Prognostication in Pulmonary Arterial Hypertension with Submaximal Exercise Testing
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Results
| N = 65 | |
|---|---|
| Demographics | |
| Age, years, median [IQR] | 62 [50–70] |
| Sex, % male | 20% |
| WHO diagnostic group | |
| IPAH, % | 52% |
| APAH, % | 48% |
| WHO functional class, % | |
| I | 9% |
| II | 35% |
| III | 52% |
| IV | 3% |
| Right heart catheterization | |
| mPAP, mmHg | 48 [39–57] |
| PVR, Woods unit | 8.5 [4.8–14] |
| RAP, mmHg | 8 [5–11] |
| PAOP, mmHg | 13 [9–16] |
| CI, L/min/m2 | 2.4 [1.8–3.6] |
| Other clinical measures | |
| 6MWD, m | 367 [284–450] |
| BNP, pg/mL | 82 [38–246] |
| DLCO, % | 59% [45–69] |
| REVEAL registry risk score | |
| Median [IQR] | 7 [5–9] |
| Highest risk (10), % | 20% |
| Submaximal exercise test | |
| VO2, mL/kg/min | 11 [8.8–13] |
| VE/VCO2 | 39 [29–47] |
| PETCO2-b, mmHg | 33 [29–36] |
| PETCO2-ex, mmHg | 34 [26–37] |
| SET Variables | Spearman correlation, r | p-value |
|---|---|---|
| VO2 | −0.51 | <0.0001 |
| VE/VCO2 | 0.57 | <0.0001 |
| PETCO2-b | −0.28 | 0.0002 |
| PETCO2-ex | −0.45 | 0.026 |
| Delta* PETCO2 | −0.41 | 0.0007 |
| SET Variables Median [IQR] | Highest Risk | Lower Risk | p-value |
|---|---|---|---|
| (RRRS ≥ 10) | (RRRS < 10) | ||
| N =13 | N = 52 | ||
| VO2 | 9 [6–11] | 11 [10–14] | 0.005 |
| VE/VCO2 | 48 [43–83] | 35 [28–43] | 0.0002 |
| PETCO2-b | 30 [27–34] | 34 [30–36] | 0.033 |
| PETCO2-ex | 25 [19–33] | 34 [30–38] | 0.0023 |
| Delta PETCO2(ex-b) | −3 [−7.7 to −0.9] | 0.9 [−4 to 2] | 0.0044 |
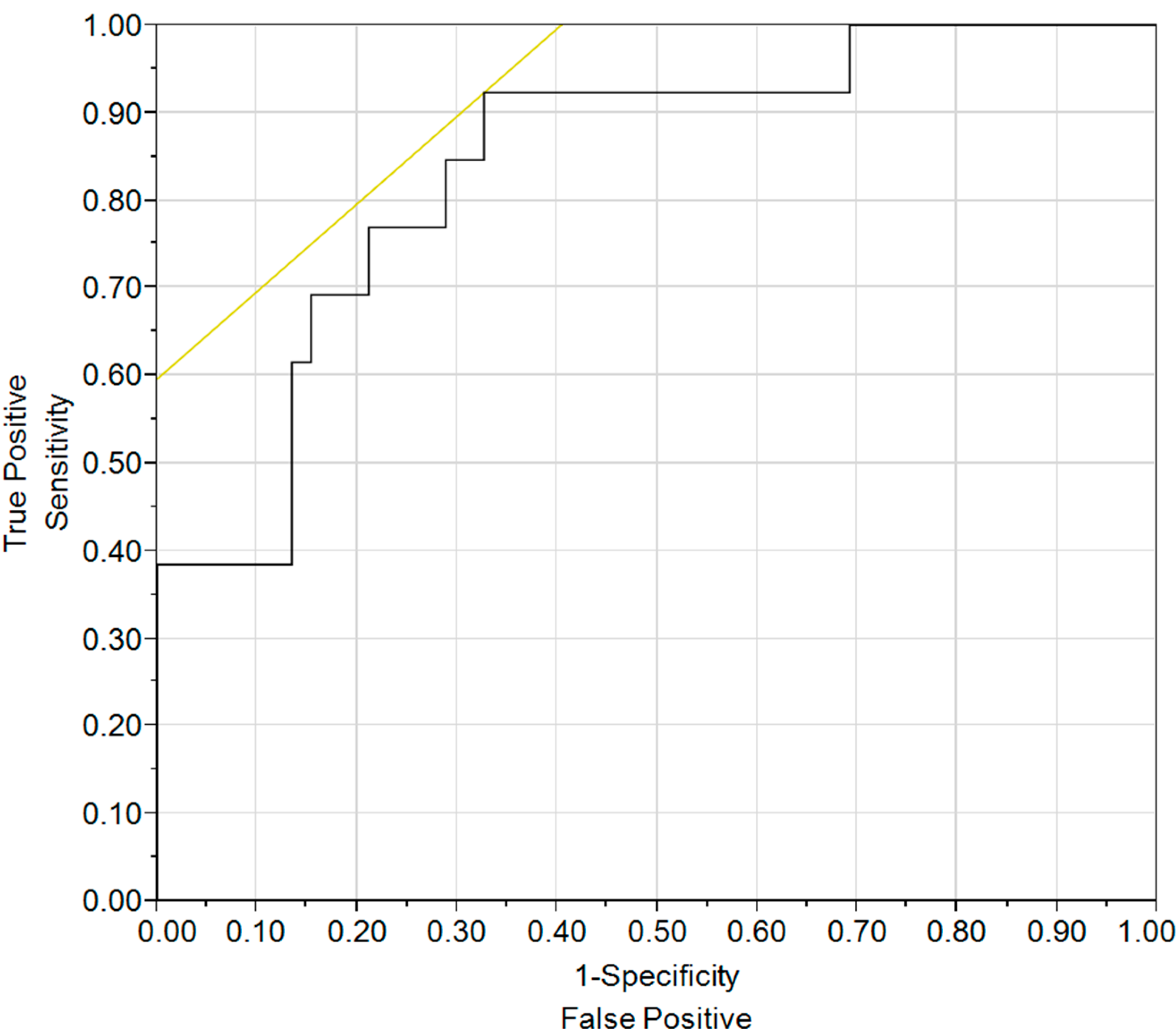
| VE/VCO2 | Positive | Negative | Positive | Negative |
|---|---|---|---|---|
| 29.9 | 0.27 | 1.00 | 1.44 | 0.00 |
| 40.6 | 0.41 | 0.97 | 2.82 | 0.11 |
| 42.7 | 0.42 | 0.93 | 2.86 | 0.32 |
3.2. Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliott, G.; et al. Predicting survival in pulmonary artery hypertension: Insights from the registry to evaluate early and long-term pulmonary artery disease management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar]
- Benza, R.L.; Gomberg-Maitland, M.; Miller, D.P.; Frost, A.; Frantz, R.P.; Foreman, A.J.; Badesch, D.B.; McGoon, M.D. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary artery hypertension. Chest 2012, 141, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kawut, S.M.; Taichman, D.B.; Ahya, V.N.; Kaplan, S.; Archer-Chicko, C.L.; Kimmel, S.E.; Palevsky, H.I. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transplant. 2005, 11, 1107–1111. [Google Scholar] [CrossRef]
- Mathai, S.C.; Hummers, L.K.; Champion, H.C.; Wigley, F.M.; Zaiman, A.; Hassoun, P.M.; Girgis, R.E. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum. 2009, 60, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Sanchez, M.A.G.; Kumar, R.K.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D34–D41. [Google Scholar]
- McLaughlin, V.V.; Gaine, S.P.; Howard, L.S.; Leuchte, H.H.; Mathier, M.A.; Mehta, S.; Palazzini, M.; Park, M.H.; Tapson, V.F.; Sitbon, O. Treatment Goals of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62, D73–D81. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Paolillo, S.; Costanzo, P.; D’Amore, C.; Cecere, M.; Losco, T.; Musella, F.; Gargiulo, P.; Marciano, C.; Perrone-Filardi, P. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J. Am. Coll. Cardiol. 2012, 60, 1192–1201. [Google Scholar] [CrossRef]
- Wensel, R.; Opitz, C.F.; Anker, S.D.; Winkler, J.; Höffken, G.; Kleber, F.X.; Sharma, R.; Hummel, M.; Hetzer, R.; Ewert, R. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation 2002, 106, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Groepenhoff, H.; Vonk-Noordegraaf, A.; Boonstra, A.; Spreeuwenberg, M.D.; Postmus, P.E.; Bogaard, H.J. Exercise testing to estimate survival in pulmonary hypertension. Med. Sci. Sport. Exer. 2008, 40, 1725–1732. [Google Scholar] [CrossRef]
- Deboeck, G.; Scoditti, C.; Huez, S.; Vachiéry, J.-L.; Lamotte, M.; Sharples, L.; Melot, C.; Naeije, R. Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur. Respir. J. 2012, 40, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Woods, P.R.; Frantz, R.P.; Taylor, B.J.; Olson, T.P.; Johnson, B.D. The usefulness of submaximal exercise gas exchange to define pulmonary arterial hypertension. J. Heart Lung Transplant. 2011, 30, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Yasunobu, Y.; Oudiz, R.J.; Sun, X.G.; Hansen, J.E.; Wasserman, K. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest 2005, 127, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Oudiz, R.J.; Midde, R.; Hovenesyan, A.; Sun, X.G.; Roveran, G.; Hansen, J.; Wasserman, K. Usefulness of right-to-left shunting and poor exercise gas exchange for predicting prognosis in patients with pulmonary arterial hypertension. Am. J. Cardiol. 2010, 105, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Hansen, J.E.; Oudiz, R.J.; Wasserman, K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation 2001, 104, 429–435. [Google Scholar] [CrossRef] [PubMed]
- M’elot, C.; Naeije, R. Pulmonary Vascular disease. Compr. Physiol. 2011, 1, 593–619. [Google Scholar] [PubMed]
- Neal, J.E.; Lee, A.S.; Burger, C.D. Submaximal exercise testing may be superior to the 6-min walk test in assessing pulmonary arterial hypertension disease severity. Clin. Respir. J. 2014, 8, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar]
- Arena, R.; Lavie, C.J.; Milani, R.V.; Myers, J.; Guazzi, M. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: An evidence-based review. J. Heart Lung Transplant. 2010, 29, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Thomson, S. The role of exercise testing in the modern management of pulmonary arterial hypertension. Diseases 2014, 2, 120–147. [Google Scholar] [CrossRef]
- Wensel, R.; Francis, D.P.; Meyer, F.J.; Opitz, C.F.; Bruch, L.; Halank, M.; Winkler, J.; Seyfarth, H.J.; Glaser, S.; Blumberg, F.; et al. Incremental prognostic value of cardiopulmonary exercise testing and resting haemodynamics in pulmonary arterial hypertension. Int. J. Cardiol. 2013, 167, 1193–1198. [Google Scholar]
- Groepenhoff, H.; Vonk-Noordegraaf, A.; van de Veerdonk, M.C.; Boonstra, A.; Westerhof, N.; Bogaard, H.J. Prognostic relevance of changes in exercise test variables in pulmonary arterial hypertension. PLoS One 2013, 8, e72013. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Hansen, J.; Sue, D.; Stringer, W.; Whipp, B. Principles of Exercise Testing and Interpretation; 4th ed.; Lippincott Williams & Wilkens: Baltimore, MD, USA, 2004. [Google Scholar]
- Schwaiblmair, M.; Faul, C.; von Scheidt, W.; Berghaus, T.M. Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulm. Med. 2012, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.M.; Taylor, A.L.; Ross, H.; Sillau, S.; Ivy, D.D. Ventilatory efficiency slope correlates with functional capacity, outcomes, and disease severity in pediatric patients with pulmonary hypertension. Int. J. Cardiol. 2013, 169, 445–448. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatri, V.; Neal, J.E.; Burger, C.D.; Lee, A.S. Prognostication in Pulmonary Arterial Hypertension with Submaximal Exercise Testing. Diseases 2015, 3, 15-23. https://doi.org/10.3390/diseases3010015
Khatri V, Neal JE, Burger CD, Lee AS. Prognostication in Pulmonary Arterial Hypertension with Submaximal Exercise Testing. Diseases. 2015; 3(1):15-23. https://doi.org/10.3390/diseases3010015
Chicago/Turabian StyleKhatri, Vinod, Jennifer E. Neal, Charles D. Burger, and Augustine S. Lee. 2015. "Prognostication in Pulmonary Arterial Hypertension with Submaximal Exercise Testing" Diseases 3, no. 1: 15-23. https://doi.org/10.3390/diseases3010015





