Analytical Enantio-Separation of Linagliptin in Linagliptin and Metformin HCl Dosage Forms by Applying Two-Level Factorial Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Equipment and Chromatographic Conditions
2.3. Preparation of Standard Solution
2.4. Preparation of Test Solution (LGP–MET HCl-2.5/500 mg, 2.5/850 mg, 2.5/1000 mg)
3. Results
3.1. Development and Method optimization
Optimization of Column and Mobile Phase
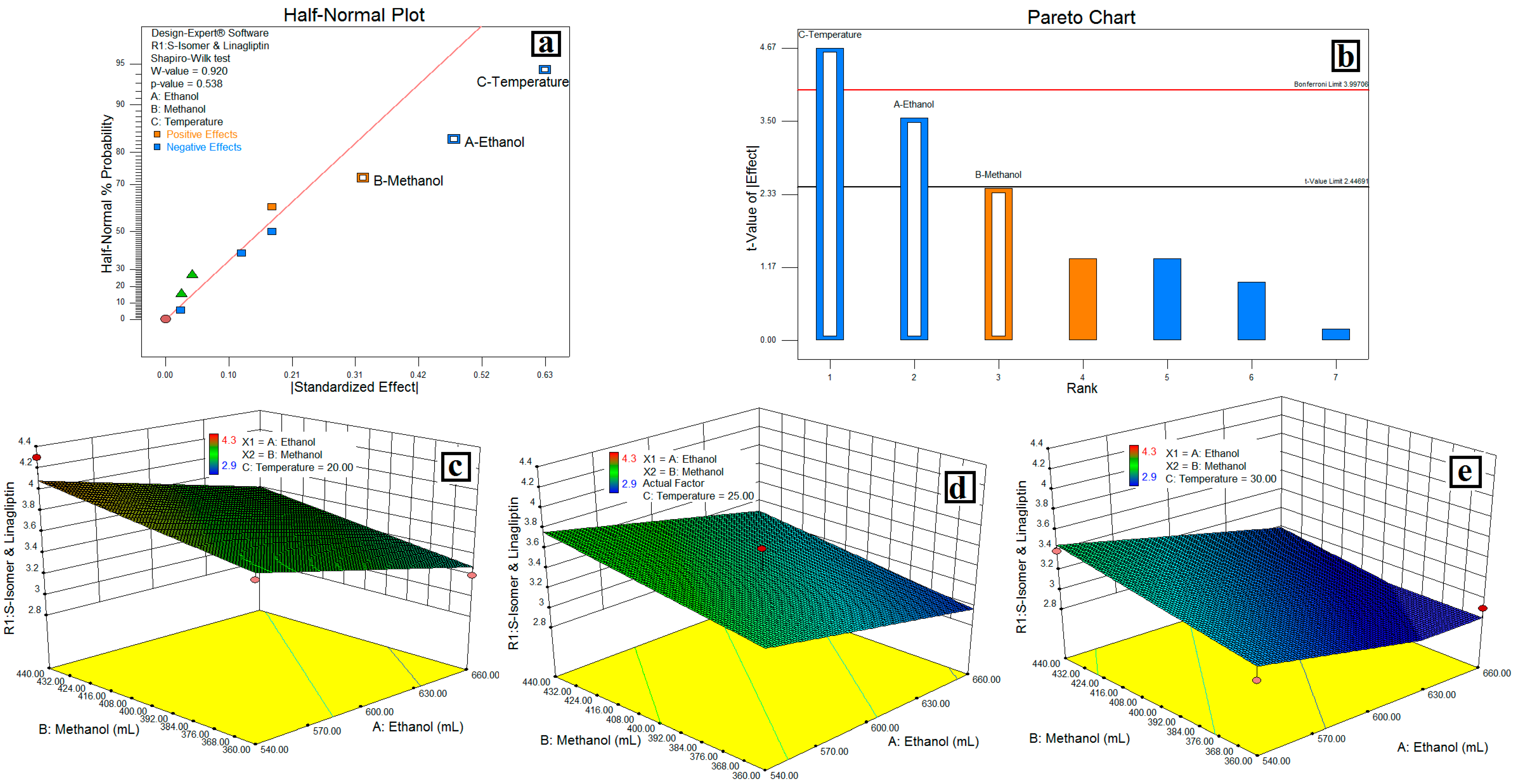
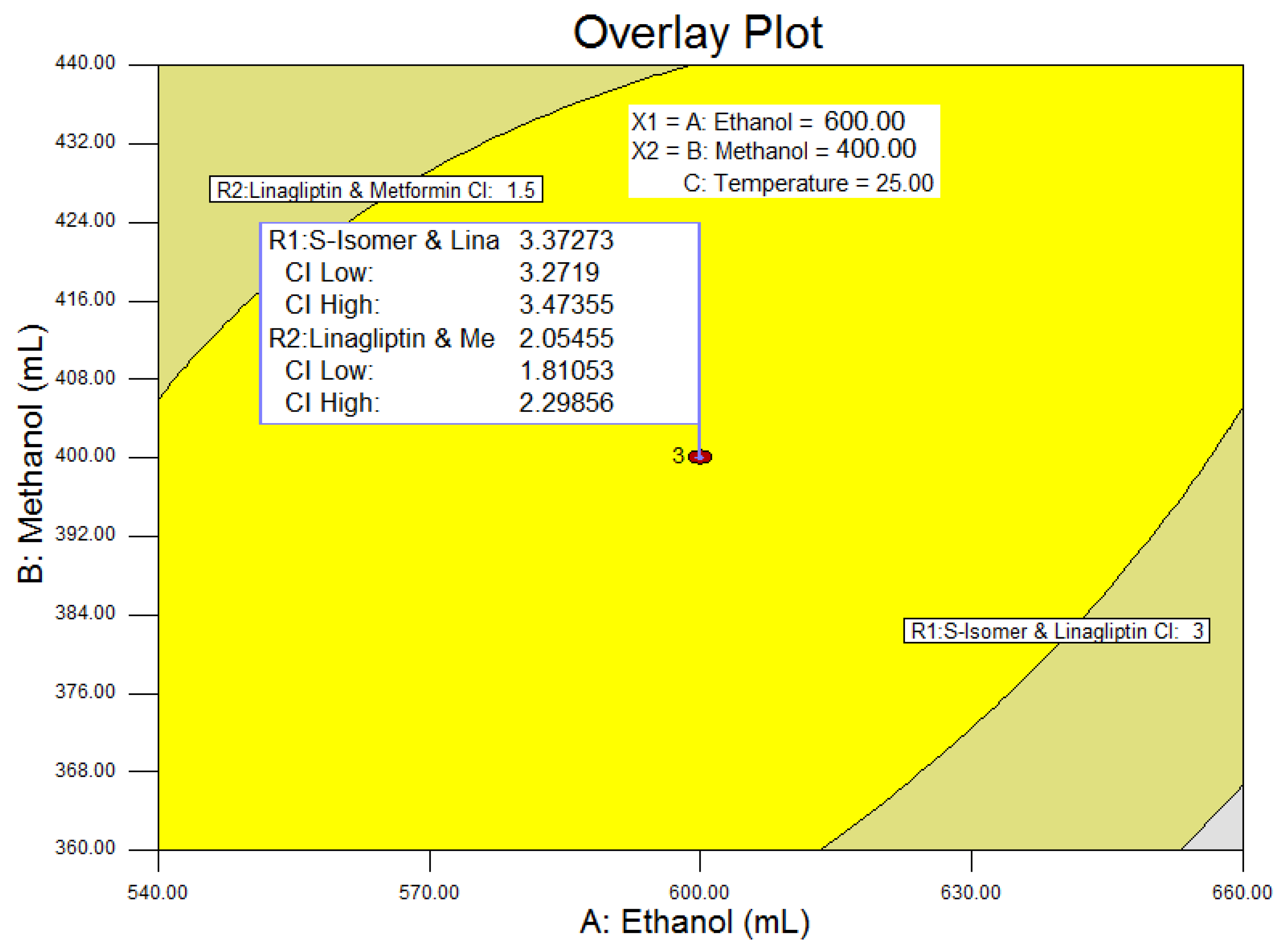
3.2. Design of Experiments
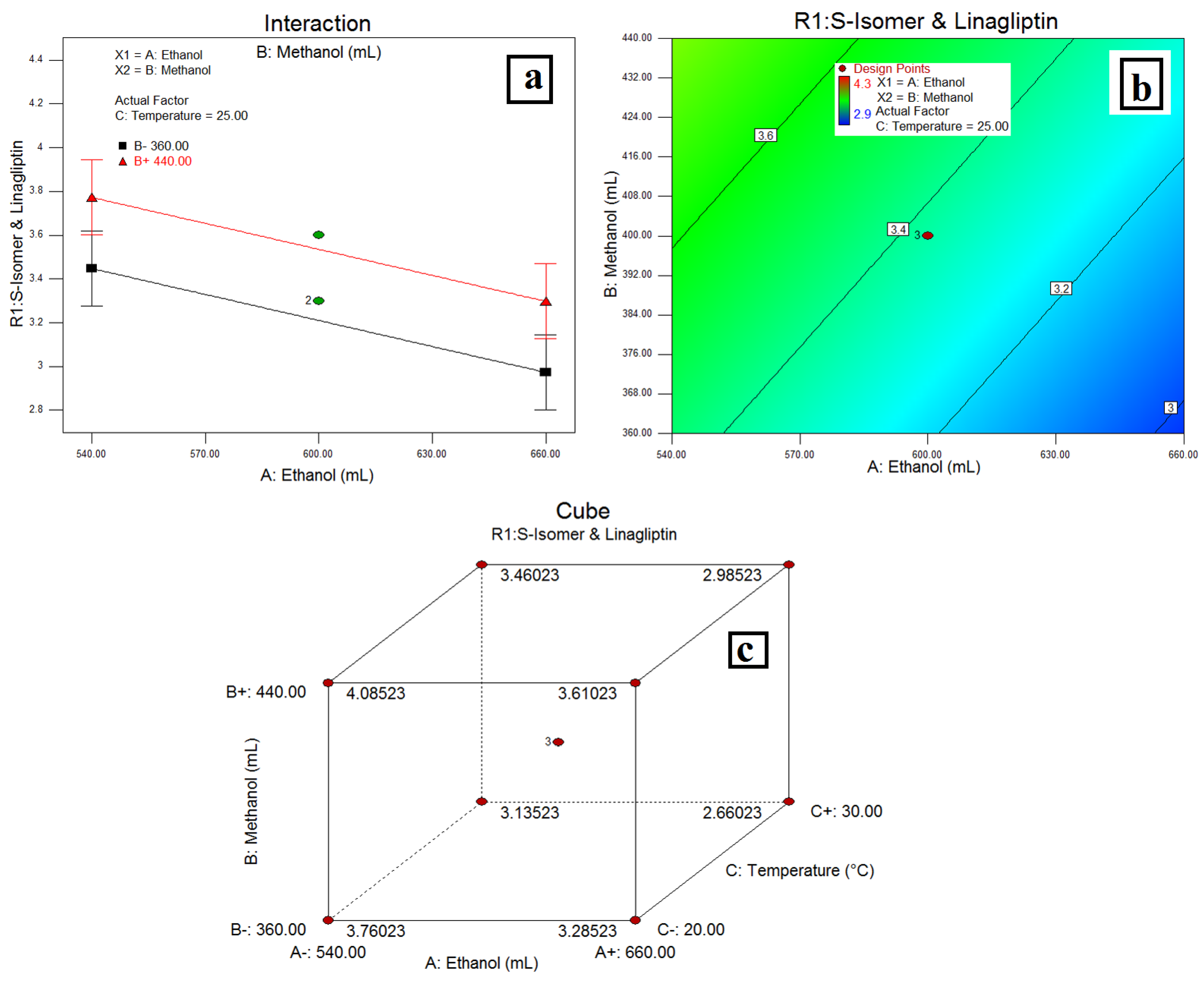
3.3. Resolution between the S-isomer and LGP-(R1)
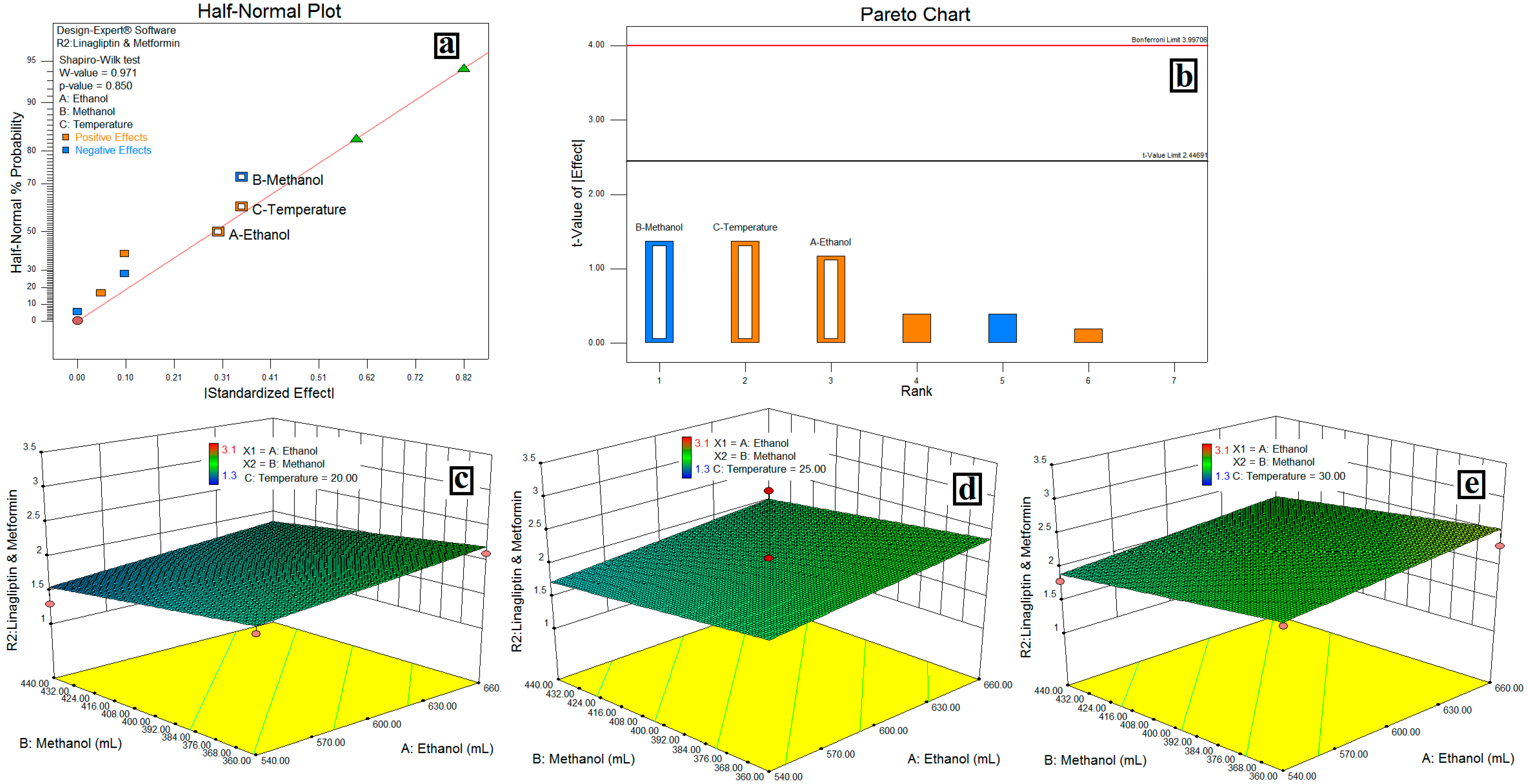
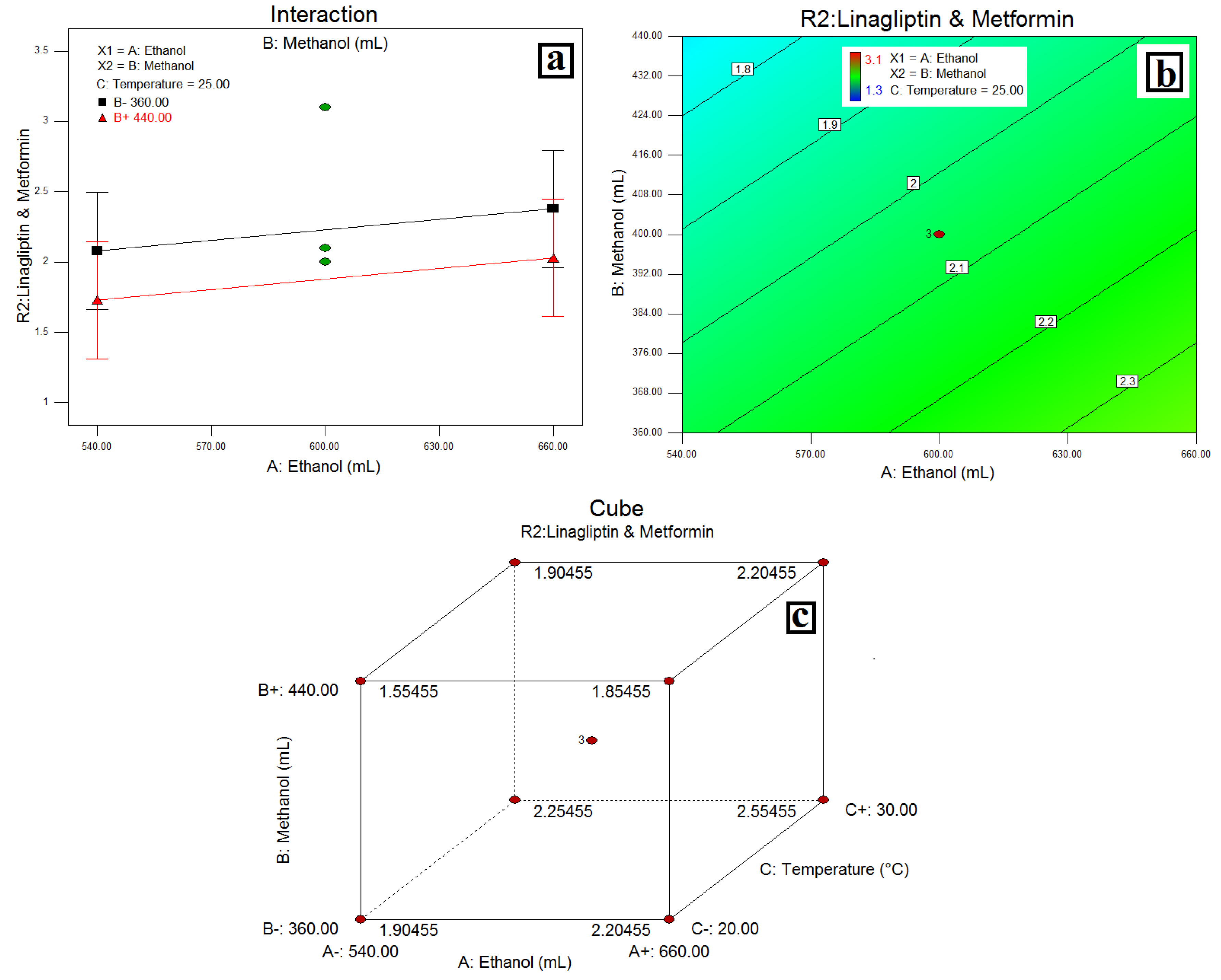
3.4. Resolution between LGP and MET-(R2)
3.5. Method Validation
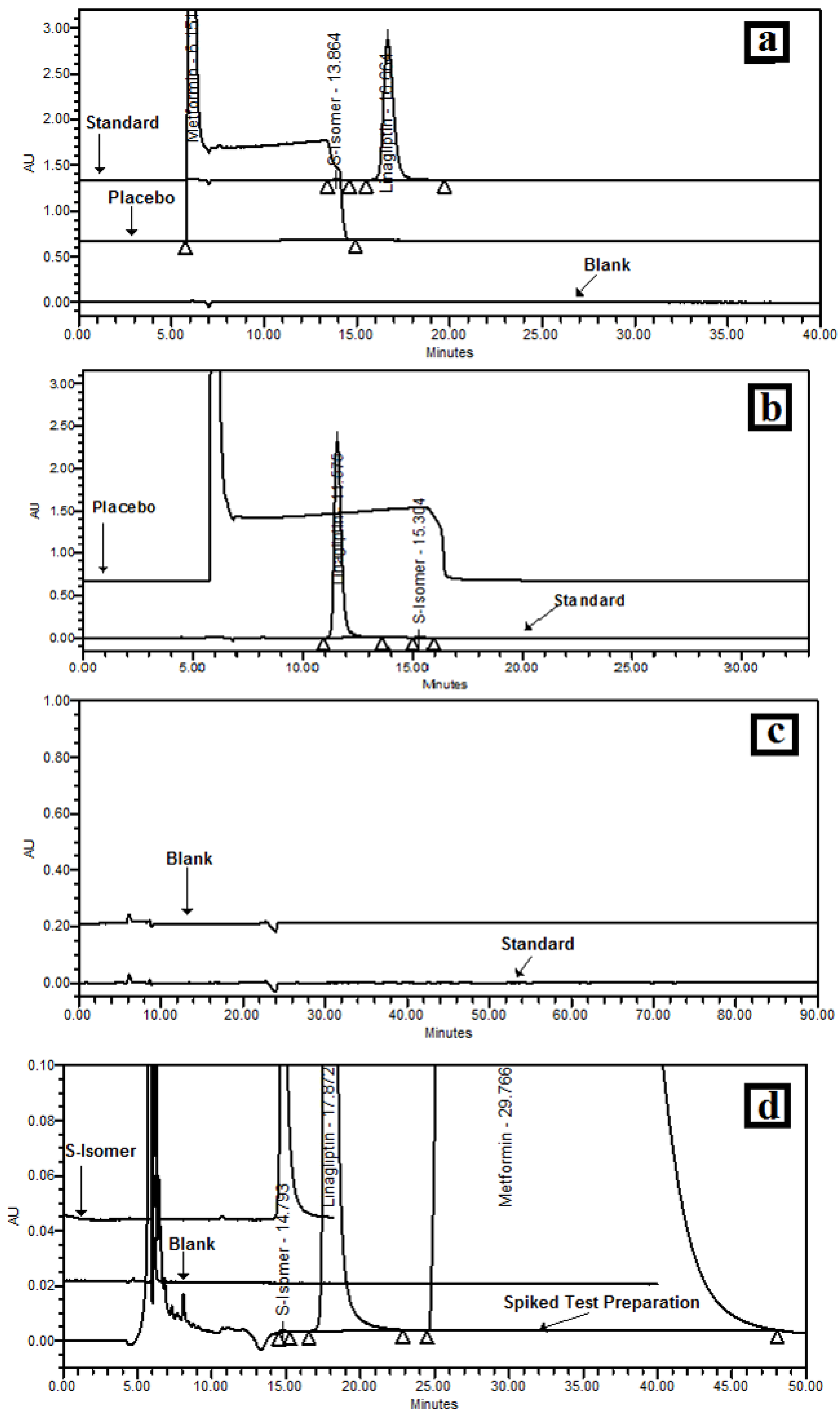
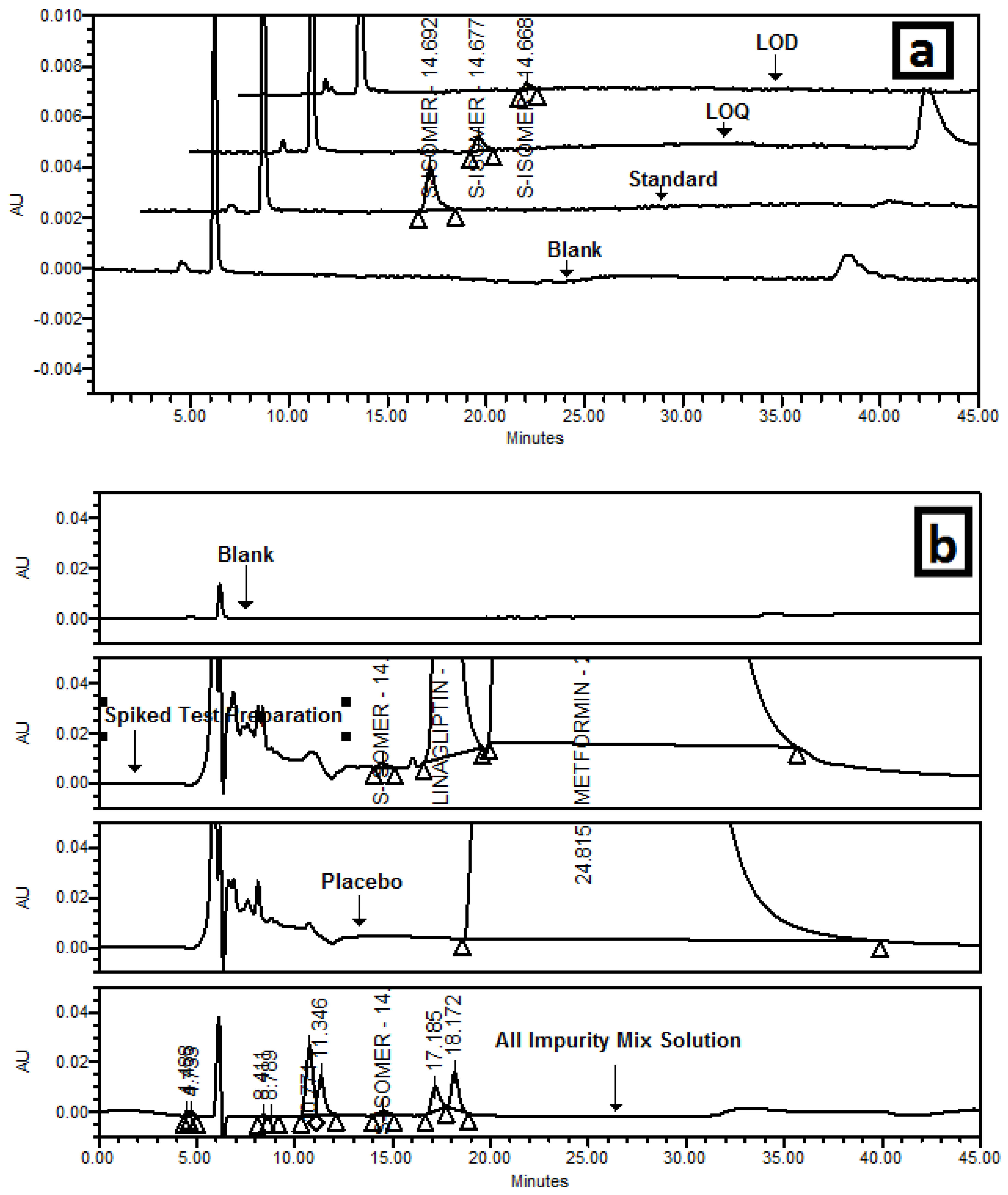
3.5.1. Specificity/Selectivity
3.5.2. Precision
3.5.3. Limit of Detection and Limit of Quantitation
3.5.4. Accuracy
3.5.5. Linearity and Range
3.5.6. Robustness
3.5.7. Solution and Mobile Phase Stability
3.5.8. Filter Compatibility
4. Discussion
4.1. Design of Experiments
4.2. Resolution between the S-Isomer and LGP-(R1)
4.3. Resolution between LGP and MET-(R2)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McIntosh, C.H.; Demuth, H.U.; Pospisilik, J.A.; Pederson, R. Dipeptidyl peptidase IV inhibitors: How do they work as new antidiabetic agents? Regul. Pept. 2005, 128, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Behme, M.T.; Dupré, J.; McDonald, T.J. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr. Disord. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupre, J.; Behme, M.T.; Hramiak, I.M.; McFarlane, P.; Williamson, M.P.; Zabel, P.; McDonald, T.J. Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes 1995, 44, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Global Guideline for Type 2 Diabetes International Diabetes Federation 2012; Clinical Guidelines Task Force: Brussels, Belgium, 2012.
- National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update); Royal College of Physicians: London, UK, 2008. [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2009, 32 (Suppl. 1), S13–S61. [Google Scholar]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycaemia in type 2 diabetes: A patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012, 55, 1577–1596. [Google Scholar] [CrossRef] [PubMed]
- Phung, O.J.; Scholle, J.M.; Talwar, M.; Coleman, C.L. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010, 303, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, T.; Paschos, P.; Paletas, K.; Matthews, D.R.; Tsapas, A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: Systematic review and meta-analysis. BMJ 2012, 344, e1369. [Google Scholar] [CrossRef] [PubMed]
- Amiel, S.A.; Dixon, T.; Mann, R.; Jameson, K. Hypoglycaemia in Type 2 diabetes. Diabet. Med. 2008, 25, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Tu, Y.K.; Chien, M.N.; Chien, K.L. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: A network meta-analysis. Diabetes Obes. Metab. 2012, 14, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, K.; Mortensen, L.S. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf. 2007, 30, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E. Antidiabetic medications in overweight/obese patients with type 2 diabetes: Drawbacks of current drugs and potential advantages of incretin-based treatment on body weight. Int. J. Clin. Pract. Suppl. 2007, 154, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Drug interactions of clinical importance with antihyperglycaemic agents—An update. Drug Saf. 2005, 28, 601–631. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.L.; Poole, C.D.; Evans, M.; Barnett, A.H.; Jenkins, J.S.; Currie, C.J. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 4605–4612. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Linagliptin plus metformin: A pharmacokinetic and pharmacodynamic evaluation. Expert Opin. Drug Metab. Toxicol. 2013, 9, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Product Quality Research Institute (PQRI) Workgroup Members. Process Robustness PQRI White Paper. Pharm. Eng. 2006, 26, 6. [Google Scholar]
- Jadhav, S.B.; Kumar, C.K.; Bandichhor, R.; Bhosale, P.N. Development of RP UPLC-TOF/MS, stability indicating method for omeprazole and its related substances by applying two level factorial design; and identification and synthesis of non-pharmacopoeial impurities. J. Pharm. Biomed. Anal. 2016, 118, 370–379. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeial Convention. Validation of Compendial Procedures. In United States Pharmacopoeia; The United States Pharmacopeial Convention: Rockville, MD, USA, 2016; Chapter 1225; pp. 1640–1645. [Google Scholar]
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Validation of Analytical Procedures: Text and Methodology Q2(R1).4, ICH Harmonized Tripartite Guideline. 2005.
| Structure | Chemical Name/Molecular Weight/Formula |
|---|---|
 | Linagliptin: 8-[(3R)-3-aminopiperidin-1-yl]-7-(but-2-yn-1-yl)-3-methyl-1-[(4-methylquinazolin-2-yl) methyl]-2, 3, 6, 7-tetrahydro-1H-purine-2,6-dione (472.55) C25H28N8O2 |
 | S-isomer: (S)-8-3(-aminopiperidin-1-yl]-7-(but-2-yn-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl) methyl)-1H-purine-2, 6 (3H, 7H)-dione (472.55) C25H28N8O2 |
 | Metformin HCl: 1,1-Dimethylbiguanide hydrochloride (165.63) C4H12ClN5 |
 | Impurity-A (MET HCl): 1-Cyanoguanidine (84.08) C2H4N5 |
| Factor 1 | Factor 2 | Factor 3 | Resolution-1 (R1) | Resolution-2 (R2) | |||
|---|---|---|---|---|---|---|---|
| Std. | Run | Type | A: EtOH (%) | B: MeOH (%) | C: Temperature (°C) | S-isomer and LGP | LGP and MET |
| 6 | 1 | Factorial | 660 | 360 | 30 | 2.9 | 2.3 |
| 4 | 2 | Factorial | 660 | 440 | 20 | 3.5 | 1.8 |
| 5 | 3 | Factorial | 540 | 360 | 30 | 3.0 | 2.2 |
| 11 | 4 | Center | 600 | 400 | 25 | 3.3 | 2.1 |
| 2 | 5 | Factorial | 660 | 360 | 20 | 3.2 | 2.1 |
| 9 | 6 | Center | 600 | 400 | 25 | 3.3 | 2.1 |
| 7 | 7 | Factorial | 540 | 440 | 30 | 3.4 | 1.8 |
| 8 | 8 | Factorial | 660 | 440 | 30 | 2.9 | 2.1 |
| 1 | 9 | Factorial | 540 | 360 | 20 | 3.7 | 1.8 |
| 3 | 10 | Factorial | 540 | 440 | 20 | 4.3 | 1.3 |
| 10 | 11 | Center | 600 | 400 | 25 | 3.6 | 2.0 |
| Response | Source | Sum of Squares | Degrees of Freedom | Mean Square | F Value | p-Value Prob > F | Model Status | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| S-isomer and LGP | Model | 1.44 | 3 | 0.48 | 15.45 | 0.0018 | Significant | |||
| A-EtOH % | 0.45 | 1 | 0.45 | 14.49 | 0.0067 | |||||
| B-MeOH % | 0.21 | 1 | 0.21 | 6.78 | 0.0352 | |||||
| C-Temp. °C | 0.78 | 1 | 0.78 | 25.08 | 0.0016 | |||||
| Residual | 0.22 | 7 | 0.031 | |||||||
| Lack of Fit | 0.16 | 5 | 0.032 | 1.05 | 0.5527 | Not Significant | ||||
| Pure Error | 0.06 | 2 | 0.030 | 0.8688 | 0.8125 | 0.6259 | ||||
| LGP and MET HCl | Cor total | 1.66 | 10 | |||||||
| Model | 0.67 | 3 | 0.22 | 1.22 | 0.3700 | Not Significant | ||||
| A-EtOH % | 0.18 | 1 | 0.18 | 0.99 | 0.3537 | |||||
| B-MeOH % | 0.25 | 1 | 0.25 | 1.34 | 0.2846 | |||||
| C-Temp. °C | 0.25 | 1 | 0.25 | 1.34 | 0.2846 | |||||
| Residual | 1.28 | 7 | 0.18 | |||||||
| Lack of Fit | 0.54 | 5 | 0.11 | 0.29 | 0.8852 | Not Significant | 0.8781 | 0.8477 | 0.7890 | |
| Pure Error | 0.74 | 2 | 0.37 | |||||||
| Cor total | 1.95 | 10 |
| Test Preparation | Method Precision | Intermediate Precision | LOQ Precision | Accuracy at LOQ |
|---|---|---|---|---|
| 1 | 109.7 | 109.3 | 0.053 | 114.0 |
| 2 | 105.7 | 108.0 | 0.043 | 92.5 |
| 3 | 109.0 | 106.7 | 0.042 | 90.3 |
| 4 | 108.4 | 112.7 | 0.045 | 96.8 |
| 5 | 102.4 | 110.0 | 0.046 | 98.9 |
| 6 | 107.8 | 114.0 | 0.049 | 105.4 |
| Average | 107.8 | 110.0 | 0.046 | 99.7 |
| Area % RSD | 3.1 | 2.5 | 8.8 | NA |
| Variation Parameter | Tailing Factor | Theoretical Plates | Area %RSD | Resolution between S-isomer and LGP (R1) | Resolution between LGP and MET (R2) |
|---|---|---|---|---|---|
| As per test method | 1.3 | 4511 | 1.9 | 3.2 | 1.6 |
| Flow (0.7 mL/min) | 1.4 | 3698 | 1.5 | 3.1 | 1.5 |
| Temperature: 23 °C | 1.1 | 4675 | 4.3 | 3.4 | 1.0 |
| Temperature: 33 °C | 1.4 | 5280 | 3.3 | 2.9 | 2.0 |
| Methanol: 360 mL | 1.4 | 4467 | 1.9 | 2.5 | 2.7 |
| Methanol: 440 mL | 1.4 | 4696 | 2.5 | 3.4 | 1.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, S.B.; Mane, R.M.; Narayanan, K.L.; Bhosale, P.N. Analytical Enantio-Separation of Linagliptin in Linagliptin and Metformin HCl Dosage Forms by Applying Two-Level Factorial Design. Sci. Pharm. 2016, 84, 671-684. https://doi.org/10.3390/scipharm84040671
Jadhav SB, Mane RM, Narayanan KL, Bhosale PN. Analytical Enantio-Separation of Linagliptin in Linagliptin and Metformin HCl Dosage Forms by Applying Two-Level Factorial Design. Scientia Pharmaceutica. 2016; 84(4):671-684. https://doi.org/10.3390/scipharm84040671
Chicago/Turabian StyleJadhav, Sushant B., Rahul M. Mane, Kalyanraman L. Narayanan, and Popatrao N. Bhosale. 2016. "Analytical Enantio-Separation of Linagliptin in Linagliptin and Metformin HCl Dosage Forms by Applying Two-Level Factorial Design" Scientia Pharmaceutica 84, no. 4: 671-684. https://doi.org/10.3390/scipharm84040671





