MYCN in Neuroblastoma: “Old Wine into New Wineskins”
Abstract
:1. Introduction
1.1. A Brief “Ensemble” to MYCN
1.2. The Frequency of MYCN in the Literature
1.3. A Brief Reference (and History) to Neuroblastoma
1.3.1. Clinical Characteristics of Neuroblastoma
1.3.2. Oncogenetic Mechanisms in Neuroblastoma
1.3.3. Prognosis of Neuroblastoma
1.4. Scope of the Present Work
2. The MYCN Gene
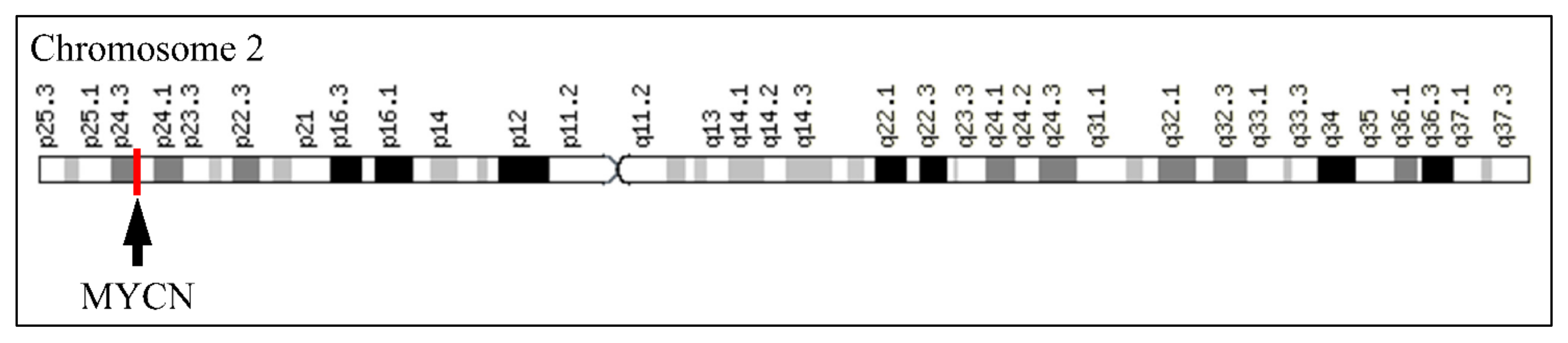
2.1. The “Anatomy” of MYCN
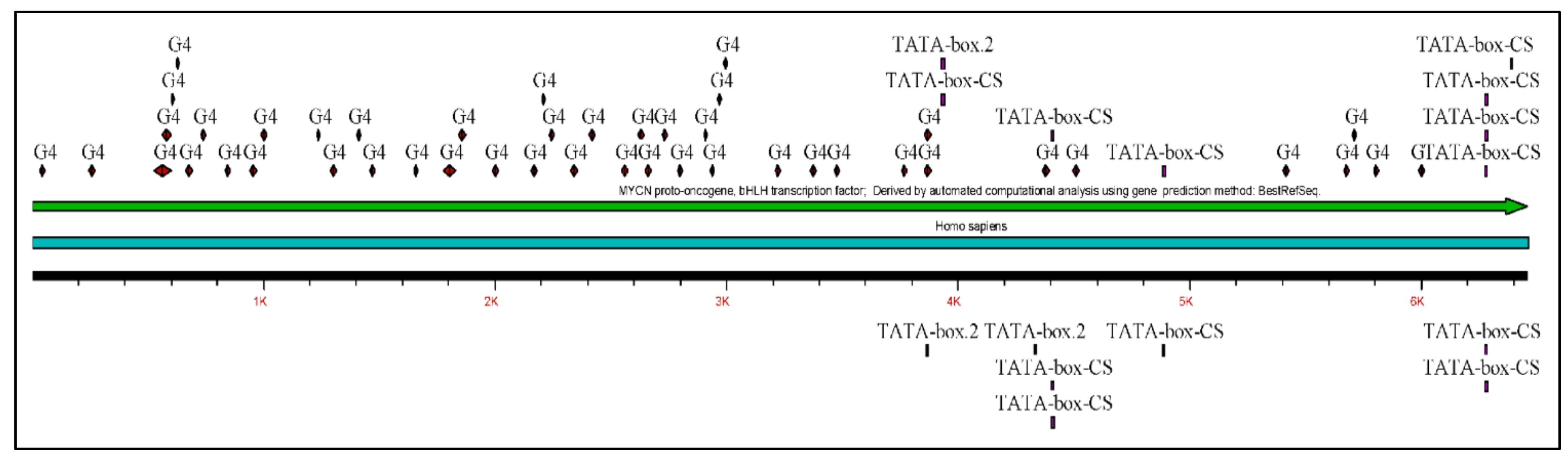
2.1.1. Transcription Factor Binding Sites
2.1.2. Gene Ontology (GO) Annotation
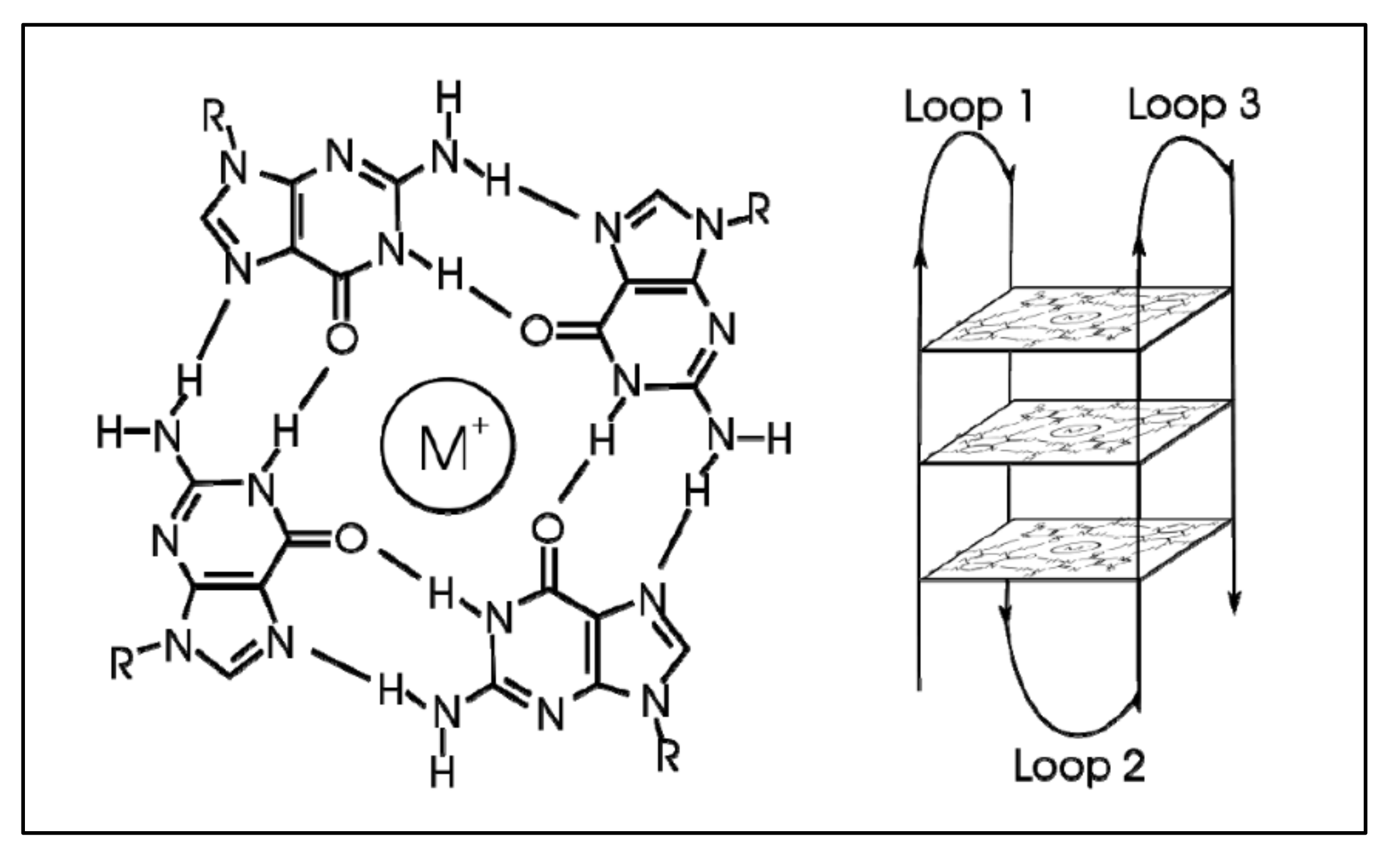
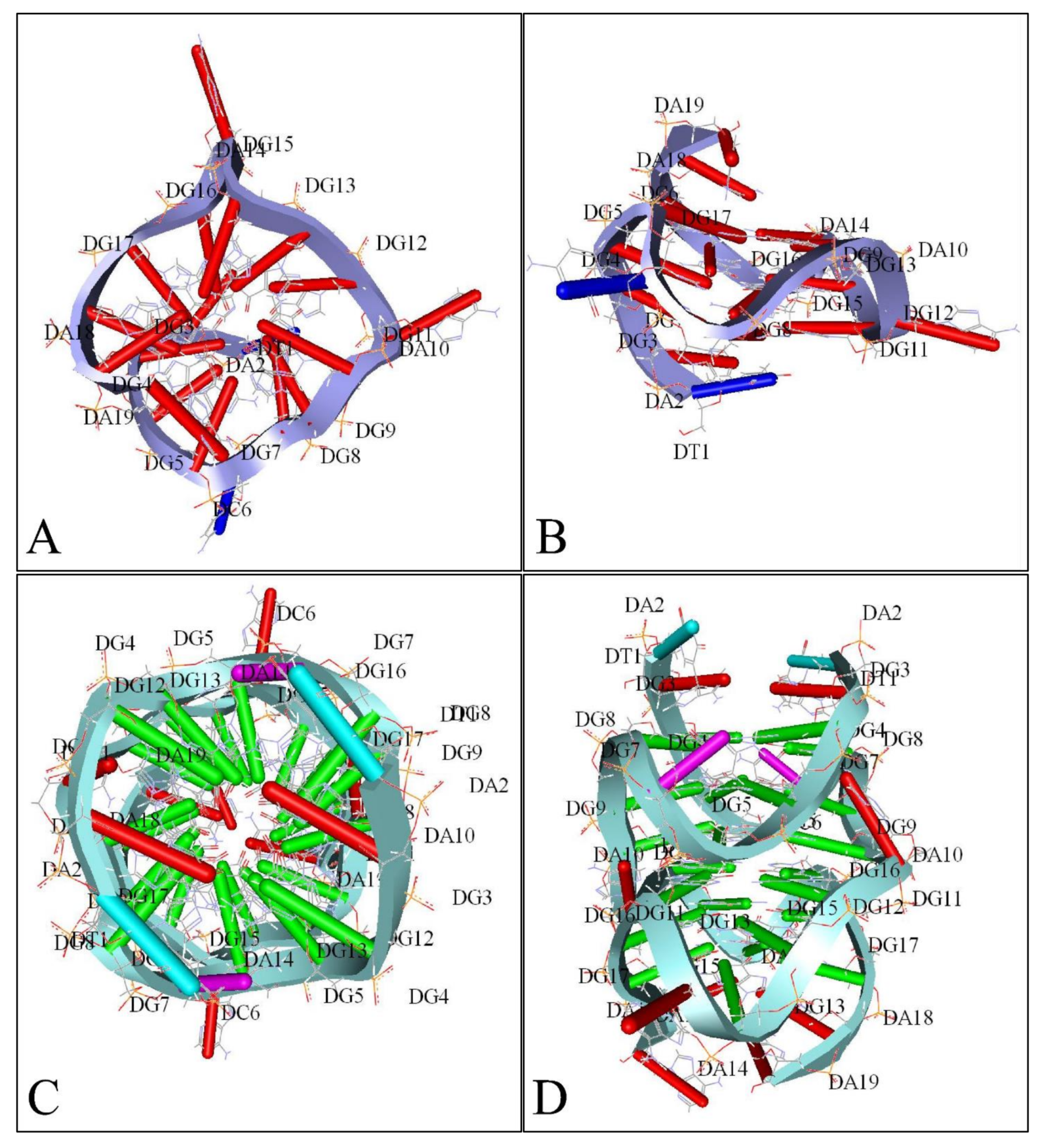
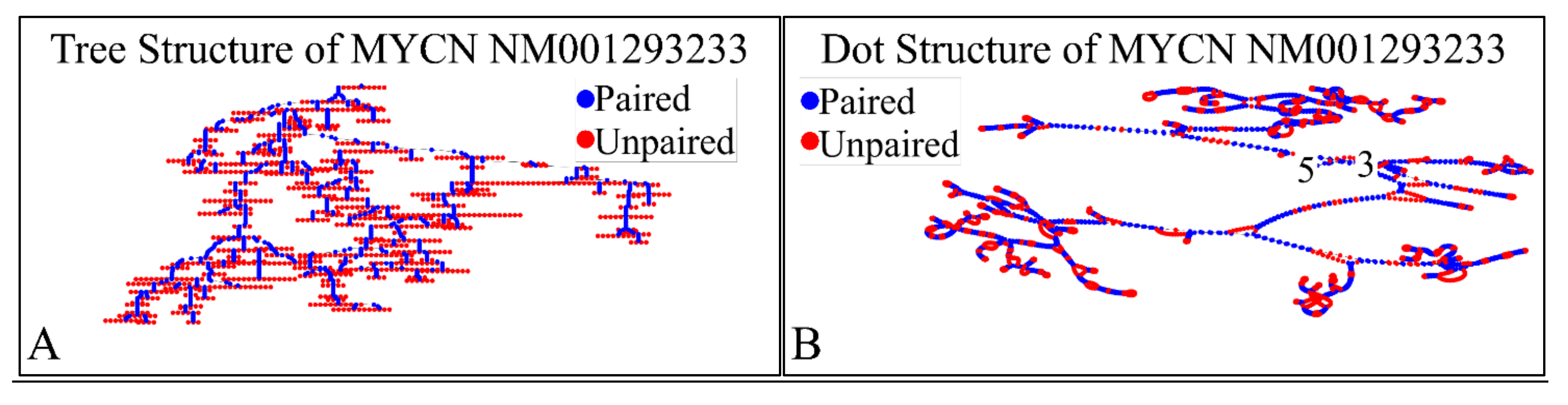
2.1.3. G-Quadraplexes in MYCN
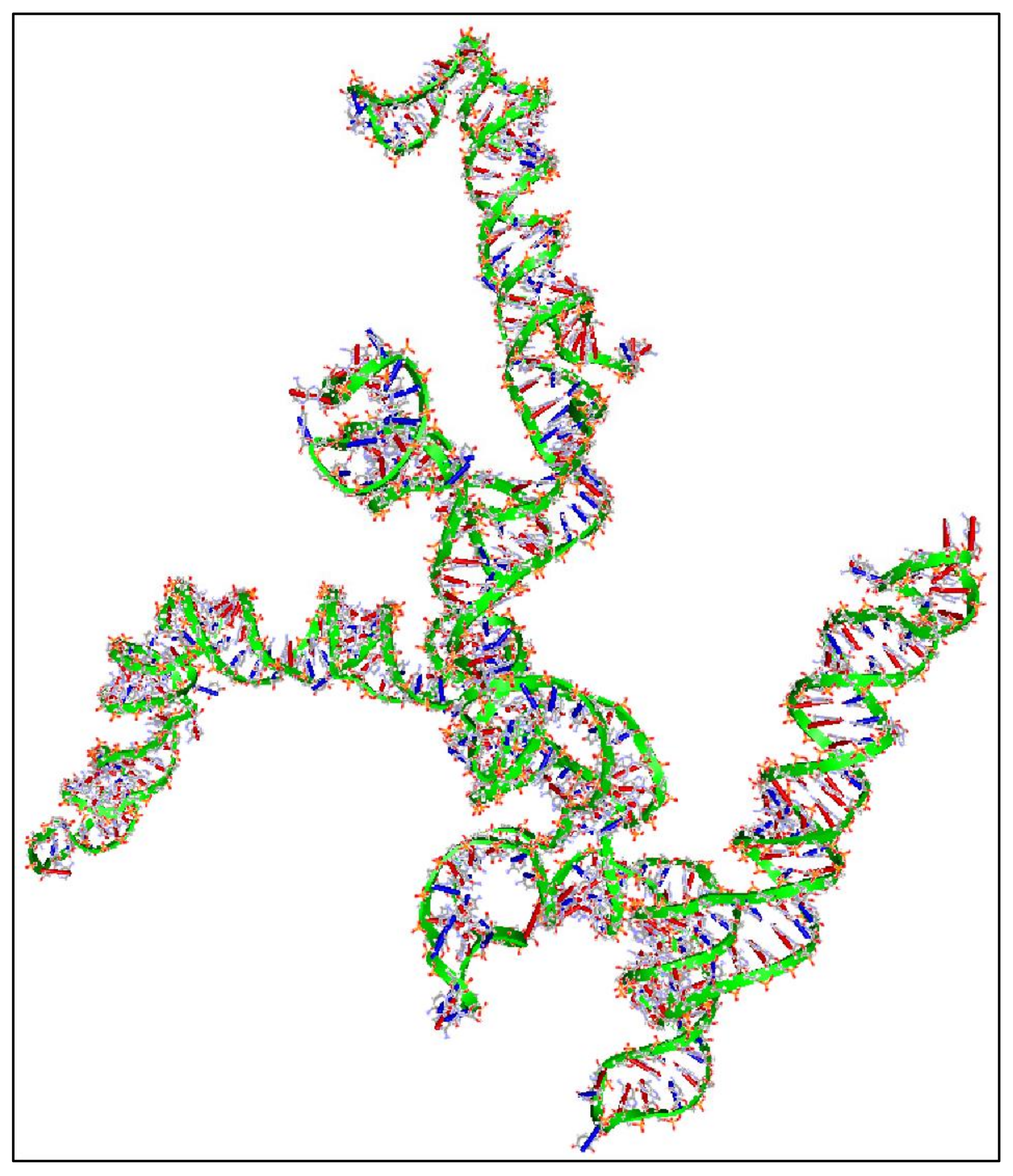
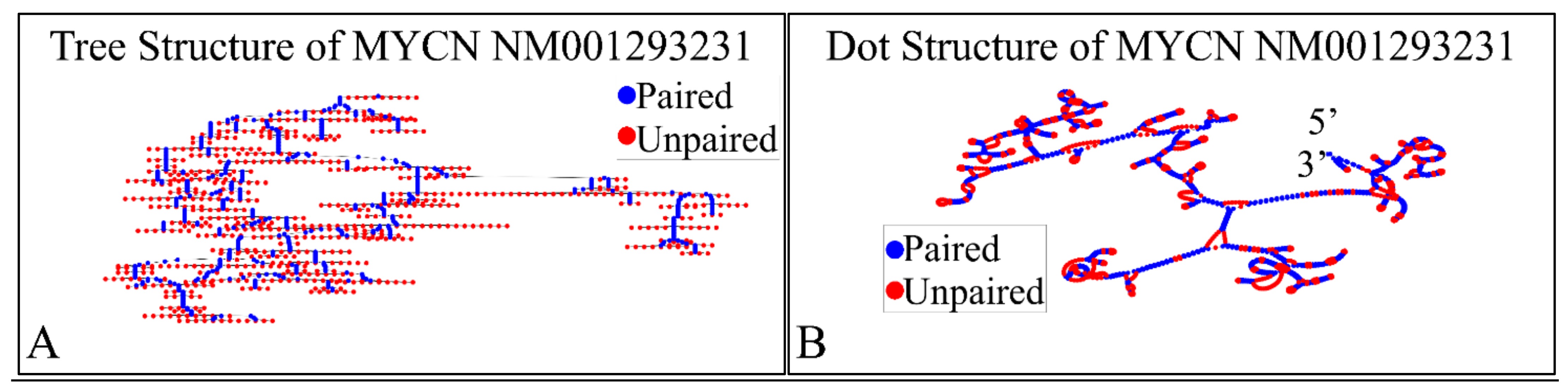
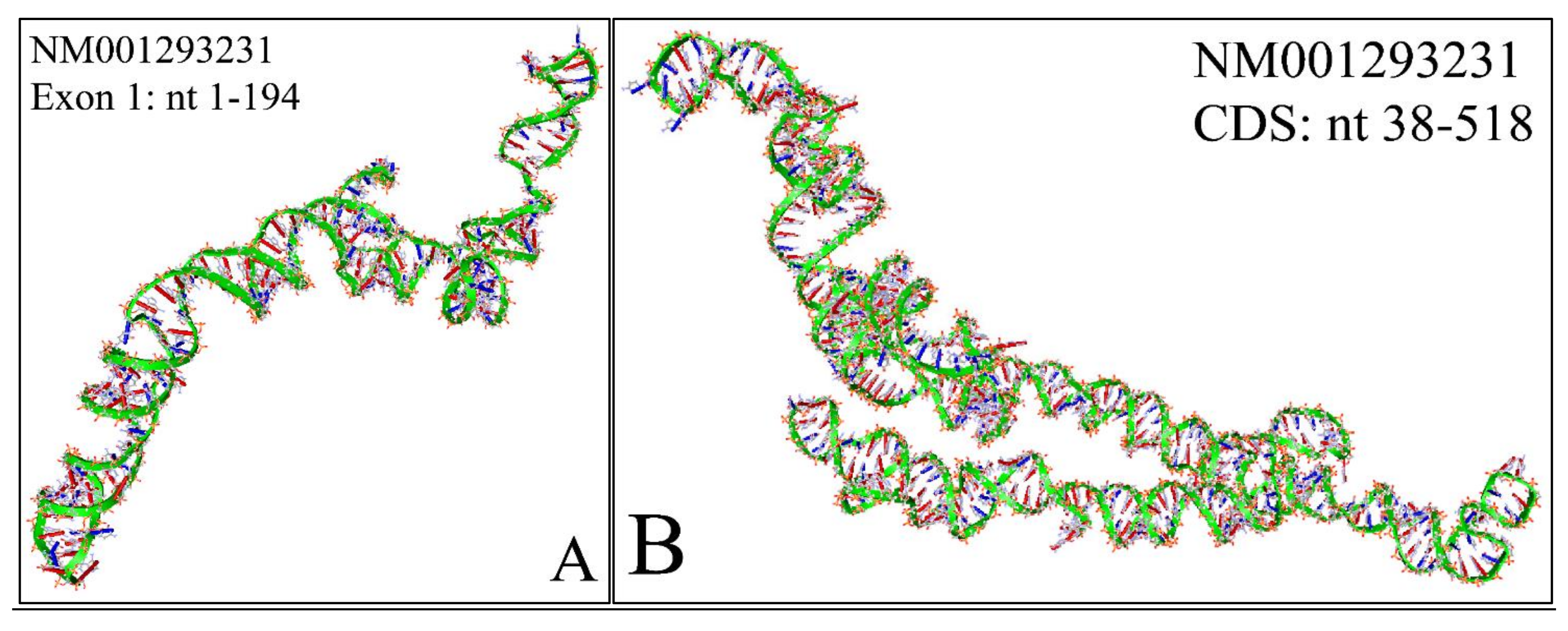
2.2. The “Anatomy” of MYCN’s Transcripts
2.2.1. MYCN Proto-Oncogene, bHLH Transcription Factor (MYCN), Transcript Variant 1, mRNA
2.2.2. MYCN Proto-Oncogene, bHLH Transcription Factor (MYCN), Transcript Variant 3, mRNA
2.2.3. MYCN Proto-Oncogene, bHLH Transcription Factor (MYCN), Transcript Variant 2, mRNA
2.2.4. MYCN Proto-Oncogene, bHLH Transcription Factor (MYCN), Transcript Variant 2, mRNA
2.3. The “Anatomy” of MYCN’s Protein
2.4. The Cellular Functions of MYCN
3. Detection of the MYCN Gene
4. Molecular Mechanistics of the MYCN Gene
4.1. Other Molecular Biomarkers Besides MYCN
4.2. Epigenetic Regulation of the MYCN Gene
4.2.1. MYCN and miRNAs
4.2.2. MYCN Methylation and Methylation-Related Mechanisms
4.2.3. Other Epigenetic and Post-Translational Mechanisms in Neuroblastoma
4.3. MYCN Gene Amplification: “The Oldest Trick in the Book”
4.4. Therapeutic Interventions with MYCN
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohl, N.E.; Kanda, N.; Schreck, R.R.; Bruns, G.; Latt, S.A.; Gilbert, F.; Alt, F.W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell 1983, 35, 359–367. [Google Scholar] [CrossRef]
- Schwab, M.; Alitalo, K.; Klempnauer, K.H.; Varmus, H.E.; Bishop, J.M.; Gilbert, F.; Brodeur, G.; Goldstein, M.; Trent, J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature 1983, 305, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Weiss, W.A. Neuroblastoma and mycn. Cold Spring Harb. Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef]
- Schwab, M.; Varmus, H.E.; Bishop, J.M. Human n-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature 1985, 316, 160–162. [Google Scholar] [CrossRef]
- Schwab, M.; Bishop, J.M. Sustained expression of the human protooncogene mycn rescues rat embryo cells from senescence. Proc. Natl. Acad. Sci. USA 1988, 85, 9585–9589. [Google Scholar] [CrossRef] [Green Version]
- Schweigerer, L.; Scheurich, P.; Fotsis, T. Enhanced mycn oncogene expression in human neuroblastoma cells results in increased susceptibility to growth inhibition by tnf alpha. Biochem. Biophys. Res. Commun. 1990, 170, 1301–1307. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Piaskowski, V.D.; Casper, J.T.; Douglass, E.C.; Von Hoff, D.D. Ability of circular extrachromosomal DNA molecules to carry amplified mycn proto-oncogenes in human neuroblastomas in vivo. J. Natl. Cancer Inst. 1990, 82, 1815–1821. [Google Scholar] [CrossRef]
- Brady, S.W.; Liu, Y.; Ma, X.; Gout, A.M.; Hagiwara, K.; Zhou, X.; Wang, J.; Macias, M.; Chen, X.; Easton, J.; et al. Pan-neuroblastoma analysis reveals age- and signature-associated driver alterations. Nat. Commun. 2020, 11, 5183. [Google Scholar] [CrossRef] [PubMed]
- Borgenvik, A.; Čančer, M.; Hutter, S.; Swartling, F.J. Targeting mycn in molecularly defined malignant brain tumors. Front. Oncol. 2020, 10, 626751. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, R.; Zakrzewski, K.; Liberski, P.P.; Zakrzewska, M. Mrna and mirna expression analyses of the myc/e2f/mir-17-92 network in the most common pediatric brain tumors. Int. J. Mol. Sci. 2021, 22, 543. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, N.; Khan, S.; Mumal, I.; Bouffet, E.; Huang, A. Embryonal tumors with multi-layered rosettes: A disease of dysregulated mirnas. J. Neuro-Oncol. 2020, 150, 63–73. [Google Scholar] [CrossRef]
- Kumar, R.; Smith, K.S.; Deng, M.; Terhune, C.; Robinson, G.W.; Orr, B.A.; Liu, A.P.Y.; Lin, T.; Billups, C.A.; Chintagumpala, M.; et al. Clinical outcomes and patient-matched molecular composition of relapsed medulloblastoma. J. Clin. Oncol. 2021, 39, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Skowron, P.; Farooq, H.; Cavalli, F.M.G.; Morrissy, A.S.; Ly, M.; Hendrikse, L.D.; Wang, E.Y.; Djambazian, H.; Zhu, H.; Mungall, K.L.; et al. The transcriptional landscape of shh medulloblastoma. Nat. Commun. 2021, 12, 1749. [Google Scholar] [CrossRef]
- Estiar, M.A.; Javan, F.; Zekri, A.; Mehrazin, M.; Mehdipour, P. Prognostic significance of mycn gene amplification and protein expression in primary brain tumors: Astrocytoma and meningioma. Cancer Biomark. 2017, 19, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Čančer, M.; Drews, L.F.; Bengtsson, J.; Bolin, S.; Rosén, G.; Westermark, B.; Nelander, S.; Forsberg-Nilsson, K.; Uhrbom, L.; Weishaupt, H.; et al. Bet and aurora kinase a inhibitors synergize against mycn-positive human glioblastoma cells. Cell Death Dis. 2019, 10, 881. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.H.; Lou, P.Y.; Chai, C.; Han, S.Y.; Ning, J.F.; Li, M. Human bone marrow-derived mesenchymal stem cell-secreted exosomes overexpressing microrna-34a ameliorate glioblastoma development via down-regulating mycn. Cell. Oncol. 2019, 42, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.S.; Clarke, S.; Veschi, V.; Thiele, C.J. Targeting mycn in pediatric and adult cancers. Front. Oncol. 2020, 10, 623679. [Google Scholar] [CrossRef]
- Nakazawa, A. Biological categories of neuroblastoma based on the international neuroblastoma pathology classification for treatment stratification. Pathol. Int. 2021, 71, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Alaminos, M.; Gerald, W.L.; Cheung, N.K. Prognostic value of mycn and id2 overexpression in neuroblastoma. Pediatr. Blood Cancer 2005, 45, 909–915. [Google Scholar] [CrossRef]
- Michalowski, M.B.; de Fraipont, F.; Plantaz, D.; Michelland, S.; Combaret, V.; Favrot, M.C. Methylation of tumor-suppressor genes in neuroblastoma: The rassf1a gene is almost always methylated in primary tumors. Pediatr. Blood Cancer 2008, 50, 29–32. [Google Scholar] [CrossRef]
- Zafar, A.; Wang, W.; Liu, G.; Xian, W.; McKeon, F.; Zhou, J.; Zhang, R. Targeting the p53-mdm2 pathway for neuroblastoma therapy: Rays of hope. Cancer Lett. 2021, 496, 16–29. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Xu, X.; Jian, B.L.; Zhang, X.; Yue, Z.X.; Guo, W.; Ma, X.L. Chromosome 10 abnormality predicts prognosis of neuroblastoma patients with bone marrow metastasis. Ital. J. Pediatr. 2021, 47, 134. [Google Scholar] [CrossRef]
- Koh, K.N.; Lee, J.Y.; Lim, J.; Shin, J.; Kang, S.H.; Suh, J.K.; Kim, H.; Im, H.J.; Namgoong, J.M.; Kim, D.Y.; et al. Genetic alterations detected by targeted next-generation sequencing and their clinical implications in neuroblastoma. Anticancer Res. 2020, 40, 7057–7065. [Google Scholar] [CrossRef]
- Higashi, M.; Sakai, K.; Fumino, S.; Aoi, S.; Furukawa, T.; Tajiri, T. The roles played by the mycn, trk, and alk genes in neuroblastoma and neural development. Surg. Today 2019, 49, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Remke, M.; Kool, M.; Hielscher, T.; Northcott, P.A.; Williamson, D.; Pfaff, E.; Witt, H.; Jones, D.T.; Ryzhova, M.; et al. Biological and clinical heterogeneity of mycn-amplified medulloblastoma. Acta Neuropathol. 2012, 123, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.H. Neurocytoma or neuroblastoma, a kind of tumor not generally recognized. J. Exp. Med. 1910, 12, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Matthay, K.K. Neuroblastoma: Biology and therapy. Oncology 1997, 11, 1857–1866, discussion 1869–1872, 1875. [Google Scholar]
- Knudson, A.G., Jr.; Strong, L. Mutation and cancer: Neuroblastoma and pheochromocytoma. Am. J. Hum. Genet. 1972, 24, 514. [Google Scholar] [PubMed]
- Smith, E.I.; Haase, G.M.; Seeger, R.C.; Brodeur, G.M. A surgical perspective on the current staging in neuroblastoma—The international neuroblastoma staging system proposal. J. Pediatr. Surg. 1989, 24, 386–390. [Google Scholar] [CrossRef]
- Wood, L.; Lowis, S. An update on neuroblastoma. Paediatr. Child Health 2008, 18, 123–128. [Google Scholar] [CrossRef]
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of multiple copies of the n-myc oncogene with rapid progression of neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Weinstein, J.N. Biomarkers in cancer staging, prognosis and treatment selection. Nat. Rev. Cancer 2005, 5, 845–856. [Google Scholar] [CrossRef]
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef]
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H. The n-myc oncogene: Maximizing its targets, regulation, and therapeutic potential. Mol. Cancer Res. 2014, 12, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futreal, P.A.; Coin, L.; Marshall, M.; Down, T.; Hubbard, T.; Wooster, R.; Rahman, N.; Stratton, M.R. A census of human cancer genes. Nat. Rev. Cancer 2004, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Knuutila, S.; Björkqvist, A.-M.; Autio, K.; Tarkkanen, M.; Wolf, M.; Monni, O.; Szymanska, J.; Larramendy, M.L.; Tapper, J.; Pere, H.; et al. DNA copy number amplifications in human neoplasms: Review of comparative genomic hybridization studies. Am. J. Pathol. 1998, 152, 1107. [Google Scholar]
- Wieland, L.; Engel, K.; Volkmer, I.; Krüger, A.; Posern, G.; Kornhuber, M.E.; Staege, M.S.; Emmer, A. Overexpression of endogenous retroviruses and malignancy markers in neuroblastoma cell lines by medium-induced microenvironmental changes. Front. Oncol. 2021, 11, 637522. [Google Scholar] [CrossRef]
- Sooksawasdi Na Ayudhya, S.; Meijer, A.; Bauer, L.; Oude Munnink, B.; Embregts, C.; Leijten, L.; Siegers, J.Y.; Laksono, B.M.; van Kuppeveld, F.; Kuiken, T.; et al. Enhanced enterovirus d68 replication in neuroblastoma cells is associated with a cell culture-adaptive amino acid substitution in vp1. mSphere 2020, 5, e00941-20. [Google Scholar] [CrossRef]
- Dang, C.V. C-myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell. Biol. 1999, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordow, S.B.; Norris, M.D.; Haber, P.S.; Marshall, G.M.; Haber, M. Prognostic significance of mycn oncogene expression in childhood neuroblastoma. J. Clin. Oncol. 1998, 16, 3286–3294. [Google Scholar] [CrossRef]
- Strieder, V.; Lutz, W. Regulation of n-myc expression in development and disease. Cancer Lett. 2002, 180, 107–119. [Google Scholar] [CrossRef]
- Maris, J.M.; Weiss, M.J.; Guo, C.; Gerbing, R.B.; Stram, D.O.; White, P.S.; Hogarty, M.D.; Sulman, E.P.; Thompson, P.M.; Lukens, J.N.; et al. Loss of heterozygosity at 1p36 independently predicts for disease progression but not decreased overall survival probability in neuroblastoma patients: A children’s cancer group study. J. Clin. Oncol. 2000, 18, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; London, W.B.; Huang, D.; Katzenstein, H.M.; Salwen, H.R.; Reinhart, T.; Madafiglio, J.; Marshall, G.M.; Norris, M.D.; Haber, M. Mycn expression is not prognostic of adverse outcome in advanced-stage neuroblastoma with nonamplified mycn. J. Clin. Oncol. 2000, 18, 3604–3613. [Google Scholar] [CrossRef]
- Sato, Y.; Kobayashi, Y.; Sasaki, H.; Toyama, T.; Kondo, S.; Kiriyama, M.; Fujii, Y. Expression of id2 mrna in neuroblastoma and normal ganglion. Eur. J. Surg. Oncol. 2003, 29, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.J.; Chen, J.L.; Wu, Z.X.; Wu, Y.M. Exportin-t: A novel prognostic predictor and potential therapeutic target for neuroblastoma. Technol. Cancer Res. Treat. 2021, 20, 15330338211039132. [Google Scholar] [CrossRef]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised neuroblastoma risk classification system: A report from the children’s oncology group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tian, Z.; Li, T.; Hu, X.; Zhu, J. Prognostic value of c-myc expression in patients with peripheral neuroblastic tumors. Int. J. Gen. Med. 2021, 14, 2901–2907. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Xu, L.; Zhang, J.; Cao, H. Age related gene dst represents an independent prognostic factor for mycn non-amplified neuroblastoma. BMC Pediatr. 2021, 21, 272. [Google Scholar] [CrossRef]
- Liao, Y.M.; Hung, T.H.; Tung, J.K.; Yu, J.; Hsu, Y.L.; Hung, J.T.; Yu, A.L. Low expression of il-15 and nkt in tumor microenvironment predicts poor outcome of mycn-non-amplified neuroblastoma. J. Pers. Med. 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.; Eilers, M. Targeting myc proteins for tumor therapy. Annu. Rev. Cancer Biol. 2020, 4, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. Webgestalt 2019: Gene set analysis toolkit with revamped uis and apis. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Vasaikar, S.; Shi, Z.; Greer, M.; Zhang, B. Webgestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017, 45, W130–W137. [Google Scholar] [CrossRef]
- Wang, J.; Duncan, D.; Shi, Z.; Zhang, B. Web-based gene set analysis toolkit (webgestalt): Update 2013. Nucleic Acids Res. 2013, 41, W77–W83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Kirov, S.; Snoddy, J. Webgestalt: An integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005, 33, W741–W748. [Google Scholar] [CrossRef]
- Richards, M.W.; Burgess, S.G.; Poon, E.; Carstensen, A.; Eilers, M.; Chesler, L.; Bayliss, R. Structural basis of n-myc binding by aurora-a and its destabilization by kinase inhibitors. Proc. Natl. Acad. Sci. USA 2016, 113, 13726–13731. [Google Scholar] [CrossRef] [Green Version]
- Bannasch, D.; Weis, I.; Schwab, M. Nmi protein interacts with regions that differ between mycn and myc and is localized in the cytoplasm of neuroblastoma cells in contrast to nuclear mycn. Oncogene 1999, 18, 6810–6817. [Google Scholar] [CrossRef] [PubMed]
- Beierle, E.A.; Trujillo, A.; Nagaram, A.; Kurenova, E.V.; Finch, R.; Ma, X.; Vella, J.; Cance, W.G.; Golubovskaya, V.M. N-myc regulates focal adhesion kinase expression in human neuroblastoma. J. Biol. Chem. 2007, 282, 12503–12516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsay, G.; Stanton, L.; Schwab, M.; Bishop, J.M. Human proto-oncogene n-myc encodes nuclear proteins that bind DNA. Mol. Cell. Biol. 1986, 6, 4450–4457. [Google Scholar] [CrossRef] [Green Version]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the gene ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Amente, S.; Gargano, B.; Diolaiti, D.; Della Valle, G.; Lania, L.; Majello, B. P14arf interacts with n-myc and inhibits its transcriptional activity. FEBS Lett. 2007, 581, 821–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannasch, D.; Mädge, B.; Schwab, M. Functional interaction of yaf2 with the central region of mycn. Oncogene 2001, 20, 5913–5919. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, M.; Poon, E.; Berry, T.; Carstensen, A.; Deubzer, H.E.; Rycak, L.; Jamin, Y.; Thway, K.; Robinson, S.P.; Roels, F.; et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of n-myc in childhood neuroblastoma. Cancer Cell 2013, 24, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-myc induces an ezh2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, W.C.; Meyerowitz, J.G.; Nekritz, E.A.; Chen, J.; Benes, C.; Charron, E.; Simonds, E.F.; Seeger, R.; Matthay, K.K.; Hertz, N.T.; et al. Drugging mycn through an allosteric transition in aurora kinase a. Cancer Cell 2014, 26, 414–427. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Tee, A.E.; Porro, A.; Smith, S.A.; Dwarte, T.; Liu, P.Y.; Iraci, N.; Sekyere, E.; Haber, M.; Norris, M.D.; et al. Activation of tissue transglutaminase transcription by histone deacetylase inhibition as a therapeutic approach for myc oncogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18682–18687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, G.M.; Liu, P.Y.; Gherardi, S.; Scarlett, C.J.; Bedalov, A.; Xu, N.; Iraci, N.; Valli, E.; Ling, D.; Thomas, W.; et al. Sirt1 promotes n-myc oncogenesis through a positive feedback loop involving the effects of mkp3 and erk on n-myc protein stability. PLoS Genet. 2011, 7, e1002135. [Google Scholar] [CrossRef] [Green Version]
- Otto, T.; Horn, S.; Brockmann, M.; Eilers, U.; Schüttrumpf, L.; Popov, N.; Kenney, A.M.; Schulte, J.H.; Beijersbergen, R.; Christiansen, H.; et al. Stabilization of n-myc is a critical function of aurora a in human neuroblastoma. Cancer Cell 2009, 15, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, B.T.; Wolf, E.; Kawauchi, D.; Gebhardt, A.; Rehg, J.E.; Finkelstein, D.; Walz, S.; Murphy, B.L.; Youn, Y.H.; Han, Y.G.; et al. The interaction of myc with miz1 defines medulloblastoma subgroup identity. Cancer Cell 2016, 29, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Morgunova, E.; Jolma, A.; Kaasinen, E.; Sahu, B.; Khund-Sayeed, S.; Das, P.K.; Kivioja, T.; Dave, K.; Zhong, F.; et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 2017, 356, eaaj2239. [Google Scholar] [CrossRef]
- De Brouwer, S.; Mestdagh, P.; Lambertz, I.; Pattyn, F.; De Paepe, A.; Westermann, F.; Schroeder, C.; Schulte, J.H.; Schramm, A.; De Preter, K.; et al. Dickkopf-3 is regulated by the mycn-induced mir-17-92 cluster in neuroblastoma. Int. J. Cancer 2012, 130, 2591–2598. [Google Scholar] [CrossRef] [Green Version]
- Kumps, C.; Fieuw, A.; Mestdagh, P.; Menten, B.; Lefever, S.; Pattyn, F.; De Brouwer, S.; Sante, T.; Schulte, J.H.; Schramm, A.; et al. Focal DNA copy number changes in neuroblastoma target mycn regulated genes. PLoS ONE 2013, 8, e52321. [Google Scholar] [CrossRef] [Green Version]
- Suenaga, Y.; Islam, S.M.; Alagu, J.; Kaneko, Y.; Kato, M.; Tanaka, Y.; Kawana, H.; Hossain, S.; Matsumoto, D.; Yamamoto, M.; et al. Ncym, a cis-antisense gene of mycn, encodes a de novo evolved protein that inhibits gsk3β resulting in the stabilization of mycn in human neuroblastomas. PLoS Genet. 2014, 10, e1003996. [Google Scholar] [CrossRef]
- Gellert, M.; Lipsett, M.N.; Davies, D.R. Helix formation by guanylic acid. Proc. Natl. Acad. Sci. USA 1962, 48, 2013–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routh, E.D.; Creacy, S.D.; Beerbower, P.E.; Akman, S.A.; Vaughn, J.P.; Smaldino, P.J. A g-quadruplex DNA-affinity approach for purification of enzymatically active g4 resolvase1. J. Vis. Exp 2017, 121, e55496. [Google Scholar] [CrossRef]
- Largy, E.; Mergny, J.L.; Gabelica, V. Role of alkali metal ions in g-quadruplex nucleic acid structure and stability. Met. Ions Life Sci. 2016, 16, 203–258. [Google Scholar]
- Sundquist, W.I.; Klug, A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature 1989, 342, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Gilbert, W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature 1988, 334, 364–366. [Google Scholar] [CrossRef]
- Han, H.; Hurley, L.H. G-quadruplex DNA: A potential target for anti-cancer drug design. Trends Pharmacol. Sci. 2000, 21, 136–142. [Google Scholar] [CrossRef]
- Wang, W.; Hu, S.; Gu, Y.; Yan, Y.; Stovall, D.B.; Li, D.; Sui, G. Human myc g-quadruplex: From discovery to a cancer therapeutic target. Biochim. Biophys. Acta. Rev. Cancer 2020, 1874, 188410. [Google Scholar] [CrossRef]
- Kikin, O.; D’Antonio, L.; Bagga, P.S. Qgrs mapper: A web-based server for predicting g-quadruplexes in nucleotide sequences. Nucleic Acids Res. 2006, 34, W676–W682. [Google Scholar] [CrossRef]
- Trajkovski, M.; da Silva, M.W.; Plavec, J. Unique structural features of interconverting monomeric and dimeric g-quadruplexes adopted by a sequence from the intron of the n-myc gene. J. Am. Chem. Soc. 2012, 134, 4132–4141. [Google Scholar] [CrossRef]
- Benabou, S.; Ferreira, R.; Aviñó, A.; González, C.; Lyonnais, S.; Solà, M.; Eritja, R.; Jaumot, J.; Gargallo, R. Solution equilibria of cytosine- and guanine-rich sequences near the promoter region of the n-myc gene that contain stable hairpins within lateral loops. Biochim. Biophys. Acta 2014, 1840, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Guo, D.; Kang, L. Study on the recognition of g-quadruplexes by two stereoisomers of alkaloids. Anal. Bioanal. Chem. 2019, 411, 5555–5561. [Google Scholar] [CrossRef]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A review of the mycotoxin enniatin b. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Zhou, J.; Yuan, G. Exploration of the selective recognition of the g-quadruplex in the n-myc oncogene by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Antczak, M.; Popenda, M.; Zok, T.; Sarzynska, J.; Ratajczak, T.; Tomczyk, K.; Adamiak, R.W.; Szachniuk, M. New functionality of rnacomposer: An application to shape the axis of mir160 precursor structure. Acta Biochim. Pol. 2016, 63, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Popenda, M.; Szachniuk, M.; Antczak, M.; Purzycka, K.J.; Lukasiak, P.; Bartol, N.; Blazewicz, J.; Adamiak, R.W. Automated 3d structure composition for large rnas. Nucleic Acids Res. 2012, 40, e112. [Google Scholar] [CrossRef]
- Besançon, R.; Valsesia-Wittmann, S.; Locher, C.; Delloye-Bourgeois, C.; Furhman, L.; Tutrone, G.; Bertrand, C.; Jallas, A.C.; Garin, E.; Puisieux, A. Upstream orf affects mycn translation depending on exon 1b alternative splicing. BMC Cancer 2009, 9, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Carter, S.; Parmar, S.; Bume, D.D.; Calabrese, D.R.; Liang, X.; Yazdani, K.; Xu, M.; Liu, Z.; Thiele, C.J.; et al. Targeting a noncanonical, hairpin-containing g-quadruplex structure from the mycn gene. Nucleic Acids Res. 2021, 49, 7856–7869. [Google Scholar] [CrossRef]
- Jacobs, J.F.; van Bokhoven, H.; van Leeuwen, F.N.; Hulsbergen-van de Kaa, C.A.; de Vries, I.J.; Adema, G.J.; Hoogerbrugge, P.M.; de Brouwer, A.P. Regulation of mycn expression in human neuroblastoma cells. BMC Cancer 2009, 9, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, L.W.; Bishop, J.M. Alternative processing of rna transcribed from nmyc. Mol. Cell. Biol. 1987, 7, 4266–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peirce, S.K.; Findley, H.W. High level mycn expression in non-mycn amplified neuroblastoma is induced by the combination treatment nutlin-3 and doxorubicin and enhances chemosensitivity. Oncol. Rep. 2009, 22, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Cage, T.A.; Chanthery, Y.; Chesler, L.; Grimmer, M.; Knight, Z.; Shokat, K.; Weiss, W.A.; Gustafson, W.C. Downregulation of mycn through pi3k inhibition in mouse models of pediatric neural cancer. Front. Oncol. 2015, 5, 111. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Pérez, M.V.; Henley, A.B.; Arsenian-Henriksson, M. The mycn protein in health and disease. Genes 2017, 8, 113. [Google Scholar] [CrossRef]
- Bell, E.; Chen, L.; Liu, T.; Marshall, G.M.; Lunec, J.; Tweddle, D.A. Mycn oncoprotein targets and their therapeutic potential. Cancer Lett. 2010, 293, 144–157. [Google Scholar] [CrossRef]
- Ham, J.; Costa, C.; Sano, R.; Lochmann, T.L.; Sennott, E.M.; Patel, N.U.; Dastur, A.; Gomez-Caraballo, M.; Krytska, K.; Hata, A.N.; et al. Exploitation of the apoptosis-primed state of mycn-amplified neuroblastoma to develop a potent and specific targeted therapy combination. Cancer Cell 2016, 29, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Cortés, C.; Kozma, S.C.; Tauler, A.; Ambrosio, S. Mycn concurrence with saha-induced cell death in human neuroblastoma cells. Cell. Oncol. 2015, 38, 341–352. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, G.; Li, B.; Vu, A.; Skuli, N.; Walton, Z.E.; Liu, X.; Mayes, P.A.; Wise, D.R.; Thompson, C.B.; Maris, J.M.; et al. Atf4 regulates myc-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 2012, 22, 631–644. [Google Scholar] [CrossRef] [Green Version]
- Pinkel, D.; Segraves, R.; Sudar, D.; Clark, S.; Poole, I.; Kowbel, D.; Collins, C.; Kuo, W.L.; Chen, C.; Zhai, Y.; et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat. Genet. 1998, 20, 207–211. [Google Scholar] [CrossRef]
- Walker, C.; Joyce, K.A.; Thompson-Hehir, J.; Davies, M.P.; Gibbs, F.E.; Halliwell, N.; Lloyd, B.H.; Machell, Y.; Roebuck, M.M.; Salisbury, J.; et al. Characterisation of molecular alterations in microdissected archival gliomas. Acta Neuropathol. 2001, 101, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Knudson, A.G. Mechanism and relevance of ploidy in neuroblastoma. Genes Chromosomes Cancer 2000, 29, 89–95. [Google Scholar] [CrossRef]
- Ladenstein, R.; Ambros, I.M.; Potschger, U.; Amann, G.; Urban, C.; Fink, F.M.; Schmitt, K.; Jones, R.; Slociak, M.; Schilling, F.; et al. Prognostic significance of DNA di-tetraploidy in neuroblastoma. Med. Pediatr. Oncol. 2001, 36, 83–92. [Google Scholar] [CrossRef]
- Fujita, T.; Igarashi, J.; Okawa, E.R.; Gotoh, T.; Manne, J.; Kolla, V.; Kim, J.; Zhao, H.; Pawel, B.R.; London, W.B.; et al. Chd5, a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J. Natl. Cancer Inst. 2008, 100, 940–949. [Google Scholar] [CrossRef] [Green Version]
- Schwab, M.; Westermann, F.; Hero, B.; Berthold, F. Neuroblastoma: Biology and molecular and chromosomal pathology. Lancet Oncol 2003, 4, 472–480. [Google Scholar] [CrossRef]
- Van Roy, N.; De Preter, K.; Hoebeeck, J.; Van Maerken, T.; Pattyn, F.; Mestdagh, P.; Vermeulen, J.; Vandesompele, J.; Speleman, F. The emerging molecular pathogenesis of neuroblastoma: Implications for improved risk assessment and targeted therapy. Genome Med 2009, 1, 74. [Google Scholar] [CrossRef] [Green Version]
- White, P.S.; Thompson, P.M.; Gotoh, T.; Okawa, E.R.; Igarashi, J.; Kok, M.; Winter, C.; Gregory, S.G.; Hogarty, M.D.; Maris, J.M.; et al. Definition and characterization of a region of 1p36.3 consistently deleted in neuroblastoma. Oncogene 2005, 24, 2684–2694. [Google Scholar] [CrossRef] [Green Version]
- Michels, E.; Hoebeeck, J.; De Preter, K.; Schramm, A.; Brichard, B.; De Paepe, A.; Eggert, A.; Laureys, G.; Vandesompele, J.; Speleman, F. Cadm1 is a strong neuroblastoma candidate gene that maps within a 3.72 mb critical region of loss on 11q23. BMC Cancer 2008, 8, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosse, Y.P.; Diskin, S.J.; Wasserman, N.; Rinaldi, K.; Attiyeh, E.F.; Cole, K.; Jagannathan, J.; Bhambhani, K.; Winter, C.; Maris, J.M. Neuroblastomas have distinct genomic DNA profiles that predict clinical phenotype and regional gene expression. Genes Chromosomes Cancer 2007, 46, 936–949. [Google Scholar] [CrossRef]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The alk(f1174l) mutation potentiates the oncogenic activity of mycn in neuroblastoma. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Cazes, A.; Lopez-Delisle, L.; Tsarovina, K.; Pierre-Eugene, C.; De Preter, K.; Peuchmaur, M.; Nicolas, A.; Provost, C.; Louis-Brennetot, C.; Daveau, R.; et al. Activated alk triggers prolonged neurogenesis and ret upregulation providing a therapeutic target in alk-mutated neuroblastoma. Oncotarget 2014, 5, 2688–2702. [Google Scholar] [CrossRef] [Green Version]
- George, R.E.; Sanda, T.; Hanna, M.; Frohling, S.; Luther, W., 2nd; Zhang, J.; Ahn, Y.; Zhou, W.; London, W.B.; McGrady, P.; et al. Activating mutations in alk provide a therapeutic target in neuroblastoma. Nature 2008, 455, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Janoueix-Lerosey, I.; Lequin, D.; Brugieres, L.; Ribeiro, A.; de Pontual, L.; Combaret, V.; Raynal, V.; Puisieux, A.; Schleiermacher, G.; Pierron, G.; et al. Somatic and germline activating mutations of the alk kinase receptor in neuroblastoma. Nature 2008, 455, 967–970. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of alk as a major familial neuroblastoma predisposition gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, S.; Takita, J.; Sanada, M.; Hayashi, Y. Oncogenic mutations of alk in neuroblastoma. Cancer Sci 2011, 102, 302–308. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, J.H.; Lindner, S.; Bohrer, A.; Maurer, J.; De Preter, K.; Lefever, S.; Heukamp, L.; Schulte, S.; Molenaar, J.; Versteeg, R.; et al. Mycn and alkf1174l are sufficient to drive neuroblastoma development from neural crest progenitor cells. Oncogene 2013, 32, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.; Dalevi, D.; Nethander, M.; Jornsten, R.; De Preter, K.; Vermeulen, J.; Stallings, R.; Kogner, P.; Maris, J.; Nilsson, S. A 6-gene signature identifies four molecular subgroups of neuroblastoma. Cancer Cell Int. 2011, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, G.M.; Iyer, R.; Croucher, J.L.; Zhuang, T.; Higashi, M.; Kolla, V. Therapeutic targets for neuroblastomas. Expert Opin. Targets 2014, 18, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. Metazoan micrornas. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. Micrornas: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mrna translation and stability by micrornas. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [Green Version]
- Misiak, D.; Hagemann, S.; Bell, J.L.; Busch, B.; Lederer, M.; Bley, N.; Schulte, J.H.; Hüttelmaier, S. The microrna landscape of mycn-amplified neuroblastoma. Front. Oncol. 2021, 11, 647737. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Improving microrna target prediction by modeling with unambiguously identified microrna-target pairs from clip-ligation studies. Bioinformatics 2016, 32, 1316–1322. [Google Scholar] [CrossRef]
- Wong, N.; Wang, X. Mirdb: An online resource for microrna target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–D152. [Google Scholar] [CrossRef]
- Wang, X.; El Naqa, I.M. Prediction of both conserved and nonconserved microrna targets in animals. Bioinformatics 2008, 24, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Wang, X. Mirdb: A microrna target prediction and functional annotation database with a wiki interface. RNA 2008, 14, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. Mirbase: From microrna sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. Mirbase: Annotating high confidence micrornas using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozomara, A.; Griffiths-Jones, S. Mirbase: Integrating microrna annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef] [Green Version]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. Mirbase: Tools for microrna genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [Green Version]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. Mirbase: Microrna sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S. The microrna registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The mir-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Buechner, J.; Tømte, E.; Haug, B.H.; Henriksen, J.R.; Løkke, C.; Flægstad, T.; Einvik, C. Tumour-suppressor micrornas let-7 and mir-101 target the proto-oncogene mycn and inhibit cell proliferation in mycn-amplified neuroblastoma. Br. J. Cancer 2011, 105, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ma, X.; Shelton, S.D.; Sung, D.C.; Li, M.; Hernandez, D.; Zhang, M.; Losiewicz, M.D.; Chen, Y.; Pertsemlidis, A.; et al. A combined gene expression and functional study reveals the crosstalk between n-myc and differentiation-inducing micrornas in neuroblastoma cells. Oncotarget 2016, 7, 79372–79387. [Google Scholar] [CrossRef]
- Cole, K.A.; Attiyeh, E.F.; Mosse, Y.P.; Laquaglia, M.J.; Diskin, S.J.; Brodeur, G.M.; Maris, J.M. A functional screen identifies mir-34a as a candidate neuroblastoma tumor suppressor gene. Mol. Cancer Res. 2008, 6, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, D.; Pastorino, F.; Brignole, C.; Corrias, M.V.; Emionite, L.; Cilli, M.; Tamma, R.; Priddy, L.; Amaro, A.; Ferrari, D.; et al. Combined replenishment of mir-34a and let-7b by targeted nanoparticles inhibits tumor growth in neuroblastoma preclinical models. Small 2020, 16, e1906426. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.; Gupta, S.; Gokhale, S.S.; Dey, R.; Gunjal, A.D.; Kumar, V.A.; Pillai, B. Detection and knockdown of microrna-34a using thioacetamido nucleic acid. Nucleic Acid Ther. 2013, 23, 195–202. [Google Scholar] [CrossRef]
- Stallings, R.L. Microrna involvement in the pathogenesis of neuroblastoma: Potential for microrna mediated therapeutics. Curr. Pharm. Des. 2009, 15, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.S.; Song, Y.K.; Durinck, S.; Chen, Q.R.; Cheuk, A.T.; Tsang, P.; Zhang, Q.; Thiele, C.J.; Slack, A.; Shohet, J.; et al. The mycn oncogene is a direct target of mir-34a. Oncogene 2008, 27, 5204–5213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.G.; He, J.H.; Yu, L.; Hang, Z.P.; Li, W.; Shun, W.H.; Huang, G.X. Microrna-202 suppresses mycn expression under the control of e2f1 in the neuroblastoma cell line lan-5. Mol. Med. Rep. 2014, 9, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.; Fay, J.; Meehan, M.; Bryan, K.; Watters, K.M.; Murphy, D.M.; Stallings, R.L. Mirna-335 suppresses neuroblastoma cell invasiveness by direct targeting of multiple genes from the non-canonical tgf-β signalling pathway. Carcinogenesis 2012, 33, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Li, G.L.; Lei, P.C. [effects of mir-144 on proliferation, apoptosis and cisplatin resistance by targeting mycn in pediatric neuroblastoma]. Zhonghua Zhong Liu Za Zhi 2019, 41, 516–521. [Google Scholar]
- Ramaiah, M.J.; Pushpavalli, S.N.; Lavanya, A.; Bhadra, K.; Haritha, V.; Patel, N.; Tamboli, J.R.; Kamal, A.; Bhadra, U.; Pal-Bhadra, M. Novel anthranilamide-pyrazolo [1,5-a]pyrimidine conjugates modulate the expression of p53-mycn associated micro rnas in neuroblastoma cells and cause cell cycle arrest and apoptosis. Bioorgan. Med. Chem. Lett. 2013, 23, 5699–5706. [Google Scholar] [CrossRef]
- Gan, L.; Xiu, R.; Ren, P.; Yue, M.; Su, H.; Guo, G.; Xiao, D.; Yu, J.; Jiang, H.; Liu, H.; et al. Metabolic targeting of oncogene myc by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene 2016, 35, 3037–3048. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Farazi, T.A.; Ostrovnaya, I.; Xu, H.; Tran, H.; Mihailovic, A.; Tuschl, T.; Cheung, N.K. Deep microrna sequencing reveals downregulation of mir-29a in neuroblastoma central nervous system metastasis. Genes Chromosomes Cancer 2014, 53, 803–814. [Google Scholar] [CrossRef]
- Teitz, T.; Inoue, M.; Valentine, M.B.; Zhu, K.; Rehg, J.E.; Zhao, W.; Finkelstein, D.; Wang, Y.D.; Johnson, M.D.; Calabrese, C.; et al. Th-mycn mice with caspase-8 deficiency develop advanced neuroblastoma with bone marrow metastasis. Cancer Res. 2013, 73, 4086–4097. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Xu, L.; Deng, L.; Xue, L.; Meng, Q.; Wei, F.; Wang, J. Rna n(6)-methyladenosine modification is required for mir-98/mycn axis-mediated inhibition of neuroblastoma progression. Sci. Rep. 2020, 10, 13624. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, K.; Ma, L.; Zhang, H. Microrna-145 overexpression inhibits neuroblastoma tumorigenesis in vitro and in vivo. Bioengineered 2020, 11, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Lovén, J.; Zinin, N.; Wahlström, T.; Müller, I.; Brodin, P.; Fredlund, E.; Ribacke, U.; Pivarcsi, A.; Påhlman, S.; Henriksson, M. Mycn-regulated micrornas repress estrogen receptor-alpha (esr1) expression and neuronal differentiation in human neuroblastoma. Proc. Natl. Acad. Sci. USA 2010, 107, 1553–1558. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.A.; Hald, Ø.H.; Fuchs, S.; Løkke, C.; Mikkola, I.; Flægstad, T.; Schulte, J.; Einvik, C. Microrna-193b-3p represses neuroblastoma cell growth via downregulation of cyclin d1, mcl-1 and mycn. Oncotarget 2018, 9, 18160–18179. [Google Scholar] [CrossRef] [Green Version]
- Schulte, J.H.; Horn, S.; Otto, T.; Samans, B.; Heukamp, L.C.; Eilers, U.C.; Krause, M.; Astrahantseff, K.; Klein-Hitpass, L.; Buettner, R.; et al. Mycn regulates oncogenic micrornas in neuroblastoma. Int. J. Cancer 2008, 122, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Samaraweera, L.; Spengler, B.A.; Ross, R.A. Reciprocal antagonistic regulation of n-myc mrna by mir-17 and the neuronal-specific rna-binding protein hud. Oncol. Rep. 2017, 38, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Claeys, S.; Denecker, G.; Durinck, K.; Decaesteker, B.; Mus, L.M.; Loontiens, S.; Vanhauwaert, S.; Althoff, K.; Wigerup, C.; Bexell, D.; et al. Alk positively regulates mycn activity through repression of hbp1 expression. Oncogene 2019, 38, 2690–2705. [Google Scholar] [CrossRef]
- Naraparaju, K.; Kolla, V.; Zhuang, T.; Higashi, M.; Iyer, R.; Kolla, S.; Okawa, E.R.; Blobel, G.A.; Brodeur, G.M. Role of micrornas in epigenetic silencing of the chd5 tumor suppressor gene in neuroblastomas. Oncotarget 2016, 7, 15977–15985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buechner, J.; Henriksen, J.R.; Haug, B.H.; Tømte, E.; Flaegstad, T.; Einvik, C. Inhibition of mir-21, which is up-regulated during mycn knockdown-mediated differentiation, does not prevent differentiation of neuroblastoma cells. Differ. Res. Biol. Divers. 2011, 81, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Westerlund, I.; Shi, Y.; Toskas, K.; Fell, S.M.; Li, S.; Surova, O.; Södersten, E.; Kogner, P.; Nyman, U.; Schlisio, S.; et al. Combined epigenetic and differentiation-based treatment inhibits neuroblastoma tumor growth and links hif2α to tumor suppression. Proc. Natl. Acad. Sci. USA 2017, 114, E6137–E6146. [Google Scholar] [CrossRef] [Green Version]
- Parodi, F.; Carosio, R.; Ragusa, M.; Di Pietro, C.; Maugeri, M.; Barbagallo, D.; Sallustio, F.; Allemanni, G.; Pistillo, M.P.; Casciano, I.; et al. Epigenetic dysregulation in neuroblastoma: A tale of mirnas and DNA methylation. Biochim. Biophys. Acta 2016, 1859, 1502–1514. [Google Scholar] [CrossRef] [PubMed]
- Uekusa, S.; Kawashima, H.; Sugito, K.; Yoshizawa, S.; Shinojima, Y.; Igarashi, J.; Ghosh, S.; Wang, X.; Fujiwara, K.; Ikeda, T.; et al. Nr4a3, a possibile oncogenic factor for neuroblastoma associated with cpgi methylation within the third exon. Int. J. Oncol. 2014, 44, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asada, K.; Watanabe, N.; Nakamura, Y.; Ohira, M.; Westermann, F.; Schwab, M.; Nakagawara, A.; Ushijima, T. Stronger prognostic power of the cpg island methylator phenotype than methylation of individual genes in neuroblastomas. JPN J. Clin. Oncol. 2013, 43, 641–645. [Google Scholar] [CrossRef] [Green Version]
- Hoebeeck, J.; Michels, E.; Pattyn, F.; Combaret, V.; Vermeulen, J.; Yigit, N.; Hoyoux, C.; Laureys, G.; De Paepe, A.; Speleman, F.; et al. Aberrant methylation of candidate tumor suppressor genes in neuroblastoma. Cancer Lett. 2009, 273, 336–346. [Google Scholar] [CrossRef]
- Yan, P.; Mühlethaler, A.; Bourloud, K.B.; Beck, M.N.; Gross, N. Hypermethylation-mediated regulation of cd44 gene expression in human neuroblastoma. Genes Chromosomes Cancer 2003, 36, 129–138. [Google Scholar] [CrossRef]
- Korshunov, A.; Okonechnikov, K.; Schmitt-Hoffner, F.; Ryzhova, M.; Sahm, F.; Stichel, D.; Schrimpf, D.; Reuss, D.E.; Sievers, P.; Suwala, A.K.; et al. Molecular analysis of pediatric cns-pnet revealed nosologic heterogeneity and potent diagnostic markers for cns neuroblastoma with foxr2-activation. Acta Neuropathol. Commun. 2021, 9, 20. [Google Scholar] [CrossRef]
- Schmitt-Hoffner, F.; van Rijn, S.; Toprak, U.H.; Mauermann, M.; Rosemann, F.; Heit-Mondrzyk, A.; Hübner, J.M.; Camgöz, A.; Hartlieb, S.; Pfister, S.M.; et al. Foxr2 stabilizes mycn protein and identifies non-mycn-amplified neuroblastoma patients with unfavorable outcome. J. Clin. Oncol. 2021, 39, 3217–3228. [Google Scholar] [CrossRef]
- Colmenero-Repiso, A.; Gómez-Muñoz, M.A.; Rodríguez-Prieto, I.; Amador-Álvarez, A.; Henrich, K.O.; Pascual-Vaca, D.; Okonechnikov, K.; Rivas, E.; Westermann, F.; Pardal, R.; et al. Identification of vrk1 as a new neuroblastoma tumor progression marker regulating cell proliferation. Cancers 2020, 12, 3465. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chuang, J.H.; Wang, P.W.; Lin, T.K.; Wu, M.T.; Hsu, W.M.; Chuang, H.C. 5-aza-2’-deoxycytidine induces a rig-i-related innate immune response by modulating mitochondria stress in neuroblastoma. Cells 2020, 9, 1920. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Doyle, K.; Mosbruger, T.L.; Butterfield, A.; Weston, A.; Ast, A.; Kaadige, M.; Verma, A.; Sharma, S. Reversible lsd1 inhibition with hci-2509 induces the p53 gene expression signature and disrupts the mycn signature in high-risk neuroblastoma cells. Oncotarget 2018, 9, 9907–9924. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.; Tee, A.E.L.; Milazzo, G.; Bell, J.L.; Poulos, R.C.; Atmadibrata, B.; Sun, Y.; Jing, D.; Ho, N.; Ling, D.; et al. The histone methyltransferase dot1l promotes neuroblastoma by regulating gene transcription. Cancer Res. 2017, 77, 2522–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.Y.; Atmadibrata, B.; Mondal, S.; Tee, A.E.; Liu, T. Ncym is upregulated by lncusmycn and modulates n-myc expression. Int. J. Oncol. 2016, 49, 2464–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, K.O.; Bender, S.; Saadati, M.; Dreidax, D.; Gartlgruber, M.; Shao, C.; Herrmann, C.; Wiesenfarth, M.; Parzonka, M.; Wehrmann, L.; et al. Integrative genome-scale analysis identifies epigenetic mechanisms of transcriptional deregulation in unfavorable neuroblastomas. Cancer Res. 2016, 76, 5523–5537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, J.; Opitz, D.; Althoff, K.; Lodrini, M.; Hero, B.; Volland, R.; Beckers, A.; de Preter, K.; Decock, A.; Patil, N.; et al. Mycn and hdac5 transcriptionally repress cd9 to trigger invasion and metastasis in neuroblastoma. Oncotarget 2016, 7, 66344–66359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikram, F.; Ackermann, S.; Kahlert, Y.; Volland, R.; Roels, F.; Engesser, A.; Hertwig, F.; Kocak, H.; Hero, B.; Dreidax, D.; et al. Transcription factor activating protein 2 beta (tfap2b) mediates noradrenergic neuronal differentiation in neuroblastoma. Mol. Oncol. 2016, 10, 344–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Bell, J.L.; Carter, D.; Gherardi, S.; Poulos, R.C.; Milazzo, G.; Wong, J.W.; Al-Awar, R.; Tee, A.E.; Liu, P.Y.; et al. Wdr5 supports an n-myc transcriptional complex that drives a protumorigenic gene expression signature in neuroblastoma. Cancer Res. 2015, 75, 5143–5154. [Google Scholar] [CrossRef] [Green Version]
- Yáñez, Y.; Grau, E.; Rodríguez-Cortez, V.C.; Hervás, D.; Vidal, E.; Noguera, R.; Hernández, M.; Segura, V.; Cañete, A.; Conesa, A.; et al. Two independent epigenetic biomarkers predict survival in neuroblastoma. Clin. Epigenetics 2015, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Gomez, P.; Bello, M.J.; Lomas, J.; Arjona, D.; Alonso, M.E.; Amiñoso, C.; Lopez-Marin, I.; Anselmo, N.P.; Sarasa, J.L.; Gutierrez, M.; et al. Aberrant methylation of multiple genes in neuroblastic tumours. Relationship with mycn amplification and allelic status at 1p. Eur. J. Cancer 2003, 39, 1478–1485. [Google Scholar] [CrossRef]
- Dreidax, D.; Bannert, S.; Henrich, K.O.; Schröder, C.; Bender, S.; Oakes, C.C.; Lindner, S.; Schulte, J.H.; Duffy, D.; Schwarzl, T.; et al. P19-ink4d inhibits neuroblastoma cell growth, induces differentiation and is hypermethylated and downregulated in mycn-amplified neuroblastomas. Hum. Mol. Genet. 2014, 23, 6826–6837. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Kamijo, T.; Nakagawara, A.; Kaneko, Y. Rassf1a methylation may have two biological roles in neuroblastoma tumorigenesis depending on the ploidy status and age of patients. Cancer Lett. 2014, 348, 167–176. [Google Scholar] [CrossRef]
- Lázcoz, P.; Muñoz, J.; Nistal, M.; Pestaña, A.; Encío, I.; Castresana, J.S. Frequent promoter hypermethylation of rassf1a and casp8 in neuroblastoma. BMC Cancer 2006, 6, 254. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zage, P.; Kagan, D.; Tian, Y.; Seshadri, R.; Salwen, H.R.; Liu, S.; Chlenski, A.; Cohn, S.L. Association of epigenetic inactivation of rassf1a with poor outcome in human neuroblastoma. Clin. Cancer Res. 2004, 10, 8493–8500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S.; Poremba, C.; Berwanger, B.; Häcker, S.; Eilers, M.; Christiansen, H.; Hero, B.; Debatin, K.M. Loss of caspase-8 expression does not correlate with mycn amplification, aggressive disease, or prognosis in neuroblastoma. Cancer Res. 2006, 66, 10016–10023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casciano, I.; Banelli, B.; Croce, M.; De Ambrosis, A.; di Vinci, A.; Gelvi, I.; Pagnan, G.; Brignole, C.; Allemanni, G.; Ferrini, S.; et al. Caspase-8 gene expression in neuroblastoma. Ann. N. Y. Acad. Sci. 2004, 1028, 157–167. [Google Scholar] [CrossRef]
- Sugito, K.; Kawashima, H.; Yoshizawa, S.; Uekusa, S.; Hoshi, R.; Furuya, T.; Kaneda, H.; Hosoda, T.; Konuma, N.; Masuko, T.; et al. Non-promoter DNA hypermethylation of zygote arrest 1 (zar1) in neuroblastomas. J. Pediatr. Surg. 2013, 48, 782–788. [Google Scholar] [CrossRef]
- Casalà, C.; Gil-Guiñón, E.; Ordóñez, J.L.; Miguel-Queralt, S.; Rodríguez, E.; Galván, P.; Lavarino, C.; Munell, F.; de Alava, E.; Mora, J.; et al. The calcium-sensing receptor is silenced by genetic and epigenetic mechanisms in unfavorable neuroblastomas and its reactivation induces erk1/2-dependent apoptosis. Carcinogenesis 2013, 34, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Decock, A.; Ongenaert, M.; Hoebeeck, J.; De Preter, K.; Van Peer, G.; Van Criekinge, W.; Ladenstein, R.; Schulte, J.H.; Noguera, R.; Stallings, R.L.; et al. Genome-wide promoter methylation analysis in neuroblastoma identifies prognostic methylation biomarkers. Genome Biol. 2012, 13, R95. [Google Scholar] [CrossRef] [Green Version]
- Lau, D.T.; Hesson, L.B.; Norris, M.D.; Marshall, G.M.; Haber, M.; Ashton, L.J. Prognostic significance of promoter DNA methylation in patients with childhood neuroblastoma. Clin. Cancer Res. 2012, 18, 5690–5700. [Google Scholar] [CrossRef] [Green Version]
- Sugito, K.; Kawashima, H.; Uekusa, S.; Yoshizawa, S.; Hoshi, R.; Furuya, T.; Kaneda, H.; Hosoda, T.; Masuko, T.; Ohashi, K.; et al. Identification of aberrant methylation regions in neuroblastoma by screening of tissue-specific differentially methylated regions. Pediatr. Blood Cancer 2013, 60, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Djos, A.; Martinsson, T.; Kogner, P.; Carén, H. The rassf gene family members rassf5, rassf6 and rassf7 show frequent DNA methylation in neuroblastoma. Mol. Cancer 2012, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Misawa, A.; Tanaka, S.; Yagyu, S.; Tsuchiya, K.; Iehara, T.; Sugimoto, T.; Hosoi, H. Rassf1a hypermethylation in pretreatment serum DNA of neuroblastoma patients: A prognostic marker. Br. J. Cancer 2009, 100, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Gumy-Pause, F.; Pardo, B.; Khoshbeen-Boudal, M.; Ansari, M.; Gayet-Ageron, A.; Sappino, A.P.; Attiyeh, E.F.; Ozsahin, H. Gstp1 hypermethylation is associated with reduced protein expression, aggressive disease and prognosis in neuroblastoma. Genes Chromosomes Cancer 2012, 51, 174–185. [Google Scholar] [CrossRef]
- Nair, P.N.; McArdle, L.; Cornell, J.; Cohn, S.L.; Stallings, R.L. High-resolution analysis of 3p deletion in neuroblastoma and differential methylation of the sema3b tumor suppressor gene. Cancer Genet. Cytogenet. 2007, 174, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Lázcoz, P.; Muñoz, J.; Nistal, M.; Pestaña, A.; Encío, I.J.; Castresana, J.S. Loss of heterozygosity and microsatellite instability on chromosome arm 10q in neuroblastoma. Cancer Genet. Cytogenet. 2007, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Erdreich-Epstein, A.; Singh, A.R.; Joshi, S.; Vega, F.M.; Guo, P.; Xu, J.; Groshen, S.; Ye, W.; Millard, M.; Campan, M.; et al. Association of high microvessel alphavbeta3 and low pten with poor outcome in stage 3 neuroblastoma: Rationale for using first in class dual pi3k/brd4 inhibitor, sf1126. Oncotarget 2017, 8, 52193–52210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Jeong, Y.J.; Yu, A.R.; Yoon, K.S.; Choe, W.; Ha, J.; Kim, S.S.; Yeo, E.J.; Kang, I. Fluoxetine induces apoptosis through endoplasmic reticulum stress via mitogen-activated protein kinase activation and histone hyperacetylation in sk-n-be(2)-m17 human neuroblastoma cells. Apoptosis Int. J. Program. Cell Death 2017, 22, 1079–1097. [Google Scholar] [CrossRef]
- Wang, S.S.; Hsiao, R.; Limpar, M.M.; Lomahan, S.; Tran, T.A.; Maloney, N.J.; Ikegaki, N.; Tang, X.X. Destabilization of myc/mycn by the mitochondrial inhibitors, metaiodobenzylguanidine, metformin and phenformin. Int. J. Mol. Med. 2014, 33, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegarty, S.V.; Togher, K.L.; O’Leary, E.; Solger, F.; Sullivan, A.M.; O’Keeffe, G.W. Romidepsin induces caspase-dependent cell death in human neuroblastoma cells. Neurosci. Lett. 2017, 653, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Fabian, J.; Lodrini, M.; Oehme, I.; Schier, M.C.; Thole, T.M.; Hielscher, T.; Kopp-Schneider, A.; Opitz, L.; Capper, D.; von Deimling, A.; et al. Grhl1 acts as tumor suppressor in neuroblastoma and is negatively regulated by mycn and hdac3. Cancer Res. 2014, 74, 2604–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.K.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of n-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef]
- Chen, L.; Iraci, N.; Gherardi, S.; Gamble, L.D.; Wood, K.M.; Perini, G.; Lunec, J.; Tweddle, D.A. P53 is a direct transcriptional target of mycn in neuroblastoma. Cancer Res. 2010, 70, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Helmsauer, K.; Valieva, M.E.; Ali, S.; Chamorro González, R.; Schöpflin, R.; Röefzaad, C.; Bei, Y.; Dorado Garcia, H.; Rodriguez-Fos, E.; Puiggròs, M.; et al. Enhancer hijacking determines extrachromosomal circular mycn amplicon architecture in neuroblastoma. Nat. Commun. 2020, 11, 5823. [Google Scholar] [CrossRef]
- George, R.E.; London, W.B.; Cohn, S.L.; Maris, J.M.; Kretschmar, C.; Diller, L.; Brodeur, G.M.; Castleberry, R.P.; Look, A.T. Hyperdiploidy plus nonamplified mycn confers a favorable prognosis in children 12 to 18 months old with disseminated neuroblastoma: A pediatric oncology group study. J. Clin. 2005, 23, 6466–6473. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr. Opin. Pediatr. 2005, 17, 7–13. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Lal, A.; Seeger, R.C.; Maris, J.M.; Shimada, H.; O’Leary, M.; Gerbing, R.B.; Matthay, K.K. Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified mycn neuroblastoma: A children’s cancer group study. J. Clin. Oncol. 2005, 23, 6474–6480. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The international neuroblastoma risk group (inrg) classification system: An inrg task force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Godfried, M.B.; Veenstra, M.; v Sluis, P.; Boon, K.; v Asperen, R.; Hermus, M.C.; v Schaik, B.D.; Voute, T.P.; Schwab, M.; Versteeg, R.; et al. The n-myc and c-myc downstream pathways include the chromosome 17q genes nm23-h1 and nm23-h2. Oncogene 2002, 21, 2097–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhavanfard, S.; Nohr, E.; AlNajjar, M.; Haughn, M.; Hashimoto, S.; Deeg, C.; Pfau, R.; Brundler, M.A.; Reshmi, S.C. 5′ alk amplification in neuroblastoma: A case report. Case Rep. Oncol. 2021, 14, 585–591. [Google Scholar] [CrossRef]
- Bellini, A.; Pötschger, U.; Bernard, V.; Lapouble, E.; Baulande, S.; Ambros, P.F.; Auger, N.; Beiske, K.; Bernkopf, M.; Betts, D.R.; et al. Frequency and prognostic impact of alk amplifications and mutations in the european neuroblastoma study group (siopen) high-risk neuroblastoma trial (hr-nbl1). J. Clin. Oncol. 2021, 39, 3377–3390. [Google Scholar] [CrossRef]
- Javanmardi, N.; Fransson, S.; Djos, A.; Umapathy, G.; Östensson, M.; Milosevic, J.; Borenäs, M.; Hallberg, B.; Kogner, P.; Martinsson, T.; et al. Analysis of alk, mycn, and the alk ligand alkal2 (fam150b/augalpha) in neuroblastoma patient samples with chromosome arm 2p rearrangements. Genes Chromosomes Cancer 2019, 59, 50–57. [Google Scholar] [CrossRef]
- Janoueix-Lerosey, I.; Schleiermacher, G.; Michels, E.; Mosseri, V.; Ribeiro, A.; Lequin, D.; Vermeulen, J.; Couturier, J.; Peuchmaur, M.; Valent, A.; et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J. Clin. Oncol. 2009, 27, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Lastowska, M.; Viprey, V.; Santibanez-Koref, M.; Wappler, I.; Peters, H.; Cullinane, C.; Roberts, P.; Hall, A.G.; Tweddle, D.A.; Pearson, A.D.; et al. Identification of candidate genes involved in neuroblastoma progression by combining genomic and expression microarrays with survival data. Oncogene 2007, 26, 7432–7444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; Baudis, M.; De Preter, K.; Van Roy, N.; Ambros, P.; Bown, N.; Brinkschmidt, C.; Christiansen, H.; Combaret, V.; Lastowska, M.; et al. Unequivocal delineation of clinicogenetic subgroups and development of a new model for improved outcome prediction in neuroblastoma. J. Clin. Oncol. 2005, 23, 2280–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitz, R.; Hero, B.; Ernestus, K.; Berthold, F. Fish analyses for alterations in chromosomes 1, 2, 3, and 11 define high-risk groups in neuroblastoma. Med. Pediatr. Oncol. 2003, 41, 30–35. [Google Scholar] [CrossRef]
- Chen, Q.R.; Bilke, S.; Wei, J.S.; Whiteford, C.C.; Cenacchi, N.; Krasnoselsky, A.L.; Greer, B.T.; Son, C.G.; Westermann, F.; Berthold, F.; et al. Cdna array-cgh profiling identifies genomic alterations specific to stage and mycn-amplification in neuroblastoma. BMC Genom. 2004, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Fang, Y.F.; Wu, D.M.; Lin, Y.; Zhang, B.; Liu, M.K.; Bai, J.X.; Chen, F. Comparison of the efficacy of minimally invasive and open surgery on children with neuroblastoma: A meta-analysis. J. Laparoendosc. Adv. Surg. Tech. Part A 2021, 31, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Brisse, H.J.; McCarville, M.B.; Granata, C.; Krug, K.B.; Wootton-Gorges, S.L.; Kanegawa, K.; Giammarile, F.; Schmidt, M.; Shulkin, B.L.; Matthay, K.K.; et al. Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the international neuroblastoma risk group project. Radiology 2011, 261, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Pearson, A.; Lunec, J. The mycn oncoprotein as a drug development target. Cancer Lett. 2003, 197, 125–130. [Google Scholar] [CrossRef]
- Kling, M.J.; Griggs, C.N.; McIntyre, E.M.; Alexander, G.; Ray, S.; Challagundla, K.B.; Joshi, S.S.; Coulter, D.W.; Chaturvedi, N.K. Synergistic efficacy of inhibiting mycn and mtor signaling against neuroblastoma. BMC Cancer 2021, 21, 1061. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, S.; Colicchia, V.; Pastorino, F.; Pedretti, F.; Fabretti, F.; Nicolis di Robilant, V.; Ramponi, V.; Scafetta, G.; Moretti, M.; Licursi, V.; et al. A combination of parp and chk1 inhibitors efficiently antagonizes mycn-driven tumors. Oncogene 2021, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ding, J.; Zhu, S.; Alptekin, A.; Dong, Z.; Yan, C.; Zha, Y.; Ding, H.F. Therapeutic targeting of both dihydroorotate dehydrogenase and nucleoside transport in mycn-amplified neuroblastoma. Cell Death Dis. 2021, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, L.; Wang, T.; Hu, J.; Wang, J.; Tu, R.; Su, H.; Jiang, J.; Qing, G.; Liu, H. Synergistic targeting of chk1 and mtor in myc-driven tumors. Carcinogenesis 2021, 42, 448–460. [Google Scholar] [CrossRef]
- Waetzig, R.; Matthes, M.; Leister, J.; Penkivech, G.; Heise, T.; Corbacioglu, S.; Sommer, G. Comparing mtor inhibitor rapamycin with torin-2 within the rist molecular-targeted regimen in neuroblastoma cells. Int. J. Med Sci. 2021, 18, 137–149. [Google Scholar] [CrossRef]
- Dedoni, S.; Marras, L.; Olianas, M.C.; Ingianni, A.; Onali, P. The neurotrophin receptor trkc as a novel molecular target of the antineuroblastoma action of valproic acid. Int. J. Mol. Sci. 2021, 22, 7790. [Google Scholar] [CrossRef]
- Takatori, A.; Hossain, M.S.; Ogura, A.; Akter, J.; Nakamura, Y.; Nakagawara, A. Nlrr1 is a potential therapeutic target in neuroblastoma and mycn-driven malignant cancers. Front. Oncol. 2021, 11, 669667. [Google Scholar] [CrossRef]
- Liu, C.; Gen, Y.; Tanimoto, K.; Muramatsu, T.; Inoue, J.; Inazawa, J. Concurrent targeting of map3k3 and brd4 by mir-3140-3p overcomes acquired resistance to bet inhibitors in neuroblastoma cells. Mol. Ther. Nucleic Acids 2021, 25, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.R.; Swanson, M.A.; Dowling, T.C.; Bachmann, A.S. Probenecid increases renal retention and antitumor activity of dfmo in neuroblastoma. Cancer Chemother. Pharmacol. 2021, 88, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.M.; Krytska, K.; Lochmann, T.L.; Sano, R.; Casey, C.; D’Aulerio, A.; Khan, Q.A.; Crowther, G.S.; Coon, C.; Cai, J.; et al. Venetoclax-based rational combinations are effective in models of mycn-amplified neuroblastoma. Mol. Cancer Ther. 2021, 20, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Nakagawara, A. Acceleration or brakes: Which is rational for cell cycle-targeting neuroblastoma therapy? Biomolecules 2021, 11, 750. [Google Scholar] [CrossRef]
- Umapathy, G.; Mendoza-Garcia, P.; Hallberg, B.; Palmer, R.H. Targeting anaplastic lymphoma kinase in neuroblastoma. Acta Pathol. Microbiol. Immunol. Scand. 2019, 127, 288–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Cheng, C.; Wang, Y.; Chen, T.; Tu, J.; Niu, C.; Xing, R.; Wang, Y.; Xu, Y. Synergistic effect of statins and abiraterone acetate on the growth inhibition of neuroblastoma via targeting androgen receptor. Front. Oncol. 2021, 11, 595285. [Google Scholar] [CrossRef] [PubMed]
- Dubiella, C.; Pinch, B.J.; Koikawa, K.; Zaidman, D.; Poon, E.; Manz, T.D.; Nabet, B.; He, S.; Resnick, E.; Rogel, A.; et al. Sulfopin is a covalent inhibitor of pin1 that blocks myc-driven tumors in vivo. Nat. Chem. Biol. 2021, 17, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Huang, M.; Zeki, J.; Gong, M.; Taylor, J.; Shimada, H.; Chiu, B. Combining inhibitors of brd4 and cyclin-dependent kinase can decrease tumor growth in neuroblastoma with mycn amplification. J. Pediatr. Surg. 2021, 56, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.M.; Lochmann, T.L.; Floros, K.V.; Calbert, M.L.; Kurupi, R.; Stein, G.T.; McClanaghan, J.; Murchie, E.; Egan, R.K.; Greninger, P.; et al. Catastrophic atp loss underlies a metabolic combination therapy tailored for mycn-amplified neuroblastoma. Proc. Natl. Acad. Sci. USA 2021, 118, e2009620118. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.B.; Kleynhans, A.; Mittra, R.; Kim, P.Y.; Holien, J.K.; Nagy, Z.; Ciampa, O.C.; Seneviratne, J.A.; Mayoh, C.; Raipuria, M.; et al. A novel combination therapy targeting ubiquitin-specific protease 5 in mycn-driven neuroblastoma. Oncogene 2021, 40, 2367–2381. [Google Scholar] [CrossRef]





| Type | Start | Stop | Strand | Comment |
|---|---|---|---|---|
| source | 1 | 6455 | 5′→3′ | /gene = MYCN /gene_synonym = MYCN-AS1; N-CYM; NCYM; NYCM /organism = Homo sapiens /mol_type = genomic DNA /db_xref = taxon:9606 /chromosome = 2 |
| gene | <1C | 1884 | 3′→5′ | /note = MYCN opposite strand; Derived by automated computational analysis /db_xref = GeneID:10408 /db_xref = HGNC:HGNC:16911 /db_xref = MIM:605374 |
| ncRNA | <425C 983C | 548 1136 | 3′→5′ | /ncRNA_class = lncRNA /product = MYCN opposite strand, transcript variant 1 /transcript_id = NR_110230.2 |
| ncRNA | <425C 983C | 548 1884 | 3′→5′ | /ncRNA_class = lncRNA /gene = MYCNOS /gene_synonym = MYCN-AS1; N-CYM; NCYM; NYCM /product = MYCN opposite strand, transcript variant 3 /transcript_id = NR_161163.1 |
| ncRNA | 425C 983C | 548 1136 | 3′→5′ | /ncRNA_class = lncRNA /gene = MYCNOS /gene_synonym = MYCN-AS1; N-CYM; NCYM; NYCM /product = MYCN opposite strand, transcript variant 2 /transcript_id = NR_161162.1 |
| mRNA | 1 1399 4944 | 504 2305 6455 | 5′→3′ | /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /product = MYCN proto-oncogene, bHLH transcription factor, transcript variant 1 /transcript_id = NM_001293228.2 |
| mRNA | 1 1399 4944 | 194 2305 6455 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /product = MYCN proto-oncogene, bHLH transcription factor, transcript variant 2 /transcript_id = NM_005378.6 /db_xref = Ensembl:ENST00000281043.4 |
| mRNA | 1 1399 4944 | 194 2305 6455 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /product = MYCN proto-oncogene, bHLH transcription factor, transcript variant 2 /transcript_id = NM_001293233.2 |
| mRNA | 1 4944 | 194 6455 | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /product = MYCN proto-oncogene, bHLH transcription factor, transcript variant 3 /transcript_id = NM_001293231.2 | |
| CDS | 38 4944 | 194 5548 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = isoform 2 is encoded by transcript variant 3; /codon_start = 1 /product = N-myc proto-oncogene protein isoform 2 /protein_id = NP_001280160.1 /db_xref = CCDS:CCDS86823.1 /translation=MRGAPGNCVGAEQALARRKRAQTVAIRGHPRPPGPPGDTRAESPPDPLQSAGDDEDDEEEDEEEEIDVVTVEKRRSSSNTKAVTTFTITVRPKNAALGPGRAQSSELILKRCLPIHQQHNYAAPSPYVESEDAPPQKKIKSEASPRPLKSVIPPKAKSLSPRNSDSEDSERRRNHNILERQRRNDLRSSFLTLRDHVPELVKNEKAAKVVILKKATEYVHSLQAEEHQLLLEKEKLQARQQQLLKKIEHARTC |
| CDS | 38 1399 | 194 1580 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = isoform 3 is encoded by transcript variant 2; /codon_start = 1 /product = N-myc proto-oncogene protein isoform 3 /protein_id = NP_001280162.1 /db_xref = GeneID:4613 /db_xref = HGNC:HGNC:7559 /db_xref = MIM:164840 /translation=MRGAPGNCVGAEQALARRKRAQTVAIRGHPRPPGPPGDTRAESPPDPLQSAGVLEVGAGPRLPRPPREGSTPGIKTNGAERSPQSPAGRRADAELLHVHHAGHDLQEPRPRV |
| CDS | 1516 4944 | 2305 5548 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = isoform 1 is encoded by transcript variant 2; /codon_start = 1 /product = N-myc proto-oncogene protein isoform 1 /protein_id = NP_005369.2 /db_xref = CCDS:CCDS1687.1 /db_xref = Ensembl:ENSP00000281043.3 /db_xref = GeneID:4613 /db_xref = HGNC:HGNC:7559 /db_xref = MIM:164840 /translation=MPSCSTSTMPGMICKNPDLEFDSLQPCFYPDEDDFYFGGPDSTPPGEDIWKKFELLPTPPLSPSRGFAEHSSEPPSWVTEMLLENELWGSPAEEDAFGLGGLGGLTPNPVILQDCMWSGFSAREKLERAVSEKLQHGRGPPTAGSTAQSPGAGAASPAGRGHGGAAGAGRAGAALPAELAHPAAECVDPAVVFPFPVNKREPAPVPAAPASAPAAGPAVASGAGIAAPAGAPGVAPPRPGGRQTSGGDHKALSTSGEDTLSDSDDEDDEEEDEEEEIDVVTVEKRRSSSNTKAVTTFTITVRPKNAALGPGRAQSSELILKRCLPIHQQHNYAAPSPYVESEDAPPQKKIKSEASPRPLKSVIPPKAKSLSPRNSDSEDSERRRNHNILERQRRNDLRSSFLTLRDHVPELVKNEKAAKVVILKKATEYVHSLQAEEHQLLLEKEKLQARQQQLLKKIEHARTC |
| misc_feature | 1570 | 1656 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = propagated from UniProtKB/Swiss-Prot (P04198.2); Region: Interactio nwith AURKA./evidence=ECO:0000269|PubMed:27837025 |
| misc_feature | 1696 | 1782 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = propagated from UniProtKB/Swiss-Prot (P04198.2); Region: Interaction with AURKA and FBXW7./evidence=ECO:0000269|PubMed:27837025 |
| misc_feature | 2296 | 2298 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = Phosphoserine, by CK2./evidence=ECO:0000269|PubMed:1425701; propagated from UniProtKB/Swiss-Prot (P04198.2); phosphorylation site |
| misc_feature | 2302 | 2304 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = Phosphoserine, by CK2./evidence=ECO:0000269|PubMed:1425701; propagated from UniProtKB/Swiss-Prot (P04198.2); phosphorylation site |
| misc_feature | 5450 | 5515 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = propagated from UniProtKB/Swiss-Prot (P04198.2); Region: Leucine-zipper |
| CDS | 1516 4944 | 2305 5548 | 5′→3′ | /gene = MYCN/ gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = isoform 1 is encoded by transcript variant 1; /codon_start = 1 /product = N-myc proto-oncogene protein isoform 1/protein_id = NP_001280157.1 /db_xref = CCDS:CCDS1687.1 /translation=MPSCSTSTMPGMICKNPDLEFDSLQPCFYPDEDDFYFGGPDSTPPGEDIWKKFELLPTPPLSPSRGFAEHSSEPPSWVTEMLLENELWGSPAEEDAFGLGGLGGLTPNPVILQDCMWSGFSAREKLERAVSEKLQHGRGPPTAGSTAQSPGAGAASPAGRGHGGAAGAGRAGAALPAELAHPAAECVDPAVVFPFPVNKREPAPVPAAPASAPAAGPAVASGAGIAAPAGAPGVAPPRPGGRQTSGGDHKALSTSGEDTLSDSDDEDDEEEDEEEEIDVVTVEKRRSSSNTKAVTTFTITVRPKNAALGPGRAQSSELILKRCLPIHQQHNYAAPSPYVESEDAPPQKKIKSEASPRPLKSVIPPKAKSLSPRNSDSEDSERRRNHNILERQRRNDLRSSFLTLRDHVPELVKNEKAAKVVILKKATEYVHSLQAEEHQLLLEKEKLQARQQQLLKKIEHARTC |
| misc_feature | 1570 | 1656 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = propagated from UniProtKB/Swiss-Prot (P04198.2); Region: Interaction with AURKA./evidence=ECO:0000269|PubMed:27837025 |
| misc_feature | 1696 | 1782 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = propagated from UniProtKB/Swiss-Prot (P04198.2); Region: Interaction with AURKA and FBXW7./evidence=ECO:0000269|PubMed:27837025 |
| misc_feature | 2296 | 2298 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = Phosphoserine, by CK2./evidence=ECO:0000269|PubMed:1425701; propagated from UniProtKB/Swiss-Prot (P04198.2); phosphorylation site |
| misc_feature | 2302 | 2304 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = Phosphoserine, by CK2./evidence=ECO:0000269|PubMed:1425701; propagated from UniProtKB/Swiss-Prot (P04198.2); phosphorylation site |
| misc_feature | 5450 | 5515 | 5′→3′ | /gene = MYCN /gene_synonym = bHLHe37; MODED; N-myc; NMYC; ODED /note = propagated from UniProtKB/Swiss-Prot (P04198.2); Region: Leucine-zipper |
| Inv. | Location (5′→3′) | Subsequence | Pattern |
|---|---|---|---|
| 1 | 3930 | TATA-box | TATAAAA |
| 2 | 4401 | TATA-box | ATATATA |
| 3 | 4402 | TATA-box | TATATAT |
| 4 | 4403 | TATA-box | ATATATA |
| 5 | 4882 | TATA-box | ATATATA |
| 6 | 4883 | TATA-box | TATATAT |
| 7 | 6271 | TATA-box | TATATAT |
| 8 | 6272 | TATA-box | ATATATA |
| 9 | 6273 | TATA-box | TATATAT |
| 10 | 6274 | TATA-box | ATATATA |
| 11 | 6275 | TATA-box | TATATAT |
| 12 | 6382 | TATA-box | TATATAT |
| miRNA | Symbol | Accession | Relation between miRNA and MYCN | miRNA Expression in Neuroblastoma | Effect on Therapy-Related Resistance | Citation |
|---|---|---|---|---|---|---|
| hsa-miR-101-3p | MIR101-1 | MI0000103 | MIR101-1 directly suppresses MYCN | Down-regulated | Unknown | Buechner et al. (2011) [136] |
| hsa-miR-449a | MIR449A | MI0001648 | MIR449A directly suppresses MYCN | Down-regulated | Unknown | Zhao et al. (2016) [137] |
| hsa-miR-34a | MIR34A | MI0000268 | MIR34A directly suppresses MYCN | Down-regulated | Induces chemo- and radiosensitivity | Cole et al. (2008) [138] Di Paolo et al. (2020) [139] Soni et al. (2013) [140] Stallings et al. (2009) [141] Wei et al. (2008) [142] |
| hsa-miR-202 | MIR202 | MI0003130 | MIR202 directly suppresses MYCN | Down-regulated | Unknown | Li et al. (2014) [143] |
| hsa-miR-335-3p | MIR335 | MI0000816 | MIR335 directly suppresses MYCN | Down-regulated | Unknown | Lynch et al. (2012) [144] |
| hsa-miR-144-3p | MIR144 | MI0000460 | MIR144 directly suppresses MYCN | Down-regulated | Induces chemo- and radiosensitivity | Liu et al. (2019) [145] |
| hsa-miR-107 | MIR107 | MI0000114 | MIR107 directly suppresses MYCN | Down-regulated | Induces apoptosis, cell cycle arrest, chemosensitivity | Ramaiah et al. (2013) [146] |
| hsa-miR-29c | MIR29C | MI0000735 | MIR29C indirectly suppresses MYCN | Down-regulated | Unknown | Gan et al. (2016) [147] |
| hsa-miR-7 | MIR7 | MI0000127 | MIR7 probably enhances MYCN indirectly | Up-regulated | Unknown | Cheung et al. (2014) [148] |
| hsa-miR-29a-3p | MIR29A | MI0000087 | MIR29A directly suppresses MYCN | Down-regulated | Unknown | Cheung et al. (2014) [148] |
| hsa-miR-29b-3p | MIR29B | MI0000105 | MIR29B directly suppresses MYCN | Down-regulated | Unknown | Teitz et al. (2013) [149] |
| hsa-miR-98-5p | MIR98 | MI0000100 | MIR98 directly suppresses MYCN | Down-regulated | Unknown | Cheng et al. (2020) [150] |
| hsa-miR-145-5p | MIR145 | MI0000461 | MIR145 directly suppresses MYCN | Down-regulated | Unknown | Zhao et al. (2020) [151] |
| hsa-miR-19a-3p | MIR19A | MI0000073 | MYCN enhances MIR19A | Up-regulated | Represses neuronal differentiation | Loven et al. (2010) [152] |
| hsa-miR-19b-3p | MIR19B | MI0000074 | MIR19B directly suppresses MYCN | Down-regulated | Unknown | Kumps et al. (2013) [72] De Brouwer et al. (2012) [71] |
| hsa-miR-193b-3p | MIR193B | MI0003137 | MIR193B directly suppresses MYCN | Down-regulated | Induces apoptosis, proliferation arrest | Roth et al. (2018) [153] |
| hsa-miR-200b-3p | MIR200B | MI0000342 | MIR200B directly suppresses MYCN | Down-regulated | Induces apoptosis, cell cycle arrest | Ramaiah et al. (2013) [146] |
| hsa-miR-106a-5p | MIR106A | MI0000113 | MYCN enhances MIR106A | Up-regulated | Promotes cell proliferation | Schulte et al. (2008) [154] |
| hsa-miR-20a-5p | MIR20A | MI0000076 | MYCN enhances MIR20A | Up-regulated | Promotes cell proliferation | Samaraweera et al. (2017) [155] |
| hsa-miR-17-5p | MIR17 | MI0000071 | MYCN enhances MIR17 | Up-regulated | Promotes cell proliferation | Claeys et al. (2019) [156] Samaraweera et al. (2017) [155] Naraparaju et al. (2016) [157] Buechner et al. (2011) [158] Loven et al. (2010) [152] Schulte et al. (2008) [154] |
| Gene | Methylation Status | MYCN Amplification | Effect on Neuroblastoma | Effect on Prognosis | Effect on Therapy-Related Resistance | Citation |
|---|---|---|---|---|---|---|
| FOXR2 | Methylated | Non-amplified | Tumor progression | Poor | Unknown | Korschunov et al. (2021) [165] Schmitt-Hoffner et al. (2021) [166] |
| VRK1 | Hypo-methylated | Amplified | Tumor progression | Poor | Unknown | Colmenero-Repiso et al. (2020) [167] |
| DDX58 | Hyper-methylated | Both | Tumor progression | Poor | Chemo-resistance | Lin et al. (2020) [168] |
| H3K9me1/2 | Methylated | Both | Tumor progression | Poor | Chemo-resistance. De-methylation confers tumor sensitivity | Gupta et al. (2018) [169] |
| NAV2, NCAM2, PRPH | Hyper-methylated | Amplified | Tumor progression | Poor | Chemo-resistance. De-methylation confers tumor sensitivity | Westerlund et al. (2017) [159] |
| H3K79 | Methylated | Amplified | Tumor progression | Poor | Chemo-resistance. De-methylation confers tumor sensitivity | Wong et al. (2017) [170] |
| LET7G, MIR124-2, MIR1490, MIR15599, MIR23B, MIR24-1, MIR27B, MIR34C, MIR34B2, MIR196A-1 | Methylated | Both | Tumor progression | Poor | Chemo-resistance. De-methylation confers tumor sensitivity | Parodi et al. (2016) [160] |
| NCYM | Methylated | Both | Tumor progression | Poor | Unknown | Liu et al. (2016) [171] |
| PCDHB, ABCB1, CACNA1G, CD44,DUSP23, PRDM2, RBP1, SFRP1 | Hyper-methylated | Both | Tumor progression | Poor | Chemo-resistance. De-methylation confers tumor sensitivity | Henrich et al. (2016) [172] |
| KRT19, PRPH, CNR1, QPCT | Methylated | Both | Tumor progression | Poor | Chemo-resistance. De-methylation confers tumor sensitivity | Henrich et al. (2016) [172] |
| CD9 | Methylated | Non-amplified | Tumor progression | Poor | Unknown | Fabian et al. (2016) [173] |
| TFAP2B | Methylated | Both | Tumor progression | Poor | Chemo-resistance. De-methylation confers tumor sensitivity | Ikram et al. (2016) [174] |
| H3K4 | Tri-methylated | Both | Tumor progression | Poor | Unknown | Sun et al. (2015) [175] |
| NNAT, TP73, CCND, RUNX32, CTSZ, DUSP2, HPN, JAK2, LRRC4, MAGEA2, MGMT, PAX8, ECRG4, RB1, TDGF1, TSPAN32 | Hyper-methylated | Both | Tumor progression | Poor | Unknown | Yanez et al. (2015) [176] Gonzalez-Gomez et al. (2003) [177] |
| p19-INK4d | Hyper-methylated | Both | Tumor progression | Poor | Unknown | Dreidax et al. (2014) [178] |
| RASSF1A, PCDHB | Methylated | Both | Tumor progression | Poor | Unknown | Haruta et al. (2014) [179] Lazcoz et al. (2007) [180] Yang et al. (2004) [181] |
| NR4A3 | Hyper-methylated Hypo-methylated | Both | Tumor inhibition Tumor Progression | Good Poor | Unknown | Uekusa et al. (2014) [161] |
| CASP8 | Methylated | Both | Tumor Progression | Poor | Unknown | Asada et al. (2013) [162] Lazcoz et al. (2007) [180] Fulda et al. (2006) [182] Casciano et al. (2004) [183] Gonzalez-Gomez et al. (2003) [177] |
| ZAR1 | Hyper-methylated | Both | Tumor Progression | Poor | Unknown | Sugito et al. (2013) [184] |
| CASR | Hyper-methylated | Both | Tumor Progression | Poor | Unknown | Casala et al. (2013) [185] |
| KRT19, FAS, PRPH, CNR1, QPCT, HIST1H3C, ACSS3, GRB10 | Methylated | Both | Tumor Progression | Poor | Unknown | Decock et al. (2012) [186] |
| HIST1H3C, GNAS | Methylated | Both | Tumor inhibition | Good | Unknown | Decock et al. (2012) [186] |
| DNAJC15, NTRK1, TNFRSF10D | Methylated | Both | Tumor Progression | Poor | Unknown (found in older patients) | Lau et al. (2012) [187] |
| DNAJC15, NTRK1, PYCARD | Hyper-methylated | Amplified | Tumor Progression | Poor | Unknown | Lau et al. (2012) [187] |
| FOLH1, MYOD1, THBS1 | Hyper-methylated | Both | Tumor Progression | Poor | Unknown | Lau et al. (2012) [187] Gonzalez-Gomez et al. (2003) [177] |
| SLC16A5, ZNF206 | Hypo-methylated | Both | Unknown | Unknown | Unknown | Sugito et al. (2013) [188] |
| RASSF family | Methylated | Both | Tumor Progression | Poor | Unknown | Djos et al. (2012) [189] Misawa et al. (2009) [190] Michalowski et al. (2008) [20] Lazcoz et al. (2007) [180] Yang et al. (2004) [181] |
| GSTP1 | Hyper-methylated | Both | Tumor Progression | Poor | Unknown | Gumy-Pause et al. (2012) [191] |
| CD44, RASSF1A, CASP8, PTEN, ZMYND10, CDH1, PRDM2 | Methylated | Both | Tumor Progression | Poor | Unknown | Hoebeeck et al. (2009) [163] |
| SEMA3B | Methylated | Both | Tumor Progression | Poor | Unknown | Nair et al. (2007) [192] |
| PTEN, MGMT, MXI1, FGFR2 | Methylated | Both | Tumor Progression | Poor | Unknown | Lazcoz et al. (2007) [193] |
| CD44 expressing CD44 not expressing | Unmethylated Hyper-methylated | Both Both | Tumor inhibition Tumor Progression | Good Poor | Unknown | Yan et al. (2003) [164] |
| Target Molecule | Inhibitor | MYCN Amplification | Citation |
|---|---|---|---|
| MYCN Proto-Oncogene, BHLH Transcription Factor (MYCN) | JQ1 OTX-015 | Yes | [219] |
| Mammalian Target of Rapamycin (mTOR) | Temsirolimus Rapamycin | Yes | [219,222,223] |
| Peroxisome Proliferator-Activated Receptors (PPAR) | Olaparib Talazoparib | Yes | [220] |
| Checkpoint Kinase 1 (CHK1) | MK-8776 PF-00477736 | Yes | [220] |
| Dihydroorotate Dehydrogenase (DHODH) | Leflunomide Brequinar sodium GSK983 | Yes and No | [221] |
| Tropomyosin Receptor Kinase C (TrkC) | Valproic Acid | Yes | [224] |
| Neuronal Leucine-Rich Repeat 1 (NLRR1) | Monoclonal Antibodies Against the Extracellular Domains Of NLRR1 (N1mAb) | Yes | [225] |
| Mitogen-Activated Protein Kinase Kinase Kinase 3 (MAP3K3) Bromodomain-Containing Protein 4 (BRD4) | miR-3140-3p | Yes | [226] |
| Ornithine Decarboxylase (ODC) | Probenecid DFMO | Yes | [227] |
| B-Cell Lymphoma 2 (BCL2) | Venetoclax | Yes | [228] |
| Cyclin Dependent Kinase 4 (CDK4) Cyclin Dependent Kinase 6 (CDK6) | Flavopiridol Roscovitine Dinaciclib P276-00 AT7519 TG02 RoniciclibR GB-286638 | Yes | [222,229] |
| ALK Receptor Tyrosine Kinase (ALK) | Crizotinib Ceritinib Alectinib Brigatinib Entrectinib Lorlatinib | [230] | |
| Androgen Receptor (AR) | Statins Abiraterone Acetate | [231] | |
| Peptidyl-Prolyl Cis/Trans Isomerase (Pin1) | Sulfopin | [232] | |
| Bromodomain-Containing Protein 4 (BRD4) | NHWD-870 BMS-986158 OTX-015 GSK-525762 | [233] | |
| Monocarboxylate Transporter 1 (MCT1) | AZD3965 | Yes | [234] |
| Histone Deacetylase (HDAC) | Suberanoyl Hydroxamic Acid (SAHA) | Yes | [235] |
| Ubiquitin Carboxyl-Terminal Hydrolase 5 (USP5) | SE486-11 | Yes | [235] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braoudaki, M.; Hatziagapiou, K.; Zaravinos, A.; Lambrou, G.I. MYCN in Neuroblastoma: “Old Wine into New Wineskins”. Diseases 2021, 9, 78. https://doi.org/10.3390/diseases9040078
Braoudaki M, Hatziagapiou K, Zaravinos A, Lambrou GI. MYCN in Neuroblastoma: “Old Wine into New Wineskins”. Diseases. 2021; 9(4):78. https://doi.org/10.3390/diseases9040078
Chicago/Turabian StyleBraoudaki, Maria, Kyriaki Hatziagapiou, Apostolos Zaravinos, and George I. Lambrou. 2021. "MYCN in Neuroblastoma: “Old Wine into New Wineskins”" Diseases 9, no. 4: 78. https://doi.org/10.3390/diseases9040078







