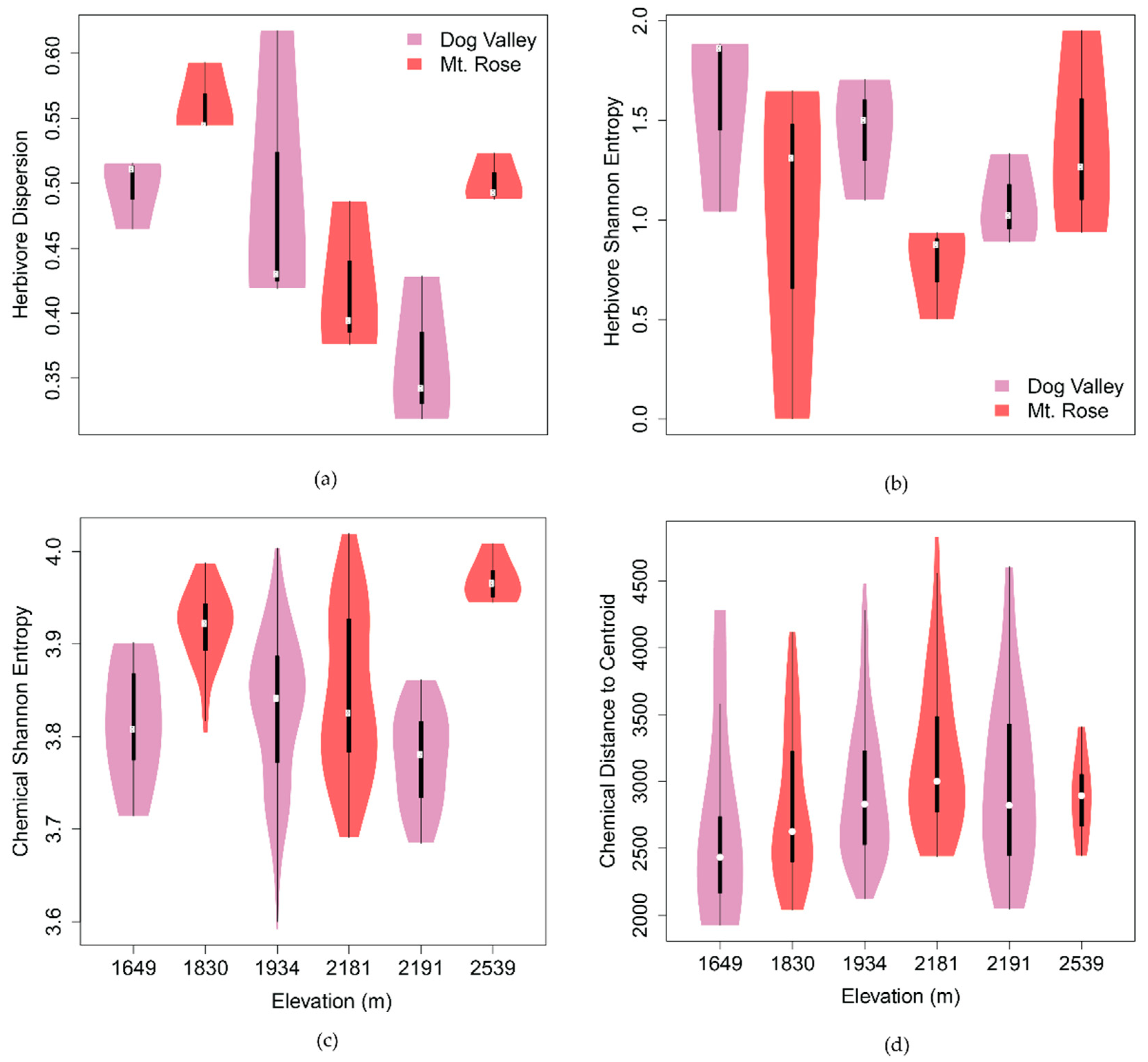
2.2.1. Spatiotemporal Chemical Variation
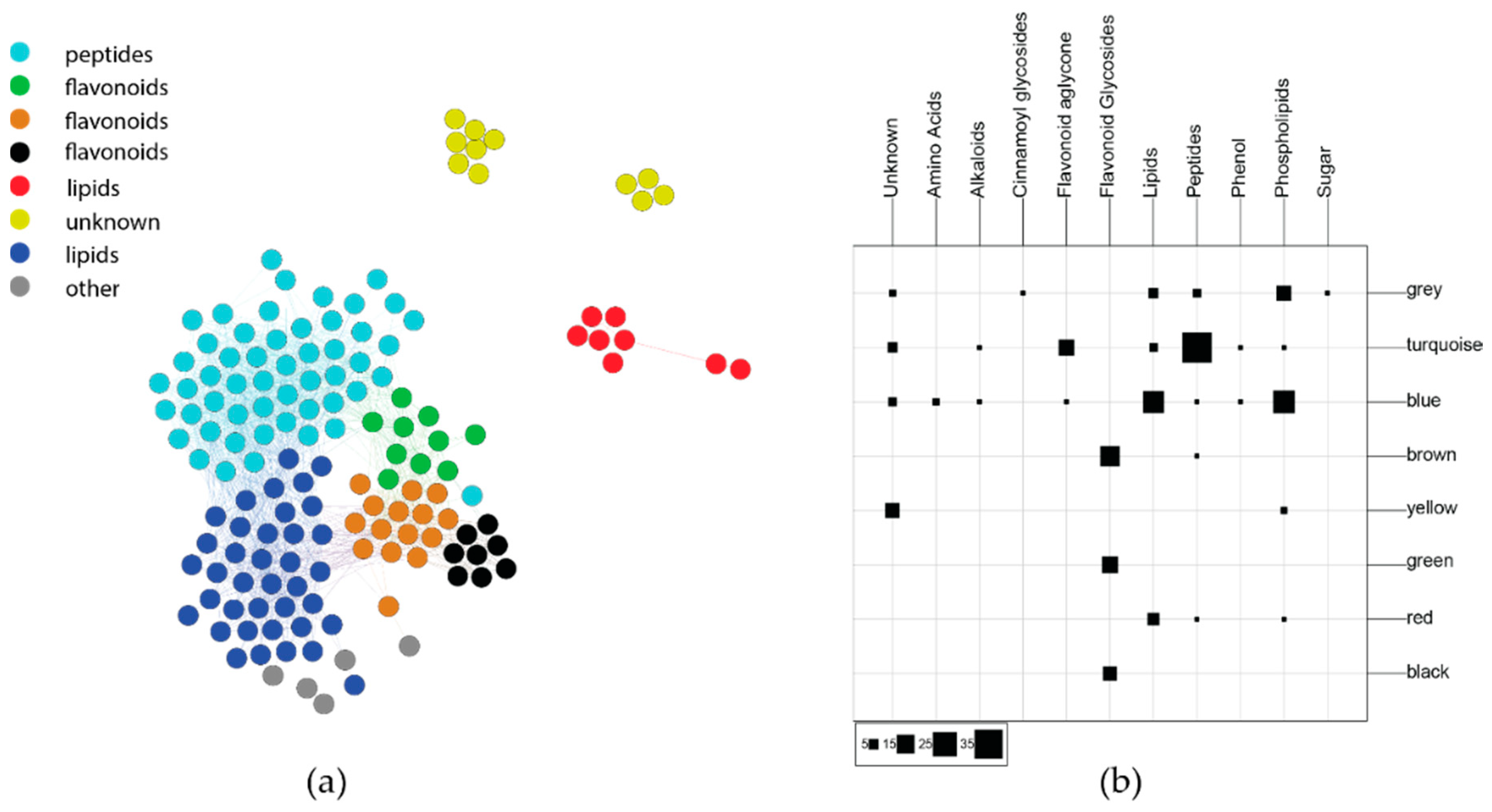
2.2.2. Phytochemical Covariance Clusters
2.3. Phytochemical Models of Tritrophic Interactions in C. velutinus Communities
3. Discussion
4. Materials and Methods
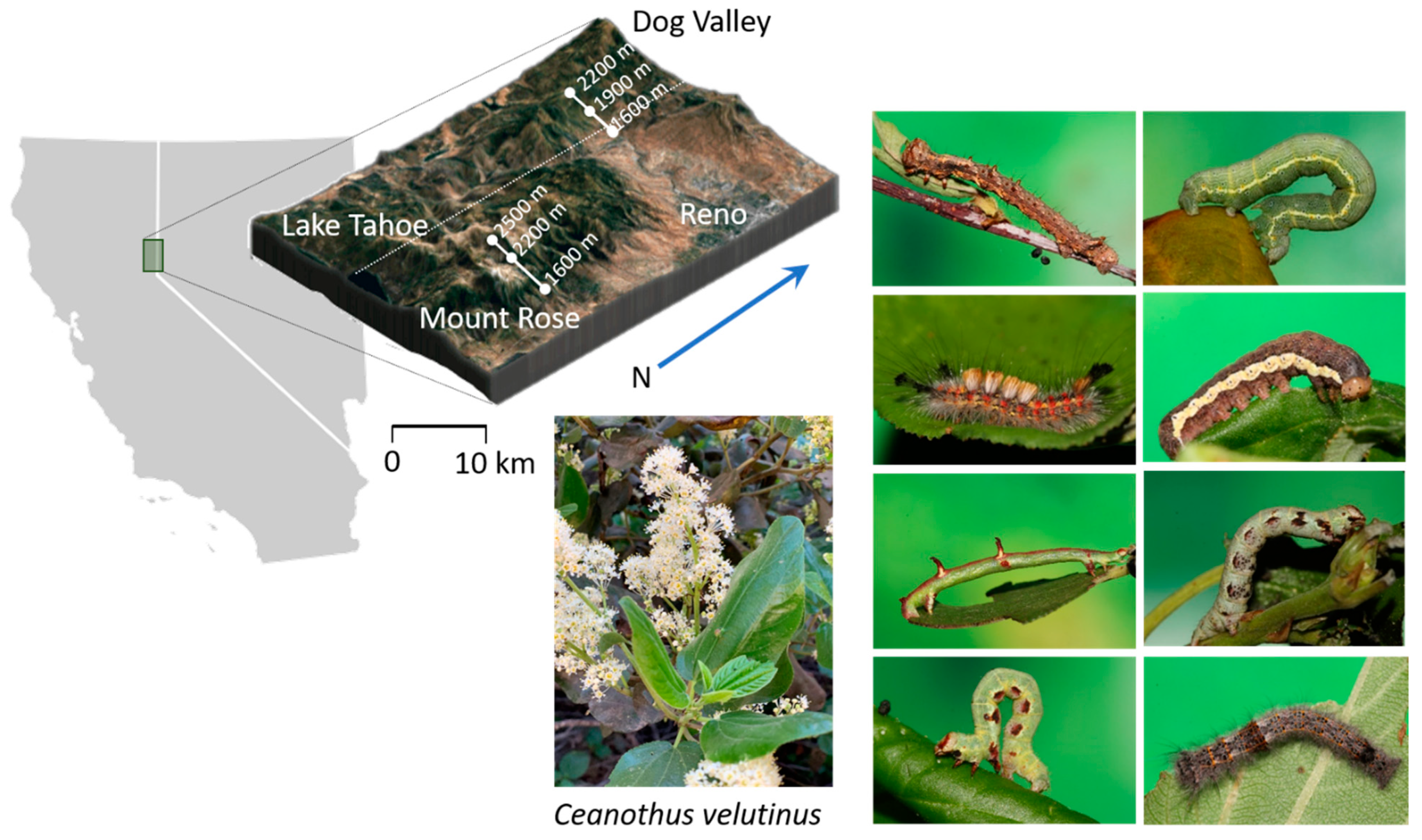
4.1. Study Site
4.2. Ecological Data
4.3. Sample Preparation
4.4. LC-TOF Analysis of Foliar Plant Tissue
4.5. Compound Classification, Annotation, and Normalization
4.6. WGCNA Correlation Networks and Model Construction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, M.D. The Phytochemical Landscape: Linking Trophic Interactions and Nutrient Dynamics; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Wetzel, W.C.; Whitehead, S.R. The many dimensions of phytochemical diversity: Linking theory to practice. Ecol. Lett. 2020, 23, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot 2011, 108, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Shirley, B.W. Flavonoid biosynthesis: ‘new’functions for an ‘old’pathway. Trends Plant. Sci. 1996, 1, 377–382. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids--biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant. Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Abdala-Roberts, L.; Rasmann, S.; Berny-Mier, Y.T.J.C.; Covelo, F.; Glauser, G.; Moreira, X. Biotic and abiotic factors associated with altitudinal variation in plant traits and herbivory in a dominant oak species. Am. J. Bot 2016, 103, 2070–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defossez, E.; Pitteloud, C.; Descombes, P.; Glauser, G.; Allard, P.M.; Walker, T.W.N.; Fernandez-Conradi, P.; Wolfender, J.L.; Pellissier, L.; Rasmann, S. Spatial and evolutionary predictability of phytochemical diversity. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, S.; Nie, Y.; Zhao, J.; Cao, X.; Dai, Y.; Zhang, Z.; Wei, F. Dietary flavonoids and the altitudinal preference of wild giant pandas in Foping National Nature Reserve, China. Glob. Ecol. Conserv. 2020, 22, e00981. [Google Scholar] [CrossRef]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Endara, M.J.; Coley, P.D.; Ghabash, G.; Nicholls, J.A.; Dexter, K.G.; Donoso, D.A.; Stone, G.N.; Pennington, R.T.; Kursar, T.A. Coevolutionary arms race versus host defense chase in a tropical herbivore-plant system. Proc. Natl. Acad. Sci. USA 2017, 114, E7499–E7505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, D.; Jaramillo, A.; Marquis, R.J. The impact of plant chemical diversity on plant–herbivore interactions at the community level. Oecologia 2016, 181, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.; Philbin, C.; Marion, Z.; Buerkle, C.; Dodson, C.; Fordyce, J.; Forister, G.; Lebeis, S.; Lucas, L.; Nice, C. Predicting patch occupancy reveals the complexity of host range expansion. Sci. Adv. 2020, 6, eabc6852. [Google Scholar] [CrossRef]
- Glassmire, A.E.; Jeffrey, C.S.; Forister, M.L.; Parchman, T.L.; Nice, C.C.; Jahner, J.P.; Wilson, J.S.; Walla, T.R.; Richards, L.A.; Smilanich, A.M. Intraspecific phytochemical variation shapes community and population structure for specialist caterpillars. New Phytol. 2016, 212, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Fortuna, T.M.; Eckert, S.; Harvey, J.A.; Vet, L.E.; Muller, C.; Gols, R. Variation in plant defences among populations of a range-expanding plant: Consequences for trophic interactions. New Phytol 2014, 204, 989–999. [Google Scholar] [CrossRef]
- Aartsma, Y.; Leroy, B.; van der Werf, W.; Dicke, M.; Poelman, E.H.; Bianchi, F.J.J.A. Intraspecific variation in herbivore-induced plant volatiles influences the spatial range of plant-parasitoid interactions. Oikos 2019, 128, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Dyer, L.A.; Philbin, C.S.; Ochsenrider, K.M.; Richards, L.A.; Massad, T.J.; Smilanich, A.M.; Forister, M.L.; Parchman, T.L.; Galland, L.M.; Hurtado, P.J.; et al. Modern approaches to study plant–insect interactions in chemical ecology. Nat. Rev. Chem. 2018, 2, 50–64. [Google Scholar] [CrossRef]
- Nyamundanda, G.; Gormley, I.C.; Fan, Y.; Gallagher, W.M.; Brennan, L. MetSizeR: Selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinform. 2013, 14, 338. [Google Scholar] [CrossRef] [Green Version]
- Forister, M.L.; Yoon, S.; Philbin, C.S.; Dodson, C.D.; Hart, B.; Harrison, J.G.; Shelef, O.; Fordyce, J.A.; Marion, Z.H.; Nice, C.C.; et al. Caterpillars on a phytochemical landscape: The case of alfalfa and the Melissa blue butterfly. Ecol. Evol. 2020, 10, 4362–4374. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.A.; Oliveira, C.; Dyer, L.A.; Rumbaugh, A.; Urbano-Muñoz, F.; Wallace, I.S.; Dodson, C.D.; Jeffrey, C.S. Shedding Light on Chemically Mediated Tri-Trophic Interactions: A 1H-NMR Network Approach to Identify Compound Structural Features and Associated Biological Activity. Front. Plant. Sci. 2018, 9, 1155. [Google Scholar] [CrossRef] [Green Version]
- Sedio, B.E.; Rojas Echeverri, J.C.; Boya, P.C.A.; Wright, S.J. Sources of variation in foliar secondary chemistry in a tropical forest tree community. Ecology 2017, 98, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Richards, L.A.; Dyer, L.A.; Forister, M.L.; Smilanich, A.M.; Dodson, C.D.; Leonard, M.D.; Jeffrey, C.S. Phytochemical diversity drives plant–insect community diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 10973–10978. [Google Scholar] [CrossRef] [Green Version]
- Marion, Z.H.; Fordyce, J.A.; Fitzpatrick, B.M. Extending the Concept of Diversity Partitioning to Characterize Phenotypic Complexity. Am. Nat. 2015, 186, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Philbin, C.S.; Dyer, L.A.; Jeffrey, C.S.; Glassmire, A.E.; Richards, L.A. Structural and compositional dimensions of phytochemical diversity in the genus Piper reflect distinct ecological modes of action. J. Ecol. 2021. [Google Scholar] [CrossRef]
- Jost, L. Partitioning Diversity into Independent Alpha and Beta Components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bates, J.D.; Sharp, R.N.; Davies, K.W. Sagebrush steppe recovery after fire varies by development phase of Juniperus occidentalis woodland. Int. J. Wildland Fire 2014, 23, 117. [Google Scholar] [CrossRef] [Green Version]
- Defrees, D.H.; Averett, J.P.; Wisdom, M.J.; Endress, B.A. Interactive effects of fuels reduction and large herbivores on shrub assemblages in dry conifer forests of the interior west, USA. For. Ecol. Manag. 2020, 463, 118031. [Google Scholar] [CrossRef]
- Johnson, D.W.; Walker, R.F.; McNulty, M.; Rau, B.M.; Miller, W.W. The Long-Term Effects of Wildfire and Post-Fire Vegetation on Sierra Nevada Forest Soils. Forests 2012, 3, 398–416. [Google Scholar] [CrossRef] [Green Version]
- Stein, C.M.; Johnson, D.W.; Miller, W.W.; Powers, R.F.; Young, D.A.; Glass, D.W. Snowbrush (Ceanothus velutinus Dougl) effects on nitrogen availability in soils and solutions from a Sierran ecosystem. Ecohydrology 2009, 3, 79–87. [Google Scholar] [CrossRef]
- Youngberg, C.T.; Wollum, A.G., II. Nitrogen Accretion in Developing Ceanothus Velutinus Stands. Soil Sci. Soc. Am. J. 1976, 40, 109–112. [Google Scholar] [CrossRef]
- Zavitkovski, J.; Newton, M. Ecological importance of snowbrush Ceanothus velutinus in the Oregon Cascades. Ecology 1968, 49, 1134–1145. [Google Scholar] [CrossRef]
- Jeong, S.-C.; Myrold, D.D. Population size and diversity of Frankia in soils of Ceanothus velutinus and Douglas-fir stands. Soil Biol. Biochem. 2001, 33, 931–941. [Google Scholar] [CrossRef]
- Ackerly, D.D. Adaptation, niche conservatism, and convergence: Comparative studies of leaf evolution in the California chaparral. Am. Nat. 2004, 163, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Karban, R. Leaf drop in evergreen Ceanothus velutinus as a means of reducing herbivory. Ecology 2008, 89, 2446–2452. [Google Scholar] [CrossRef]
- Schowalter, T.; Stafford, S.; Slagle, R. Arboreal arthropod community structure in an early successional coniferous forest ecosystem in western Oregon. Great Basin Naturalist 1988, 48, 327–333. [Google Scholar]
- Fleishman, E.; Fogarty, F. Methods for Assessment of Species Richness and Occupancy across Space, Time, Taxonomic Groups, and Ecoregions. Field Guide and Natural History of Butterflies on the Western Edge of the Great Basin; University of California-Davis Davis: Davis, CA, USA, 2018. [Google Scholar]
- Lambert, D.; Cattaneo, A.; Carignan, R. Periphyton as an early indicator of perturbation in recreational lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 258–265. [Google Scholar] [CrossRef]
- Das, K.C.; Farmer, W.J.; Weinstein, B. Phytochemical studies. IX. A new flavone, velutin. J. Org. Chem. 1970, 35, 3989–3990. [Google Scholar] [CrossRef]
- Wollenweber, E.; Dörr, M.; Bohm, B.A.; Roitman, J.N. Exudate flavonoids of eight species of Ceanothus (Rhamnaceae). Z. Nat. C J. Biosci. 2004, 59, 459–462. [Google Scholar] [CrossRef]
- Klein, F.K.; Rapoport, H. Ceanothus alkaloids. Americine. J. Am. Chem. Soc. 1968, 90, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Lagarias, J.C.; Goff, D.; Klein, F.K.; Rapoport, H. Cyclopeptide alkaloids. Phencyclopeptines from the polymorphic species Ceanothus integerrimus. J. Nat. Prod. 1979, 42, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnhoff, E.; Pradhan, S.; Ma, J. Ceanothus alkaloids: I. Isolation, separation, and characterization. Can. J. Chem. 1965, 43, 2594–2602. [Google Scholar] [CrossRef] [Green Version]
- Grytnes, J.A. Ecological interpretations of the mid-domain effect. Ecol. Lett. 2003, 6, 883–888. [Google Scholar] [CrossRef]
- Hu, L.t.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Modeling A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Oksanen, J. Multivariate analysis of ecological communities in R: Vegan tutorial. R Package Version 2011, 1, 1–43. [Google Scholar]
- Pellissier, L.; Fiedler, K.; Ndribe, C.; Dubuis, A.; Pradervand, J.-N.; Guisan, A.; Rasmann, S. Shifts in species richness, herbivore specialization, and plant resistance along elevation gradients. Ecol. Evol. 2012, 2, 1818–1825. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehm, G.; Colwell, R.K.; Kluge, J. The role of environment and mid-domain effect on moth species richness along a tropical elevational gradient. Glob. Ecol. Biogeogr. 2007, 16, 205–219. [Google Scholar] [CrossRef]
- Jones, C.G.; Firn, R.D. On the evolution of plant secondary chemical diversity. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1991, 333, 273–280. [Google Scholar]
- Baig, M.A.; Banthorpe, D.V.; Coleman, A.A.; Tampion, M.D.; Tampion, J.; White, J.J. Accumulation of tetrapeptide precursors of macrocyclic alkaloids by callus of Ceanothus americanus. Phytochemistry 1993, 34, 171–174. [Google Scholar] [CrossRef]
- Oakley, B.; North, M.; Franklin, J.F.; Hedlund, B.P.; Staley, J.T. Diversity and Distribution of Frankia Strains Symbiotic with Ceanothus in California. Appl. Environ. Microbiol. 2004, 70, 6444–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig-Müller, J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015, 37, 1325–1334. [Google Scholar] [CrossRef]
- Tanaka, A.; Tapper, B.A.; Popay, A.; Parker, E.J.; Scott, B. A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol. Microbiol. 2005, 57, 1036–1050. [Google Scholar] [CrossRef] [PubMed]
- Coley, P.D.; Bateman, M.L.; Kursar, T.A. The effects of plant quality on caterpillar growth and defense against natural enemies. Oikos 2006, 115, 219–228. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host Plant Quality and Fecundity in Herbivorous Insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Karowe, D.N.; Martin, M.M. The effects of quantity and quality of diet nitrogen on the growth, efficiency of food utilization, nitrogen budget, and metabolic rate of fifth-instar Spodoptera eridania larvae (Lepidoptera: Noctuidae). J. Insect. Physiol. 1989, 35, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Smilanich, A.M.; Dyer, L.A.; Gentry, G.L. The insect immune response and other putative defenses as effective predictors of parasitism. Ecology 2009, 90, 1434–1440. [Google Scholar] [CrossRef]
- Smilanich, A.M.; Mason, P.A.; Sprung, L.; Chase, T.R.; Singer, M.S. Complex effects of parasitoids on pharmacophagy and diet choice of a polyphagous caterpillar. Oecologia 2011, 165, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Miranda, R.; Arriola-Padilla, V.J.; Romero-Sanchez, M.E. Characterizing New Wintering Sites for Monarch Butterfly Colonies in Sierra Nevada, Mexico. Insects 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Conrad, S.G. The Role of the Genus Ceanothus in Western Forest Ecosystems; US Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Corvallis, OR, USA, 1985; Volume 182. [Google Scholar]
- Burge, D.O.; Zhukovsky, K.; Wilken, D.H. A Taxonomic Conspectus of Ceanothus subgenus Cerastes (Rhamnaceae). Syst. Bot. 2016, 40, 950–961. [Google Scholar] [CrossRef]
- Hardig, T.M.; Soltis, P.S.; Soltis, D.E. Diversification of the North American shrub genus Ceanothus (Rhamnaceae): Conflicting phylogenies from nuclear ribosomal DNA and chloroplast DNA. Am. J. Bot. 2000, 87, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Kuhl, C.; Tautenhahn, R.; Bottcher, C.; Larson, T.R.; Neumann, S. CAMERA: An integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 2011, 84, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. mclust 5: Clustering, Classification and Density Estimation Using Gaussian Finite Mixture Models. R J. 2016, 8, 289–317. [Google Scholar] [CrossRef] [Green Version]
- Ekanayaka, E.P.; Celiz, M.D.; Jones, A.D. Relative mass defect filtering of mass spectra: A path to discovery of plant specialized metabolites. Plant. Physiol. 2015, 167, 1221–1232. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the Third International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Henry, M.; Stevens, M. Vegan: Community ecology package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 31 March 2021).
- Rosseel, Y. Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philbin, C.S.; Paulsen, M.; Richards, L.A. Opposing Effects of Ceanothus velutinus Phytochemistry on Herbivore Communities at Multiple Scales. Metabolites 2021, 11, 361. https://doi.org/10.3390/metabo11060361
Philbin CS, Paulsen M, Richards LA. Opposing Effects of Ceanothus velutinus Phytochemistry on Herbivore Communities at Multiple Scales. Metabolites. 2021; 11(6):361. https://doi.org/10.3390/metabo11060361
Chicago/Turabian StylePhilbin, Casey S., Matthew Paulsen, and Lora A. Richards. 2021. "Opposing Effects of Ceanothus velutinus Phytochemistry on Herbivore Communities at Multiple Scales" Metabolites 11, no. 6: 361. https://doi.org/10.3390/metabo11060361





