Auxo-Endocrinological Approach to Celiac Children
Abstract
:1. Introduction
| SKELETAL SYSTEM: |
|
| ENDOCRINE SYSTEM: |
|
| CENTRAL NERVOUS SYSTEM: |
| Epilepsy with occipital calcifications |
|
| OTHER SYSTEMS: |
|
2. General Auxological Approach
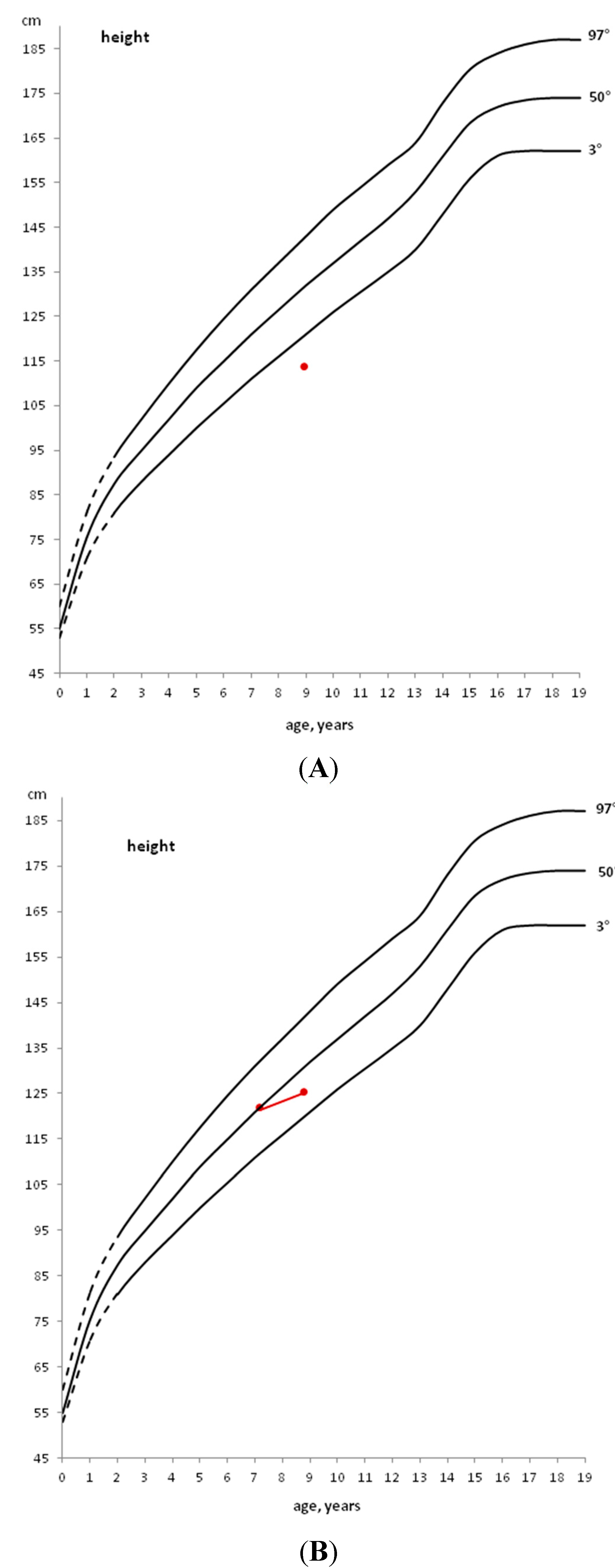
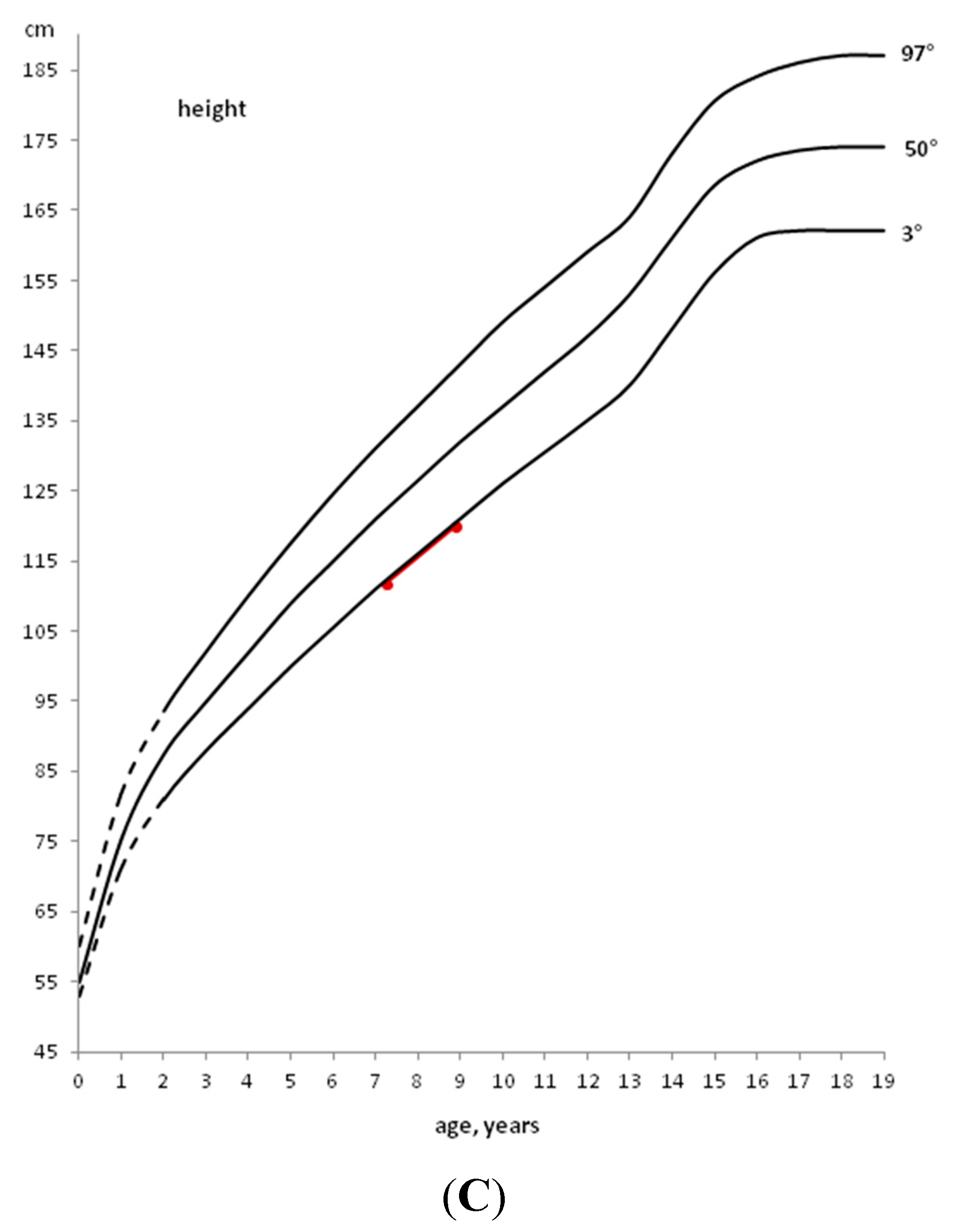

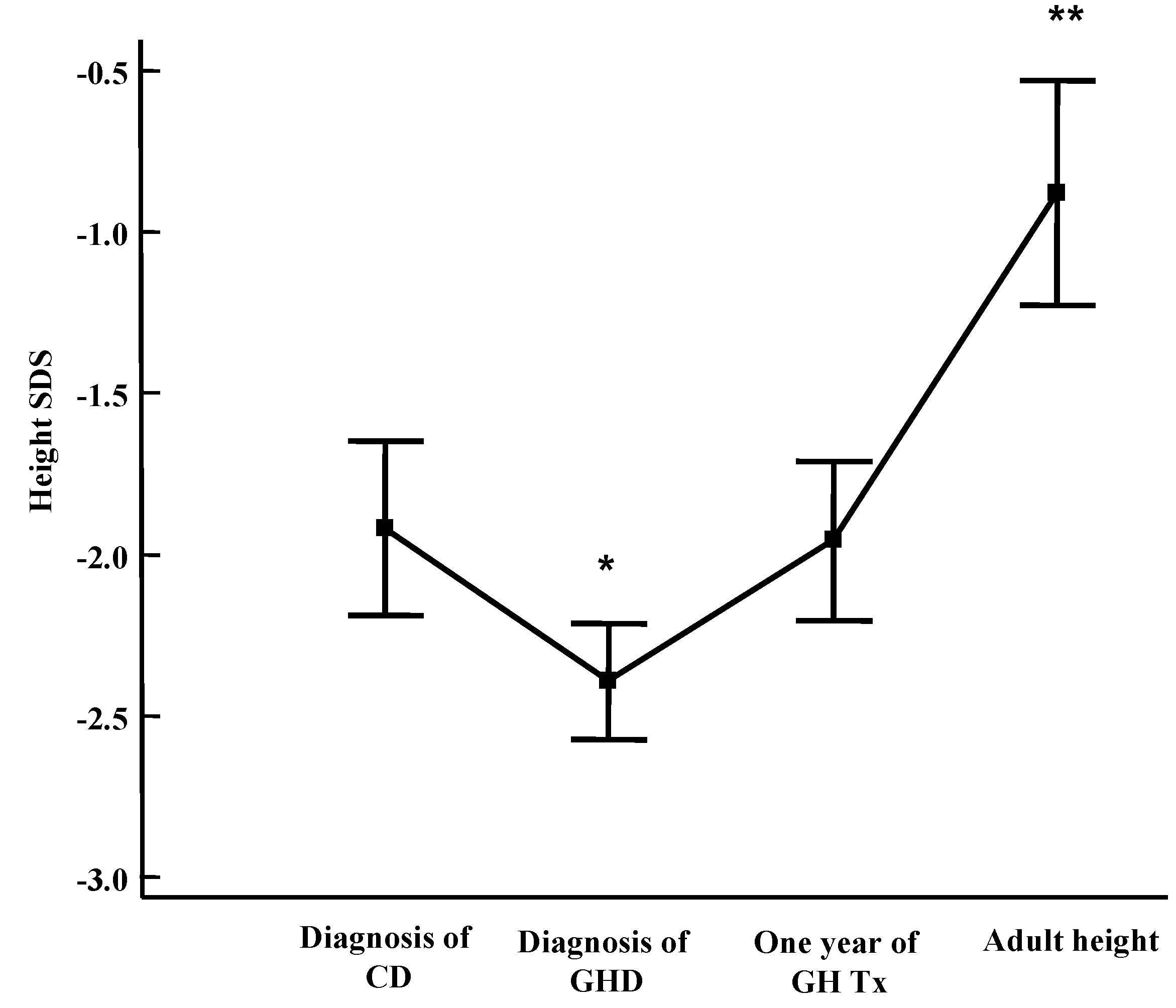
3. Development of Endocrine Autoimmune Conditions in Children with CD
3.1. Thyroiditis
3.2. Diabetes
3.3. Other Autoimmune Diseases
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fasano, A.; Catassi, C. Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Cellier, C.; Viola, S.; Colombel, J.F.; Michaud, L.; Sarles, J.; Hugot, J.P.; Ginies, J.L.; Dabadie, A.; Mouterde, O.; et al. Incidence of autoimmune diseases in celiac disease: Protective effect of the gluten-free diet. Clin. Gastroenterol. Hepatol. 2008, 6, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Fleischauer, A.T.; Bhagat, G.; Goyal, R.; Jabri, B.; Neugut, A.I. Risk of malignancy in patients with celiac disease. Am. J. Med. 2003, 115, 191–195. [Google Scholar] [CrossRef]
- Rosenbach, Y.; Dinari, G.; Zahavi, I.; Nitzan, M. Short stature as the major manifestation of coeliac disease in older children. Clin. Pediatr. (Phila) 1986, 25, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Stenhammar, L.; Fallstrom, S.P.; Jansson, G.; Jansson, U.; Lindberg, T. Coeliac disease in children with short stature without gastrointestinal symptoms. Eur. J. Pediatr. 1986, 145, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Bonamico, M.; Scirè, G.; Mariani, P.; Pasquino, A.M.; Triglione, P.; Scaccia, S.; Ballati, G.; Boscherini, B. Short stature as the primary manifestation of monosymptomatic celiac disease. J. Pediatr. Gastroenterol. Nutr. 1992, 14, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Giovenale, D.; Meazza, C.; Cardinale, G.M.; Sposito, M.; Mastrangelo, C.; Messini, B.; Citro, G.; Delvecchio, M.; di Maio, S.; Bozzola, M. The prevalence of growth hormone deficiency and celiac disease in short children. Clin. Med. Res. 2006, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Saari, A.; Harju, S.; Mäkitie, O.; Saha, M.T.; Dunkel, L.; Sankilampi, U. Systematic growth monitoring for the early detection of celiac disease in children. JAMA Pediatr. 2015, 169, e1525. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Pagani, S.; Messini, B.; Cardinale, G.M.; Mastrangelo, C.; Citro, G.; Delvecchio, M.; Tinelli, C.; Corazza, G.R.; Bozzola, M. Celiac children treated for growth hormone deficiency reach normal final height. Clin. Endocrinol. (Oxf.) 2011, 74, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Mushtaq, T.; Alvi, S. Fifteen-minute consultation: The child with short stature. Arch. Dis. Child. Educ. Pract. Ed. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Busetto, F.; Sterpa, A.; Buonomo, A. Deficit transitorio di GH in corso di morbo celiaco. Min. Ped. 1980, 32, 891–897. [Google Scholar]
- Bozzola, M.; Giovenale, D.; Bozzola, E.; Meazza, C.; Martinetti, M.; Tinelli, C.; Corazza, G.R. Growth hormone deficiency and coeliac disease: An unusual association? Clin. Endocrinol. 2005, 62, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Giovenale, D.; Meazza, C.; Cardinale, G.M.; Farinelli, E.; Mastrangelo, C.; Messini, B.; Citro, G.; Delvecchio, M.; di Maio, S.; Possenti, I.; et al. Treatment with growth hormone (GH) in prepubertal coeliac children with GH deficiency. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 433–437. [Google Scholar] [CrossRef] [PubMed]
- GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary statement of the GH research society. J. Clin. Endocrinol. Metab. 2000, 85, 3990–3993. [Google Scholar]
- Troncone, R.; Auricchio, R.; Paparo, F.; Maglio, M.; Borrelli, M.; Esposito, C. Coeliac disease and extraintestinal autoimmunity. J. Pediatr. Gastroenterol. Nutr. 2004, 39 (Suppl. S3), S740–S741. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M.; Jabri, B. Is celiac disease an autoimmune disorder? Curr. Opin. Immunol. 2005, 17, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Magazzù, G.; Greco, L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology 1999, 117, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; Bruzzi, P.; Predieri, B. Endocrine disorders and celiac disease. Expert Rev. Endocrinol. Metab. 2008, 3, 663–665. [Google Scholar] [CrossRef]
- Radetti, G. Clinical aspects of Hashimoto’s thyroiditis. Endocr. Dev. 2014, 26, 158–170. [Google Scholar] [PubMed]
- Kalyoncu, D.; Urganci, N. Antithyroid antibodies and thyroid function in pediatric patients with celiac disease. Int. J. Endocrinol. 2015, 2015, 276575. [Google Scholar] [CrossRef] [PubMed]
- Cassio, A.; Ricci, G.; Baronio, F.; Miniaci, A.; Bal, M.; Bigucci, B.; Conti, V.; Cicognani, A. Long-term clinical significance of thyroid autoimmunity in children with celiac disease. J. Pediatr. 2010, 156, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Salardi, S.; Volta, U.; Zucchini, S.; Fiorini, E.; Maltoni, G.; Vaira, B.; Cicognani, A. Prevalence of celiac disease in children with type 1 diabetes mellitus increased in the mid-1990s: An 18-year longitudinal study based on anti-endomysial antibodies. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Barera, G.; Bonfanti, R.; Viscardi, M.; Bazzigaluppi, E.; Calori, G.; Meschi, F.; Bianchi, C.; Chiumello, G. Occurrence of celiac disease after onset of type 1 diabetes: A 6-year prospective longitudinal study. Pediatrics 2002, 109, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Elfström, P.; Sundström, J.; Ludvigsson, J.F. Systematic review with meta-analysis: Associations between coeliac disease and type 1 diabetes. Aliment. Pharmacol. Ther. 2014, 40, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Bona, G.; Marinello, D.; Oderda, G. Mechanisms of abnormal puberty in coeliac disease. Horm. Res. 2002, 57 (Suppl. S2), 63–65. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; de Bellis, A.; Francavilla, R.; Rutigliano, V.; Predieri, B.; Indrio, F.; de Venuto, D.; Sinisi, A.A.; Bizzarro, A.; Bellastella, A.; et al. Anti-pituitary antibodies in children with newly diagnosed celiac disease: A novel finding contributing to linear-growth impairment. Am. J. Gastroenterol. 2010, 105, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; de Bellis, A.; Predieri, B.; Bizzarro, A.; de Simone, M.; Balli, F.; Bellastella, A.; Bernasconi, S. Growth hormone impaired secretion and antipituitary antibodies in patients with coeliac disease and poor catch-up growth after a long gluten-free diet period: A causal association? Eur. J. Pediatr. 2006, 165, 897–903. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzola, M.; Meazza, C.; Villani, A. Auxo-Endocrinological Approach to Celiac Children. Diseases 2015, 3, 111-121. https://doi.org/10.3390/diseases3020111
Bozzola M, Meazza C, Villani A. Auxo-Endocrinological Approach to Celiac Children. Diseases. 2015; 3(2):111-121. https://doi.org/10.3390/diseases3020111
Chicago/Turabian StyleBozzola, Mauro, Cristina Meazza, and Alberto Villani. 2015. "Auxo-Endocrinological Approach to Celiac Children" Diseases 3, no. 2: 111-121. https://doi.org/10.3390/diseases3020111






