Implementation of Constant Temperature–Constant Voltage Charging Method with Energy Loss Minimization for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Lithium-Ion Battery Model
2.1. Lithium-Ion Battery Equivalent Circuit Model (ECM)
2.2. ECM Characteristics
2.2.1. Introduction to the Selected Battery
2.2.2. AC Impedance Analysis
2.2.3. Constant Potential Detection for AC Impedance Analysis
2.2.4. Experimental for AC Impedance Analysis
2.2.5. AC Impedance Data Analysis
- (1)
- Choose the data presentation method for the measurement, select “Nyquist Impedance” for the Nyquist plot, and choose “z cycle” to show Nyquist plots at various remaining capacity levels.
- (2)
- Z-Fit function is found in “Analysis” option under “Electrochemical Impedance Spectroscopy” within the EC-Lab interface. For this experiment, the chosen model is the ECM. Therefore, select the equivalent model composed of the internal resistance R1, polarization resistance R2, and polarization capacitance C2 for parameter fitting.
- (3)
- The analysis of AC impedance is carried out for each 1% increment in remaining capacity. Begin by selecting the Nyquist plots corresponding to the SOC aspired and subsequently choose the fitting range.
- (4)
- After selecting the fitting range, initiate the curve fitting process by clicking “Minimize” and “Calculate,” which will yield the parameters of the fitted curve.
- (5)
- Once the fitted curve parameters are obtained, record them for further analysis.
2.3. AC Impedance Characteristics and Fitting
- (1)
- Within the Curve Fitter application, proceed to the Curve Fitter tab and access the Data section. Click on “Select Data”. In the ensuing “Select Fitting Data” dialog box, designate the X and Y data values.
- (2)
- Navigate to the Curve Fitter tab, and within the Fit Type section, click the arrow to reveal the gallery. Within the fit gallery, choose Custom Equation from the Custom group.
- (3)
- In the Fit Options pane, replace the placeholder text in the equation edit box. “The Gaussian coefficients have a straightforward interpretation, and the exponential background is well-defined”.
- In the Fit Options pane, proceed to Advanced Options.
- In the Coefficient Constraints table within the Advanced Options, adjust the Lower bound to 0, recognizing that peak amplitudes and widths cannot be negative.
- Input the StartPoint values as indicated for the specified coefficients.
3. Implementation of Constant Temperature–Constant Voltage Charging for Loss Minimization
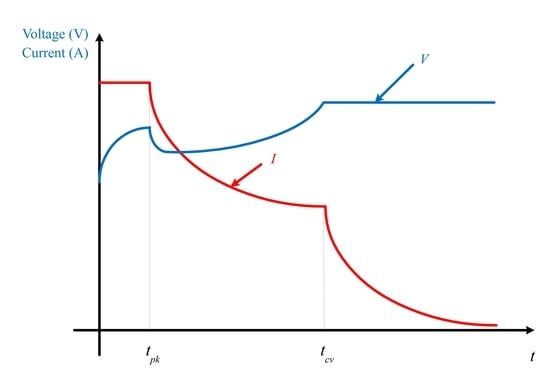
3.1. CC-CV Charging Method
3.2. CT-CV Charging Method
3.3. The Derivation of the CT-CV Charging Technique Formula
3.4. Fitness Evaluation
4. Simulation and Experimental Results
4.1. Experimental Setup
4.2. Simulation Results for Minimizing Charging Loss
4.3. Experimental Results
4.3.1. Comparison of Loss-Minimization CT-CV with Different Transition Times
4.3.2. Comparison of Different PID Parameters
4.3.3. Comparison between Loss-Minimization CT-CV and Various Cases
5. Conclusions
- This paper considers the equivalent impedance of the battery at different remaining capacities, enabling accurate estimation of CL.
- The method identifies the optimal transition time.
- It allows for the incorporation of other charging constraints for further optimization analysis.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hemavathi, S.; Shinisha, A. A study on trends and developments in electric vehicle charging technologies. J. Energy Storage 2022, 52, 105013. [Google Scholar] [CrossRef]
- Jin, X. Aging-Aware optimal charging strategy for lithium-ion batteries: Considering aging status and electro-thermal-aging dynamics. Electrochim. Acta 2022, 407, 139651. [Google Scholar] [CrossRef]
- Wassiliadis, N.; Schneider, J.; Frank, A.; Wildfeuer, L.; Lin, X.; Jossen, A.; Lienkamp, M. Review of fast charging strategies for lithium-ion battery systems and their applicability for battery electric vehicles. J. Energy Storage 2021, 44, 103306. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Xiong, R.; Shen, W.; He, H. Towards a smarter battery management system: A critical review on optimal charging methods of lithium ion batteries. Energy 2019, 183, 220–234. [Google Scholar] [CrossRef]
- Shahjalal, M.; Roy, P.K.; Shams, T.; Fly, A.; Chowdhury, J.I.; Ahmed, M.R.; Liu, K. A review on second-life of Li-ion batteries: Prospects, challenges, and issues. Energy 2022, 241, 122881. [Google Scholar] [CrossRef]
- Chen, L.-R. PLL-Based Battery Charge Circuit Topology. IEEE Trans. Ind. Electron. 2004, 51, 1344–1346. [Google Scholar] [CrossRef]
- Chen, L.-R.; Chen, J.-J.; Chu, N.-Y.; Han, G.-Y. Current pumped battery charger. IEEE Trans. Ind. Electron. 2008, 55, 2483–2488. [Google Scholar]
- Hsieh, G.-C.; Chen, L.-R.; Huang, K.-S. Fuzzy controlled Lithium-Ion Battery Charge System with Active State of Charge Controller. IEEE Trans. Ind. Electron. 2001, 48, 585–593. [Google Scholar] [CrossRef]
- Liu, H.; Naqvi, I.H.; Li, F.; Liu, C.; Shafiei, N.; Li, Y.; Pecht, M. An analytical model for the CC-CV charge of Li-ion batteries with application to degradation analysis. J. Energy Storage 2020, 29, 101342. [Google Scholar] [CrossRef]
- Chen, L.R.; Hsu, R.C.; Liu, C.S. A Design of A Grey-Predicted Lithium-Ion Battery Charge System. IEEE Trans. Ind. Electron. 2004, 51, 3692–3701. [Google Scholar]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. The Effects of Pulse Charging on Cycling Characteristics of Commercial Lithium-Ion Batteries. J. Power Sources 2001, 102, 302–309. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Notten, P.H.L. Effect of Current Pulses on Lithium Intercalation Batteries. Solid State Ion. 2002, 148, 259–268. [Google Scholar] [CrossRef]
- Chen, L.-R. A design of an optimal battery pulse charge system by frequency-varied technique. IEEE Trans. Ind. Electron. 2007, 54, 398–405. [Google Scholar] [CrossRef]
- Chen, L.-R. A design of Duty-Varied Voltage Pulse Charger for Improving Lithium-Ion Battery-Charging Response. IEEE Trans. Ind. Electron. 2009, 56, 480–487. [Google Scholar] [CrossRef]
- Niroshana, S.I.; Sirisukprasert, S. Adaptive Pulse Charger For Li-Ion Batteries. In Proceedings of the 8th International Conference of Information and Communication Technology for Embedded Systems (IC-ICTES), Chonburi, Thailand, 7–9 May 2017. [Google Scholar]
- Amanor-Boadu, J.M.; Guiseppi-Elie, A.; Sánchez-Sinencio, E. Search for Optimal Pulse Charging Parameters for Li-Ion Polymer Batteries Using Taguchi Orthogonal Arrays. IEEE Trans. Ind. Electron. 2018, 65, 8982–8992. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Hsieh, C.-H.; Luo, Y.-F. Search for an Optimal Five-Step Charging Pattern for Li-Ion Batteries Using Consecutive Orthogonal Arrays. IEEE Trans. Energy Convers. 2011, 26, 654–661. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Luo, Y.-F. Search for an optimal rapid charging pattern for Li-ion batteries using Taguchi approach. IEEE Trans. Ind. Electron. 2010, 57, 3963–3971. [Google Scholar] [CrossRef]
- Vo, T.T.; Chen, X.; Shen, W.; Kapoor, A. New charging strategy for Li-ion batteries based on the integration of Taguchi method and state of charge estimation. J. Power Sources 2015, 273, 413–422. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Teng, J.-H.; Lin, Y.-C. Search for an optimal rapid charging pattern for Li-ion batteries using ant colony system algorithm. IEEE Trans. Ind. Electron. 2005, 52, 1328–1336. [Google Scholar] [CrossRef]
- Wang, S.-C.; Liu, Y.-H. A PSO-based fuzzy-controlled searching for the optimal charge pattern of Li-ion batteries. IEEE Trans. Ind. Electron. 2015, 62, 2983–2993. [Google Scholar] [CrossRef]
- Khan, A.B.; Choi, W. Optimal Charge Pattern for the High-Performance Multistage Constant Current Charge Method for the Li-Ion Batteries. IEEE Trans. Energy Convers. 2018, 33, 1132–1140. [Google Scholar] [CrossRef]
- Dung, L.-R.; Yen, J.-H. ILP-based algorithm for Lithium-ion battery charging profile. In Proceedings of the 2010 IEEE International Symposium on Industrial Electronics, Bari, Italy, 4–7 July 2010. [Google Scholar]
- Lee, C.-H.; Chang, T.-W.; Hsu, S.-H.; Jiang, J.-A. Taguchi-based PSO for searching an optimal four-stage charge pattern of Li-ion batteries. J. Energy Storage 2018, 21, 301–309. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, M.-Y.; Hsu, S.-H.; Jiang, J.-A. Implementation of an SOC-based four-stage constant current charger for Li-ion batteries. J. Power Sources 2018, 18, 528–537. [Google Scholar] [CrossRef]
- Patnaik, L.; Praneeth, A.V.J.S.; Williamson, S.S. A Closed-Loop Constant-Temperature Constant-Voltage Charging Technique to Reduce Charge Time of Lithium-Ion Batteries. IEEE Trans. Ind. Electron. 2018, 66, 1059–1067. [Google Scholar] [CrossRef]
- Molicel INR-18650-P28A Lithium Ion Battery Specification. Available online: https://www.molicel.com/wp-content/uploads/INR18650P28A-V1-80093.pdf (accessed on 15 July 2019).
























| Molicel INR-18650-P28A Lithium-Ion Battery | |
|---|---|
| Related Capacity | 2800 mAh |
| Minimum Related capacity | 2600 mAh |
| Related Voltage | 3.6 V |
| Standard Charging | CC-CV, 2800 mA |
| Weight | 46 g |
| Suitable Temperature for Charge | 0 °C to 60 °C |
| Suitable Temperature for Discharging | −40 °C to 60 °C |
| a1 | 0.08086 | b1 | −0.6111 | c1 | 0.4565 |
| a2 | 0.07118 | b2 | 9.659 | c2 | 11.19 |
| a3 | 0.0008875 | b3 | 0.2467 | c3 | 0.124 |
| a4 | 0.001624 | b4 | 0.447 | c4 | 0.2168 |
| Charging Time (CT) | Charging Loss (CL) | Complexity | |
|---|---|---|---|
| CC-CV method | Long | High | Low |
| CT-CV method | Short | Low | High |
| C-Rate | CT (s) | CL (J) |
|---|---|---|
| 0.5 | 9062 | 566.2 |
| 4 | 3414 | 3540.5 |
| Transition Times (s) | CT (s) | Average TR (°C) | Max TR (°C) | Charging Efficiency (%) | Fitness |
|---|---|---|---|---|---|
| 267 | 5100 | 2.48 | 3.91 | 98.60 | 0.2551 |
| 282 | 4944 | 2.49 | 3.91 | 99.34 | 0.2535 |
| 296 | 4942 | 2.49 | 4.09 | 98.88 | 0.2547 |
| PID Parameter | Maximum Overshoot (°C) | Time Required for Temperature Stabilization (s) |
|---|---|---|
| kp = 8, ki = 0.005, kd = 0.1 | 0.00 | 280 |
| kp = 6, ki = 0.01, kd = 0.1 | 0.18 | 366 |
| kp = 4, ki = 0.015, kd = 0.1 | 0.18 | 783 |
| Charging Method | CT (s) | Average TR (°C) | Max TR (°C) | Charging Efficiency (%) | Fitness |
|---|---|---|---|---|---|
| Loss optimization CT-CV | 4944 | 2.49 | 3.91 | 99.34 | 0.2535 |
| 28.9 degrees fundamental CT-CV | 4868 (−1.56%) | 2.83 (+12.01%) | 5.20 (+24.81%) | 98.71 | 0.2638 |
| Same CT CC-CV (1.40C CC-CV) | 4926 (−0.37%) | 3.48 (+28.45%) | 5.39 (+27.46%) | 99.32 | 0.2828 |
| Same average TR CC-CV (1.21C CC-CV) | 6077 (+18.64%) | 2.49 (+0.00%) | 4.83 (+19.05%) | 99.48 | 0.3648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-J.; Liu, C.-L.; Liu, Y.-H.; Wang, J.-J. Implementation of Constant Temperature–Constant Voltage Charging Method with Energy Loss Minimization for Lithium-Ion Batteries. Electronics 2024, 13, 645. https://doi.org/10.3390/electronics13030645
Chen G-J, Liu C-L, Liu Y-H, Wang J-J. Implementation of Constant Temperature–Constant Voltage Charging Method with Energy Loss Minimization for Lithium-Ion Batteries. Electronics. 2024; 13(3):645. https://doi.org/10.3390/electronics13030645
Chicago/Turabian StyleChen, Guan-Jhu, Chun-Liang Liu, Yi-Hua Liu, and Jhih-Jhong Wang. 2024. "Implementation of Constant Temperature–Constant Voltage Charging Method with Energy Loss Minimization for Lithium-Ion Batteries" Electronics 13, no. 3: 645. https://doi.org/10.3390/electronics13030645