C-Reactive Protein Levels Predict Improvement in the Liver Functional Reserve by Long-Term Rifaximin Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Evaluations
2.3. Statistical Analyses
3. Results
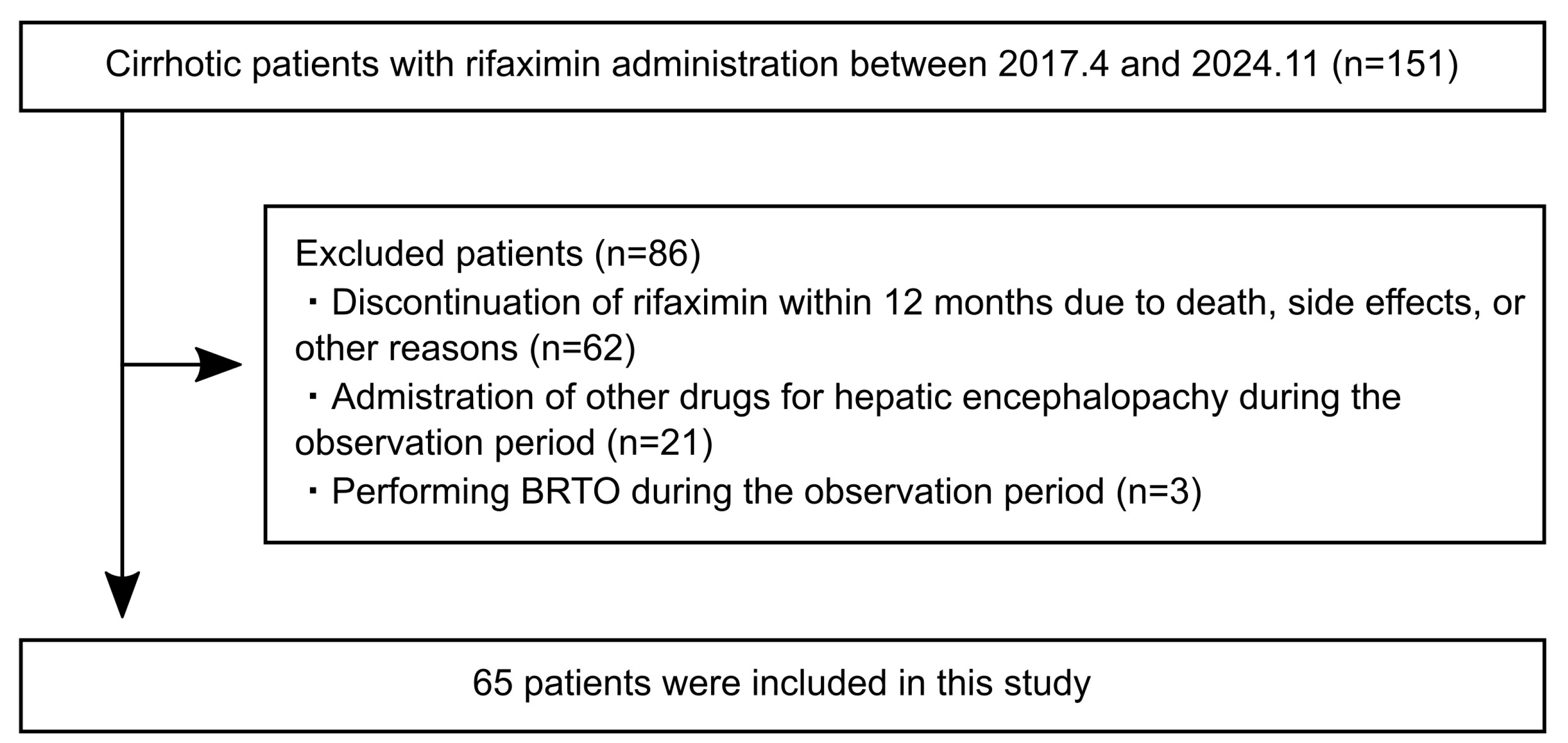
3.1. Patient Characteristics
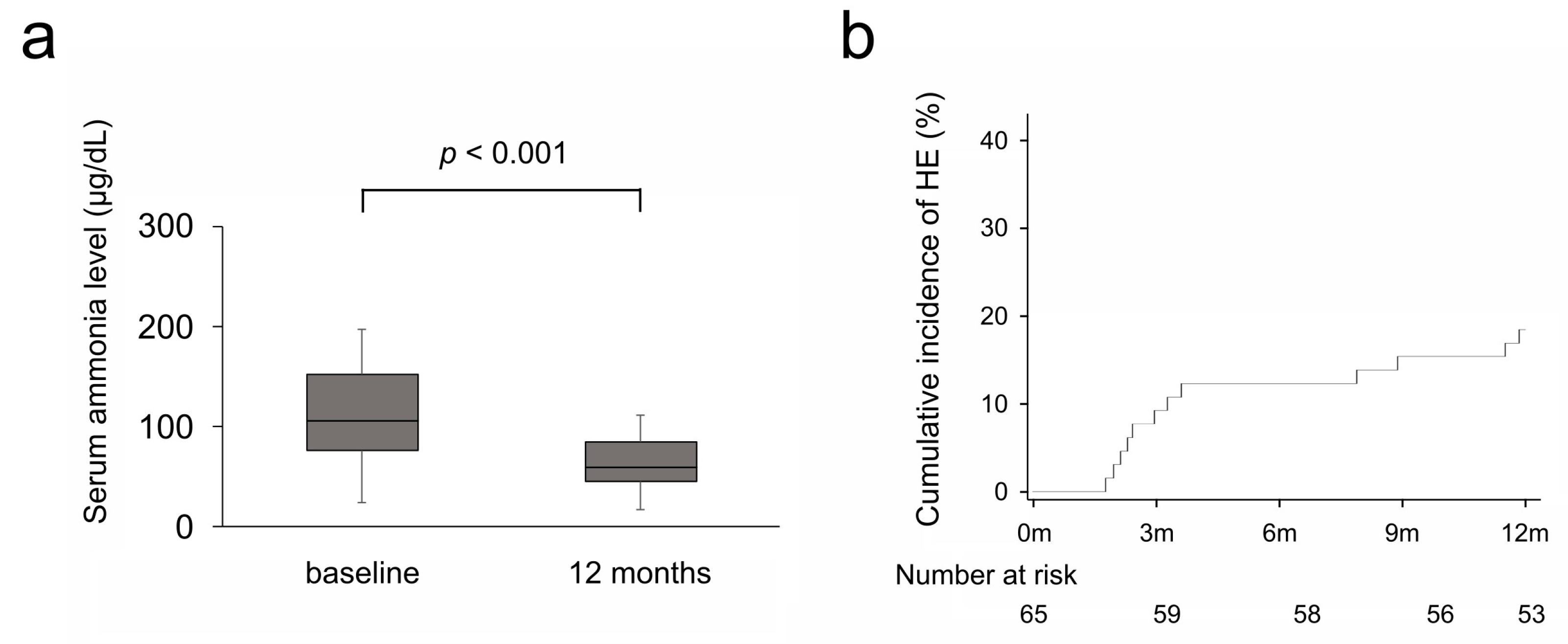
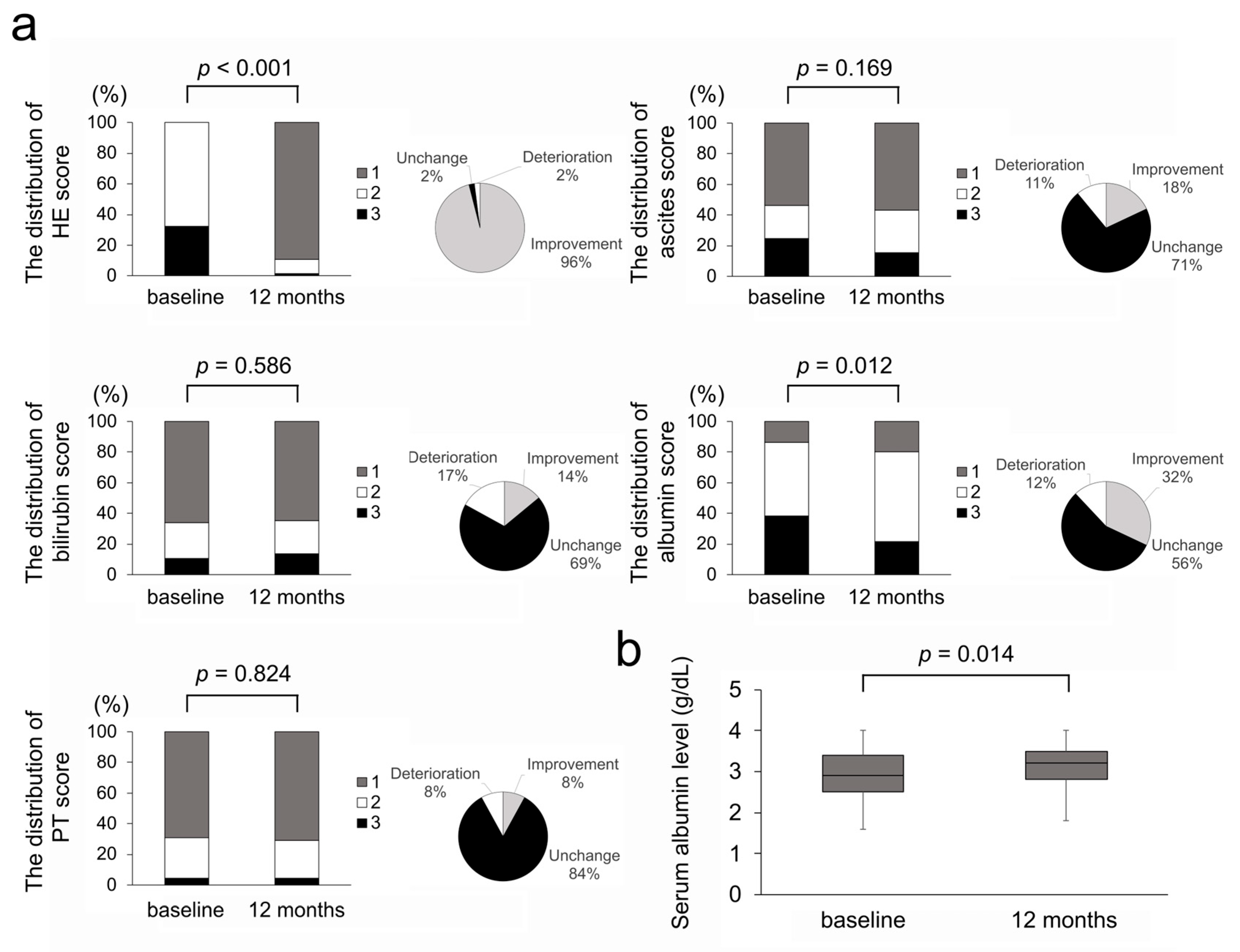
3.2. Efficacy on Serum Ammonia Levels and Hepatic Encephalopathy
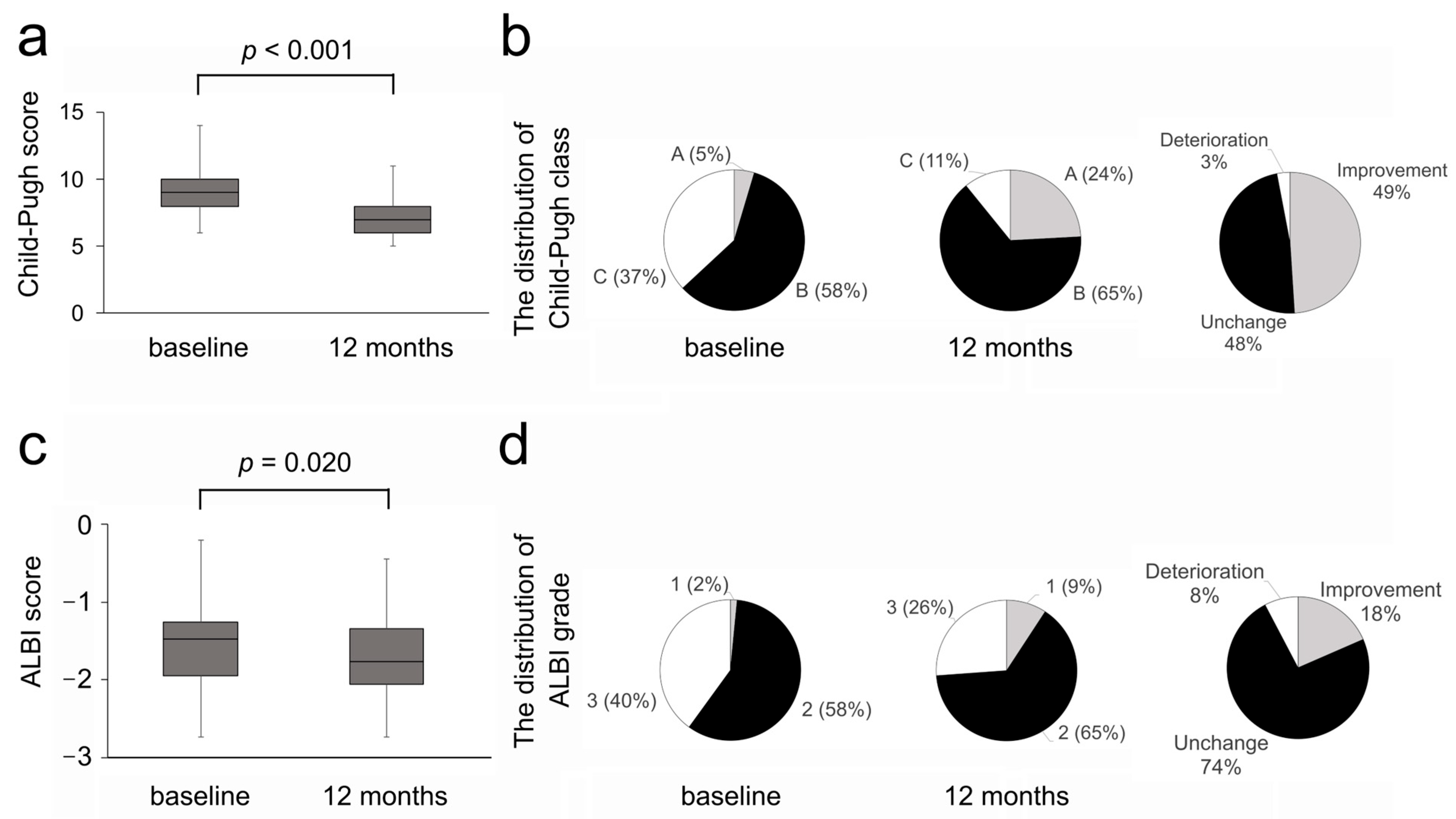
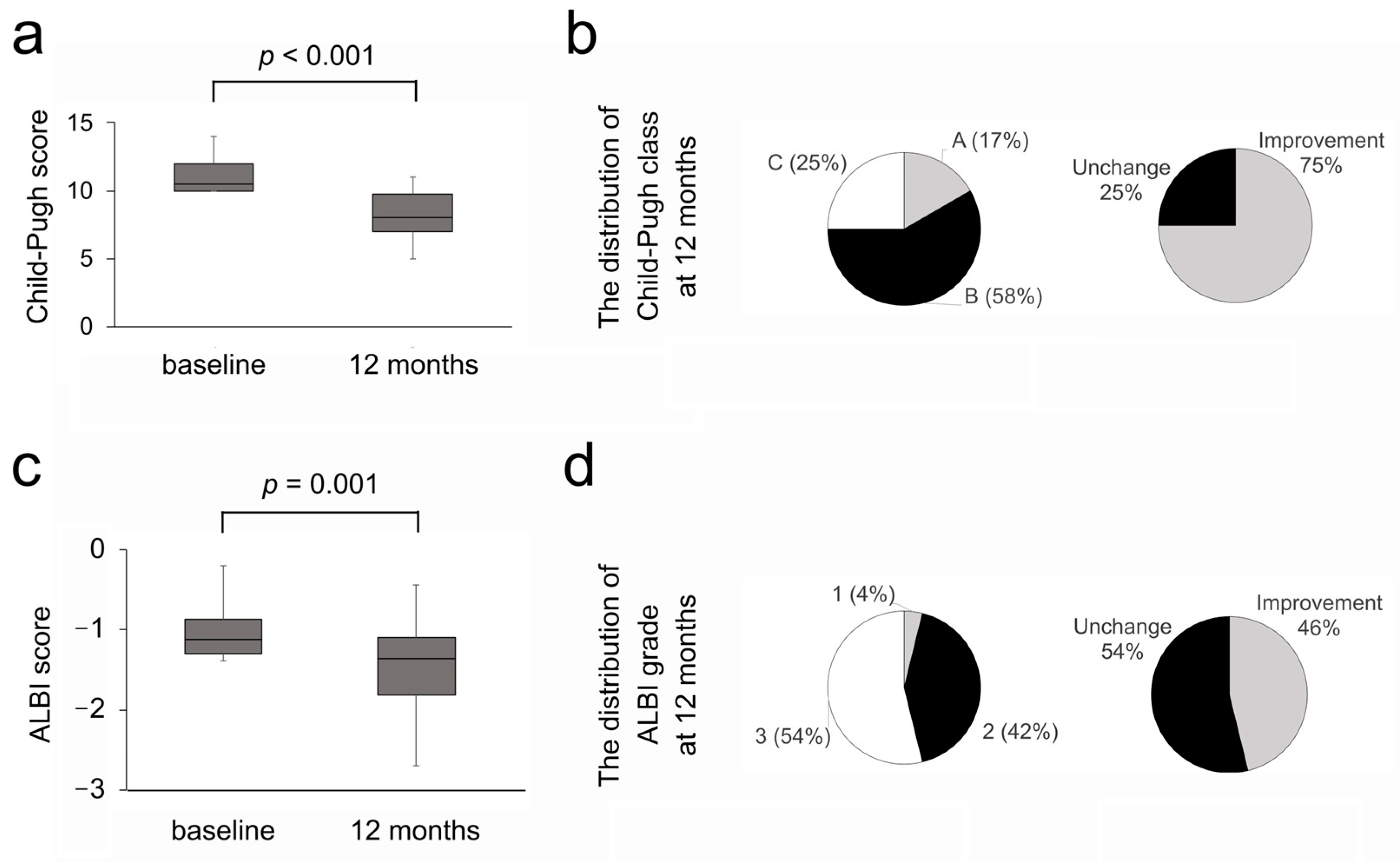
3.3. Efficacy on Liver Functional Reserve
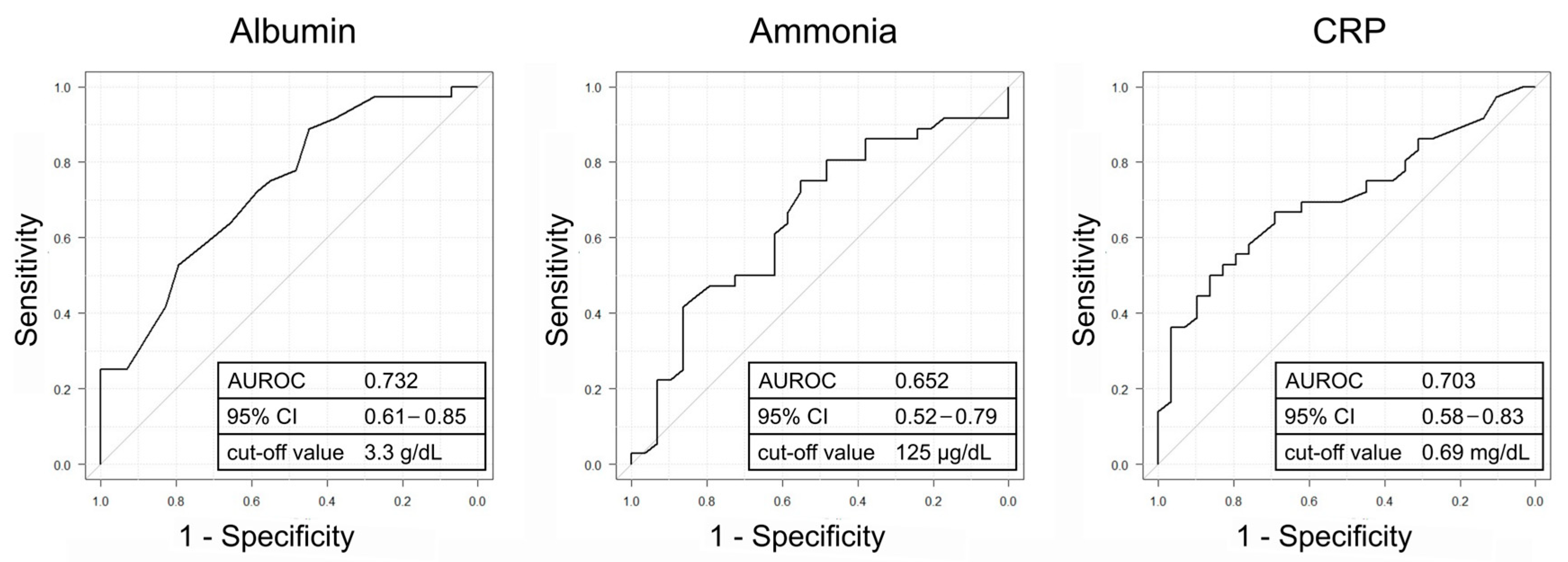
3.4. Investigation of the Predictive Factors for Improvement in the Liver Functional Reserve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Weissenborn, K. Hepatic Encephalopathy: Definition, Clinical Grading and Diagnostic Principles. Drugs 2019, 79 (Suppl. S1), 5–9. [Google Scholar] [CrossRef]
- Greinert, R.; Zipprich, A.; Simón-Talero, M.; Stangl, F.; Ludwig, C.; Wienke, A.; Praktiknjo, M.; Höhne, K.; Trebicka, J.; Genescà, J.; et al. Covert hepatic encephalopathy and spontaneous portosystemic shunts increase the risk of developing overt hepatic encephalopathy. Liver Int. 2020, 40, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Shayto, R.H.; Abou Mrad, R.; Sharara, A.I. Use of rifaximin in gastrointestinal and liver diseases. World J. Gastroenterol. 2016, 22, 6638–6651. [Google Scholar] [CrossRef]
- Yoshiji, H.; Nagoshi, S.; Akahane, T.; Asaoka, Y.; Ueno, Y.; Ogawa, K.; Kawaguchi, T.; Kurosaki, M.; Sakaida, I.; Shimizu, M.; et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J. Gastroenterol. 2021, 56, 593–619. [Google Scholar] [CrossRef]
- American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J. Hepatol. 2014, 61, 642–659. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Gerardi, V.; Pecere, S.; D’aversa, F.; Lopetuso, L.; Zocco, M.A.; Pompili, M.; Gasbarrini, A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J. Gastroenterol. 2015, 21, 12322–12333. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannakos, J.; Viazis, N.; Vasianopoulou, P.; Vafiadis, I.; Karamanolis, D.G.; Ladas, S.D. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J. Gastroenterol Hepatol. 2013, 28, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kimer, N.; Krag, A.; Møller, S.; Bendtsen, F.; Gluud, L.L. Systematic review with meta-analysis: The effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol. Ther. 2014, 40, 123–132. [Google Scholar] [CrossRef]
- Girón-González, J.A.; Martínez-Sierra, C.; Rodriguez-Ramos, C.; Macías, M.A.; Rendón, P.; Díaz, F.; Fernández-Gutiérrez, C.; Martín-Herrera, L. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004, 24, 437–445. [Google Scholar] [CrossRef]
- Thabut, D.; Massard, J.; Gangloff, A.; Carbonell, N.; Francoz, C.; Nguyen-Khac, E.; Duhamel, C.; Lebrec, D.; Poynard, T.; Moreau, R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology 2007, 46, 1872–1882. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Rama Rao, K.V.; Norenberg, M.D. Neuroinflammation in hepatic encephalopathy: Mechanistic aspects. J. Clin. Exp. Hepatol. 2015, 5 (Suppl. S1), S21–S28. [Google Scholar] [CrossRef]
- Cervoni, J.-P.; Thévenot, T.; Weil, D.; Muel, E.; Barbot, O.; Sheppard, F.; Monnet, E.; Di Martino, V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J. Hepatol. 2012, 56, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Biyik, M.; Ucar, R.; Solak, Y.; Gungor, G.; Polat, I.; Gaipov, A.; Cakir, O.O.; Ataseven, H.; Demir, A.; Turk, S.; et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Zhu, B.; Boyechko, T.; Westgate, P.M.; Chia, C.W.; Egan, J.M.; Kern, P.A. Effect of Rifaximin Treatment on Endotoxemia and Insulin Sensitivity in Humans. J. Endocr. Soc. 2019, 3, 1641–1651. [Google Scholar] [CrossRef]
- Kitsugi, K.; Kawata, K.; Noritake, H.; Chida, T.; Ohta, K.; Ito, J.; Takatori, S.; Yamashita, M.; Hanaoka, T.; Umemura, M.; et al. Rifaximin Improves Liver Functional Reserve by Regulating Systemic Inflammation. J. Clin. Med. 2023, 12, 2210. [Google Scholar] [CrossRef]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy—Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Demirtas, C.O.; D’Alessio, A.; Rimassa, L.; Sharma, R.; Pinato, D.J. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021, 3, 100347. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Infante-Rivard, C.; Esnaola, S.; Villeneuve, J.P. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology 1987, 7, 660–664. [Google Scholar] [CrossRef]
- Chen, T.-A.; Tsao, Y.-C.; Chen, A.; Lo, G.-H.; Lin, C.-K.; Yu, H.-C.; Cheng, L.-C.; Hsu, P.-I.; Tsai, W.-L. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand. J. Gastroenterol. 2009, 44, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kawaratani, H.; Tsujimoto, T.; Douhara, A.; Takaya, H.; Moriya, K.; Namisaki, T.; Noguchi, R.; Yoshiji, H.; Fujimoto, M.; Fukui, H. The effect of inflammatory cytokines in alcoholic liver disease. Mediat. Inflamm. 2013, 2013, 495156. [Google Scholar] [CrossRef]
- Kalambokis, G.N.; Tsianos, E.V. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology. 2012, 55, 655–656. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef]
- Ha, Y.E.; Kang, C.-I.; Joo, E.-J.; Joung, M.-K.; Chung, D.R.; Peck, K.R.; Lee, N.Y.; Song, J.-H. Usefulness of C-reactive protein for evaluating clinical outcomes in cirrhotic patients with bacteremia. Korean J. Intern. Med. 2011, 26, 195–200. [Google Scholar] [CrossRef]
- Di Martino, V.; Coutris, C.; Cervoni, J.; Dritsas, S.; Weil, D.; Richou, C.; Vanlemmens, C.; Thevenot, T. Prognostic value of C-reactive protein levels in patients with cirrhosis. Liver Transpl. 2015, 21, 753–760. [Google Scholar] [CrossRef]
- Luo, M.; Guo, J.Y.; Cao, W.K. Inflammation: A novel target of current therapies for hepatic encephalopathy in liver cirrhosis. World J. Gastroenterol. 2015, 21, 11815–11824. [Google Scholar] [CrossRef]
- Dondeti, M.F.; El-Maadawy, E.A.; Talaat, R.M. Hepatitis-related hepatocellular carcinoma: Insights into cytokine gene polymorphisms. World J. Gastroenterol. 2016, 22, 6800–6816. [Google Scholar] [CrossRef]
- Ma, X.; Shah, Y.M.; Guo, G.L.; Wang, T.; Krausz, K.W.; Idle, J.R.; Gonzalez, F.J. Rifaximin is a gut-specific human pregnane X receptor activator. J. Pharmacol. Exp. Ther. 2007, 322, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.-A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef]
- Lan, T.; Chen, M.; Tang, C.; Deltenre, P. Recent developments in the management of ascites in cirrhosis. United Eur. Gastroenterol. J. 2024, 12, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Kawaratani, H.; Kondo, Y.; Tatsumi, R.; Kawabe, N.; Tanabe, N.; Sakamaki, A.; Okumoto, K.; Uchida, Y.; Endo, K.; Kawaguchi, T.; et al. Long-Term Efficacy and Safety of Rifaximin in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study. J. Clin. Med. 2022, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.Y.; Ding, H.G.; Zheng, J.F.; Fan, C.L.; Li, L. Rifaximin improves survival in cirrhotic patients with refractory ascites: A real-world study. World J. Gastroenterol. 2020, 26, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Kaji, K.; Saikawa, S.; Takaya, H.; Fujinaga, Y.; Furukawa, M.; Kitagawa, K.; Ozutsumi, T.; Kaya, D.; Tsuji, Y.; Sawada, Y.; et al. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics 2020, 9, 145. [Google Scholar] [CrossRef]
- Oikonomou, T.; Goulis, I.; Kiapidou, S.; Tagkou, N.; Akriviadis, E.; Papatheodoridis, G.; Cholongitas, E. The significance of C-reactive protein to albumin ratio in patients with decompensated cirrhosis. Ann. Gastroenterol. 2020, 33, 667–674. [Google Scholar] [CrossRef]
| Variable | Results |
|---|---|
| Age [years] | 69 (60–76) |
| Sex | |
| Male gender, n (%) | 38 (59) |
| Female gender, n (%) | 27 (41) |
| Etiology, n (%) | |
| Alcohol | 27 (42) |
| MASH | 14 (21) |
| HCV | 11 (17) |
| Autoimmune | 8 (12) |
| Cryptogenic | 3 (5) |
| HBV | 2 (3) |
| Complication, n (%) | |
| Ascites | 30 (46) |
| Esophagogastric varices | 24 (37) |
| HCC | 17 (26) |
| Concomitant drug *, n (%) | |
| BCAA | 58 (89) |
| Lactulose or Lactitol | 42 (65) |
| Levocarnitine | 28 (43) |
| Zinc preparation | 7 (11) |
| Without concomitant drug | 2 (3) |
| Liver functional reserve | |
| Child–Pugh score | 9 (8–10) |
| Child–Pugh class A:B:C, n (%) | 3:38:24 (5:58:37) |
| ALBI score | −1.47 (−1.95, −1.26) |
| ALBI grade 1:2:3, n (%) | 1:38:26 (2:58:40) |
| Laboratory data | |
| Total bilirubin [mg/dL] | 1.6 (1.1–2.3) |
| Prothrombin time [%] | 67 (52–78) |
| Albumin [g/dL] | 2.9 (2.5–3.4) |
| Ammonia [μg/dL] | 106 (76–152) |
| WBC [/μL] | 4695 (3462–6035) |
| CRP [mg/dL] | 0.36 (0.16–1.01) |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Variable | Improvement Group (n = 36) | Non-Improvement Group (n = 29) | p-Value | Improvement Group (n = 21) | Non-Improvement Group (n = 21) | p-Value |
| Age [years] | 65 (60–75) | 75 (67–78) | 0.074 | 71 (64–76) | 71 (65–76) | 0.980 |
| Sex | 0.800 | 1.000 | ||||
| Male gender, n (%) | 22 (61) | 16 (55) | 12 (57) | 12 (57) | ||
| Female gender, n (%) | 14 (39) | 13 (45) | 9 (43) | 9 (43) | ||
| Etiology, n (%) | ||||||
| Alcohol | 17 (47) | 10 (35) | 0.324 | 9 (43) | 9 (43) | 1.000 |
| MASH | 6 (17) | 8 (27) | 0.367 | 4 (19) | 6 (29) | 0.719 |
| HCV | 8 (22) | 3 (10) | 0.320 | 7 (34) | 1 (4) | 0.045 |
| Autoimmune | 4 (11) | 4 (14) | 1.000 | 1 (4) | 2 (10) | 1.000 |
| Cryptogenic | 1 (3) | 2 (7) | 0.582 | 0 (0) | 2 (10) | 1.000 |
| HBV | 0 (0) | 2 (7) | 0.195 | 0 (0) | 1 (4) | 1.000 |
| Complication, n (%) | ||||||
| Ascites | 22 (61) | 8 (28) | 0.012 | 14 (67) | 6 (29) | 0.029 |
| Esophagogastric varices | 15 (42) | 9 (31) | 0.444 | 9 (43) | 6 (29) | 0.520 |
| HCC | 5 (14) | 12 (41) | 0.022 | 4 (19) | 8 (38) | 0.306 |
| Concomitant drug *, n (%) | ||||||
| BCAA | 31 (86) | 27 (93) | 0.447 | 18 (86) | 19 (91) | 1.000 |
| Lactulose or Lactitol | 20 (56) | 22 (76) | 0.119 | 15 (71) | 16 (76) | 1.000 |
| Levocarnitine | 13 (36) | 15 (52) | 0.221 | 12 (57) | 10 (48) | 0.758 |
| Zinc preparation | 5 (14) | 2 (7) | 0.447 | 3 (14) | 1 (5) | 0.606 |
| Without concomitant drug | 2 (6) | 0 (0) | 0.498 | 1 (5) | 0 (0) | 1.000 |
| Laboratory data | ||||||
| Total bilirubin [mg/dL] | 1.6 (1.1–2.3) | 1.7 (1.0–2.1) | 0.947 | 1.5 (0.9–2.4) | 1.7 (0.9–2.1) | 0.696 |
| Prothrombin time [%] | 66 (50–77) | 68 (58–79) | 0.417 | 62 (50–77) | 68 (57–79) | 0.428 |
| Albumin [g/dL] | 2.7 (2.4–3.1) | 3.2 (2.8–3.6) | 0.001 | 2.6 (2.2–3.0) | 3.2 (2.8–3.6) | 0.004 |
| Ammonia [μg/dL] | 98 (74–127) | 127 (92–161) | 0.036 | 90 (69–122) | 127 (92–198) | 0.029 |
| WBC [/μL] | 4830 (3065–6205) | 4680 (3640–5550) | 0.941 | 4620 (3110–5230) | 4380 (3620–5040) | 0.920 |
| CRP [mg/dL] | 0.62 (0.22–1.73) | 0.25 (0.10–0.41) | 0.005 | 0.69 (0.25–2.00) | 0.25 (0.10–0.44) | 0.011 |
| Variable | Categories | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Age [years] | <65 vs. ≥65 | 3.83 | 1.26–11.60 | 0.018 | 3.51 | 0.82–15.00 | 0.091 |
| Sex | Male vs. female | 1.28 | 0.47–3.44 | 0.629 | |||
| Etiology | |||||||
| Alcohol vs. others | 1.70 | 0.62–4.65 | 0.302 | ||||
| MASH vs. others | 0.53 | 0.16–1.74 | 0.291 | ||||
| HCV vs. others | 2.48 | 0.59–10.40 | 0.214 | ||||
| Autoimmune vs. others | 0.78 | 0.18–3.44 | 0.744 | ||||
| Complication | |||||||
| Ascites | Presence vs. absence | 4.12 | 1.44–11.80 | 0.009 | 1.47 | 0.39–5.57 | 0.569 |
| Esophagogastric varices | Presence vs. absence | 1.59 | 0.57–4.44 | 0.379 | |||
| HCC | Presence vs. absence | 0.23 | 0.07–0.76 | 0.016 | 0.18 | 0.03–1.12 | 0.066 |
| Concomitant drug at baseline | |||||||
| BCAA | With vs. without | 0.46 | 0.08–2.56 | 0.375 | |||
| Lactulose or Lactitol | With vs. without | 0.40 | 0.14–1.17 | 0.093 | |||
| Levocarnitine | With vs. without | 0.53 | 0.20–1.43 | 0.208 | |||
| Zinc preparation | With vs. without | 2.18 | 0.39–12.10 | 0.375 | |||
| Laboratory data | |||||||
| Total bilirubin [mg/dL] | <1.6 vs. ≥1.6 | 1.64 | 0.61–4.42 | 0.332 | |||
| Prothrombin time [%] | >67 vs. ≤67 | 0.84 | 0.31–2.22 | 0.718 | |||
| Albumin [g/dL] | <3.3 vs. ≥3.3 | 3.27 | 1.12–9.54 | 0.030 | 3.34 | 0.86–13.00 | 0.082 |
| Ammonia [μg/dL] | <125 vs. ≥125 | 3.20 | 1.14–8.99 | 0.027 | 2.32 | 0.62–8.75 | 0.213 |
| WBC [/μL] | <4695 vs. ≥4695 | 0.88 | 0.33–2.36 | 0.802 | |||
| CRP [mg/dL] | >0.69 vs. ≤0.69 | 5.00 | 1.44–17.30 | 0.011 | 8.90 | 1.45–54.50 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitsugi, K.; Kawata, K.; Murohisa, G.; Yoshizawa, Y.; Kimata, M.; Kobayashi, Y.; Unno, S.; Noritake, H.; Chida, T.; Hosoda, Y. C-Reactive Protein Levels Predict Improvement in the Liver Functional Reserve by Long-Term Rifaximin Treatment. Diseases 2025, 13, 331. https://doi.org/10.3390/diseases13100331
Kitsugi K, Kawata K, Murohisa G, Yoshizawa Y, Kimata M, Kobayashi Y, Unno S, Noritake H, Chida T, Hosoda Y. C-Reactive Protein Levels Predict Improvement in the Liver Functional Reserve by Long-Term Rifaximin Treatment. Diseases. 2025; 13(10):331. https://doi.org/10.3390/diseases13100331
Chicago/Turabian StyleKitsugi, Kensuke, Kazuhito Kawata, Go Murohisa, Yashiro Yoshizawa, Masaharu Kimata, Yosuke Kobayashi, Shuhei Unno, Hidenao Noritake, Takeshi Chida, and Yoshisuke Hosoda. 2025. "C-Reactive Protein Levels Predict Improvement in the Liver Functional Reserve by Long-Term Rifaximin Treatment" Diseases 13, no. 10: 331. https://doi.org/10.3390/diseases13100331
APA StyleKitsugi, K., Kawata, K., Murohisa, G., Yoshizawa, Y., Kimata, M., Kobayashi, Y., Unno, S., Noritake, H., Chida, T., & Hosoda, Y. (2025). C-Reactive Protein Levels Predict Improvement in the Liver Functional Reserve by Long-Term Rifaximin Treatment. Diseases, 13(10), 331. https://doi.org/10.3390/diseases13100331






