Analysis of Difference in Areal Density Aluminum Equivalent Method in Ionizing Total Dose Shielding Analysis of Semiconductor Devices
Abstract
:1. Introduction
2. Materials and Methods
3. Results
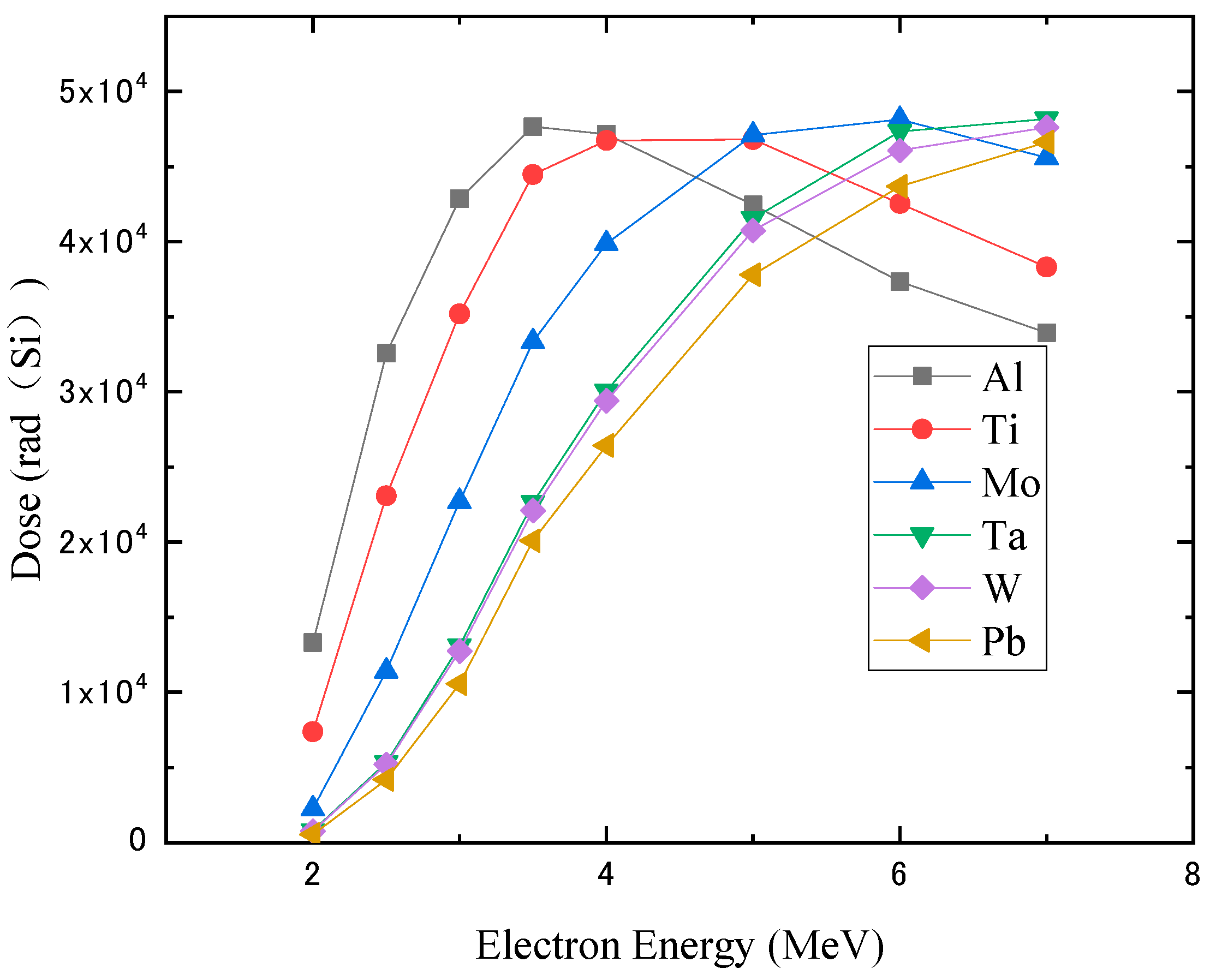
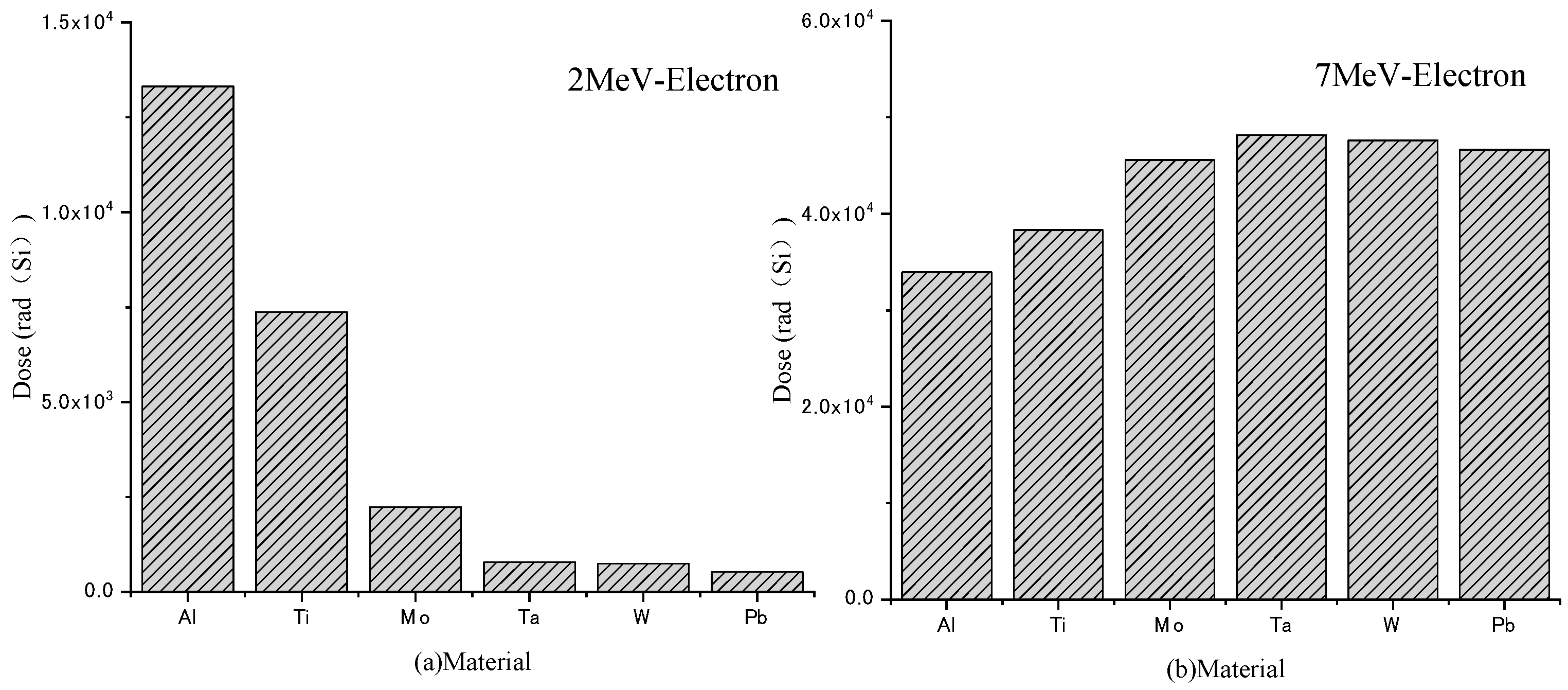
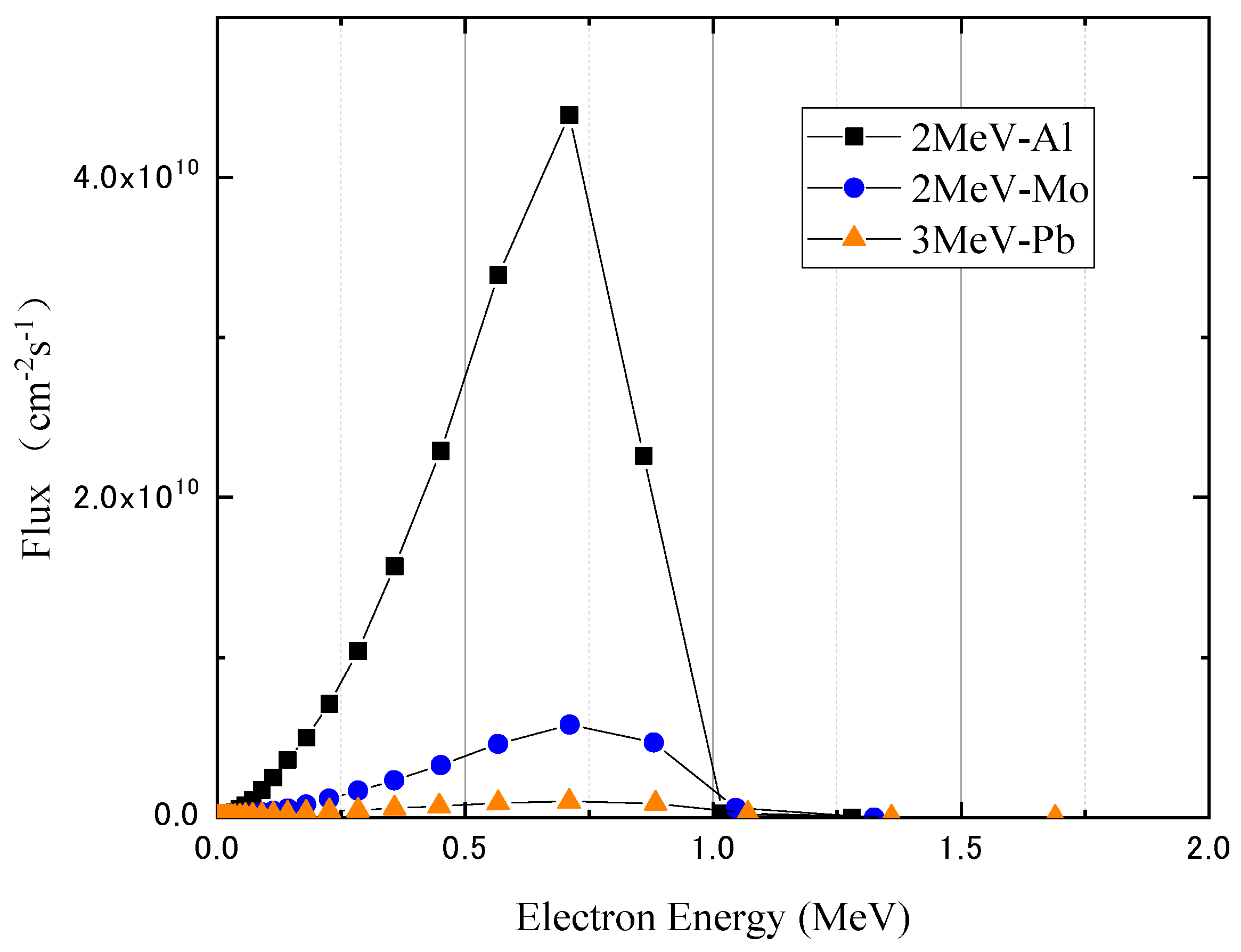
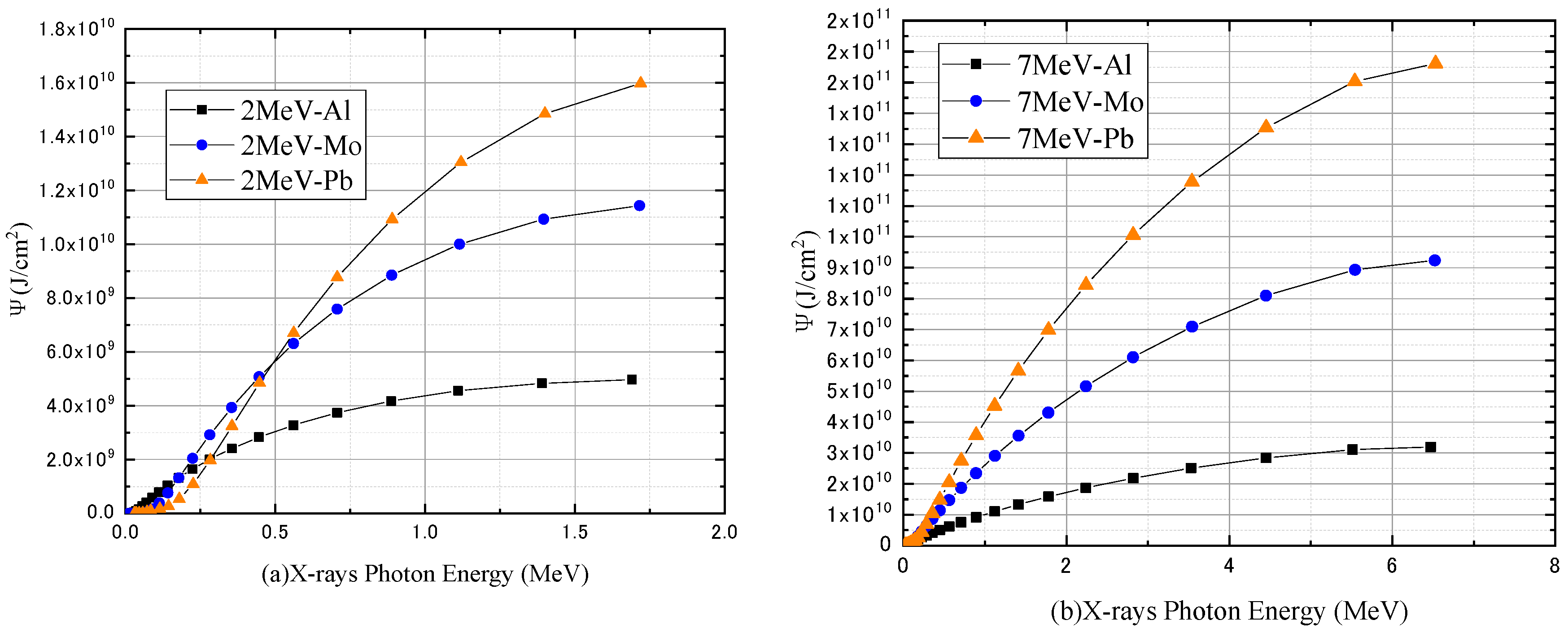
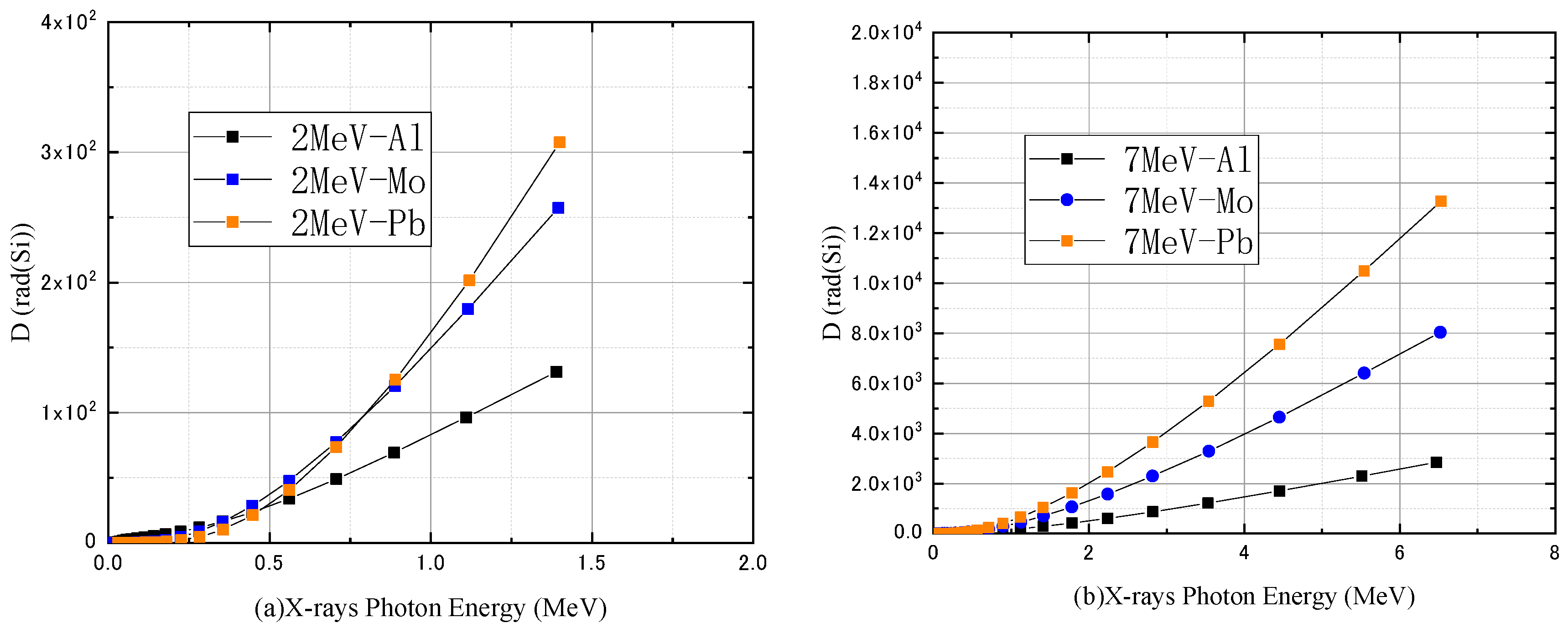
3.1. Monoenergetic Electrons
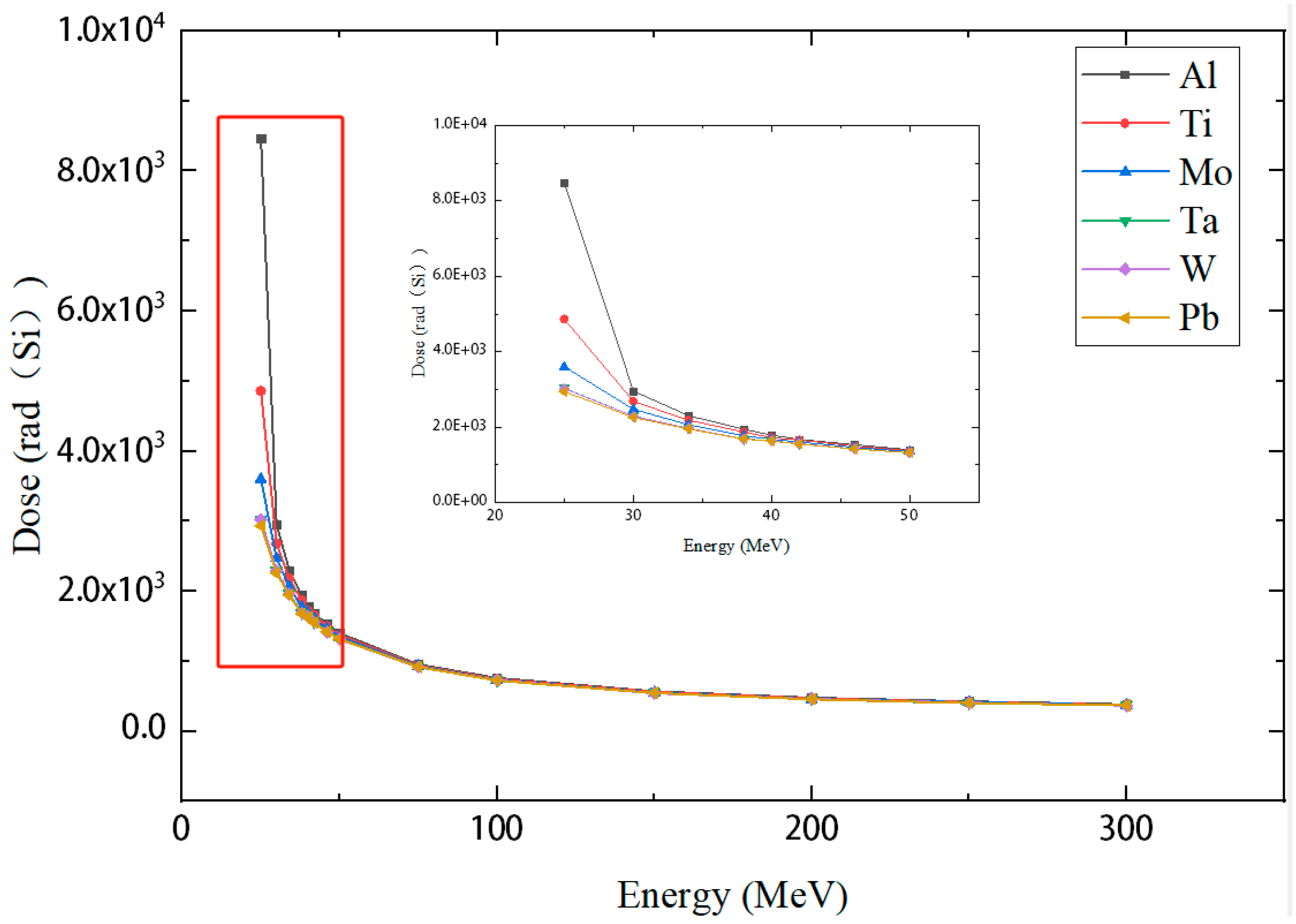
3.2. Monoenergetic Protons
4. Discussion
4.1. Monoenergetic Electrons
4.2. Monoenergetic Protons
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Hu, Y.; Zheng, Y.; Wang, H. The present situation of technologies for analysis of space radiation effects to spacecraft and some retrospection. Spacecr. Environ. Eng. 2022, 39, 4. [Google Scholar]
- Janet, L.B. Space weather effects on spacecraft systems. In Proceedings of the American Meteorological Society Annual Meeting Space Weather Symposium, Seattle, WA, USA, 13 January 2004. [Google Scholar]
- Brown, L.M. Bethe-Bloch formula with density effect correction. Nucl. Instrum. Methods 1979, 164, 15–21. [Google Scholar]
- Fujimoto, T.; Monzen, H.; Nakata, M.; Okada, T.; Yano, S.; Takakura, T.; Kuwahara, J.; Sasaki, M.; Higashimura, K.; Hiraoka, M. Dosimetric shield evaluation with tungsten sheet in 4, 6, and 9 MeV electron beams. Phys. Medica 2014, 37, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Myojoyama, A.; Saitoh, H. Bremsstrahlung and photoneutron production in a steel shield for 15-22-MeV clinical electron beams. Radiat. Prot. Dosim. 2014, 163, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Feng, Y.; Han, J.; Cai, M.; Yang, T. Simulation and validation of composite shielding for total ionizing dose. Chin. J. Space Sci. 2014, 34, 180–185. [Google Scholar] [CrossRef]
- Xun, M.; He, C.; Zheng, Y. Analysis of shielding ability of different materials for 100 MeV proton incident. Manned Spacefl. 2018, 24, 740–744. [Google Scholar] [CrossRef]
- Wang, C.; Luo, W.; Zha, Y.; Wang, C. Monte-Carlo simulation of optimization choice of shielding materials for proton radiation in space. Radiat. Prot. 2007, 27, 79–86. [Google Scholar] [CrossRef]
- Waterman, G.; Kase, K.; Orion, I.; Broisman, A.; Milstein, O. Selective shielding of bone marrow: An approach to protecting humans from external gamma radiation. Health Phys. 2017, 113, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-X.; Sun, H.-B.; He, C.-F.; Tong, Y.-P.; Ma, Y.-G.; Lu, J.-B.; Xun, M.-Z.; Hu, Y.-Q.; Cai, Z.-B.; Zhu, Z.-P. Analysis of radiation environment and its dose within sealed cabin of manned spacecraft. Spacecr. Environ. Eng. 2016, 33, 154–157. [Google Scholar]
- Seltzer, S.M. Updated Calculations for Routine Space-Shielding Radiation Dose Estimates: SHIELDOSE-2; NIST Publication NISTIR 5477; NIST Publication: Gaithersburg, MD, USA, 1994. [Google Scholar]
- Xu, S. Application of Monte-Carlo Methods in Experimental Physics; Atomic Energy Press: Beijing, China, 2006. [Google Scholar]
- Santin, G. New Geant 4 based simulation tools for space radiation shielding and effects analysis. Nucl. Phys. B 2003, 125, 69–74. [Google Scholar] [CrossRef]
- Cai, M.; Han, J. Method for Evaluating Shielding Thicknesses and Radiation Dose Inside Spacecraft Based on ProE. J. Astronaut. 2012, 33, 6. [Google Scholar] [CrossRef]
- Lei, F.; Truscott, R.R.; Dyer, C.S.; Quaghebeur, B.; Heynderickx, D.; Nieminen, R.; Evans, H.; Daly, E. MULASSIS: A Geant4-based multilayered shielding simulation tool. IEEE Trans. Nucl. Sci. 2002, 49, 2788–2793. [Google Scholar] [CrossRef]
- Attix, F.H. Introduction to Radiological Physics and Radiation Dosimetry; John Wiley & Sons: Hoboken, NJ, USA, 1986. [Google Scholar] [CrossRef]
- Fudan University; Tsinghua University; Peking University (Eds.) Experimental Methods in Nuclear Physics; Atomic Energy Press: Beijing, China, 1997. [Google Scholar]

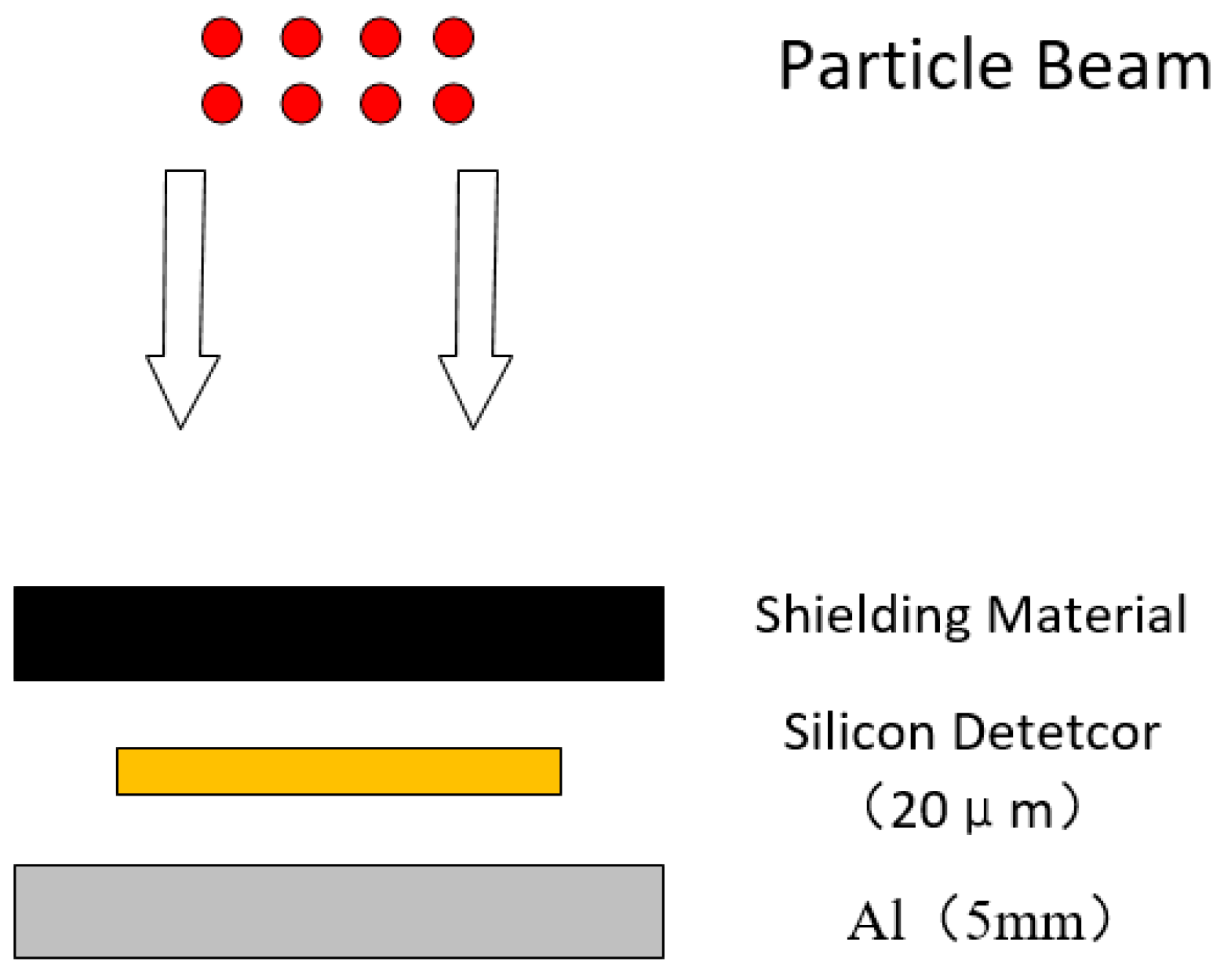




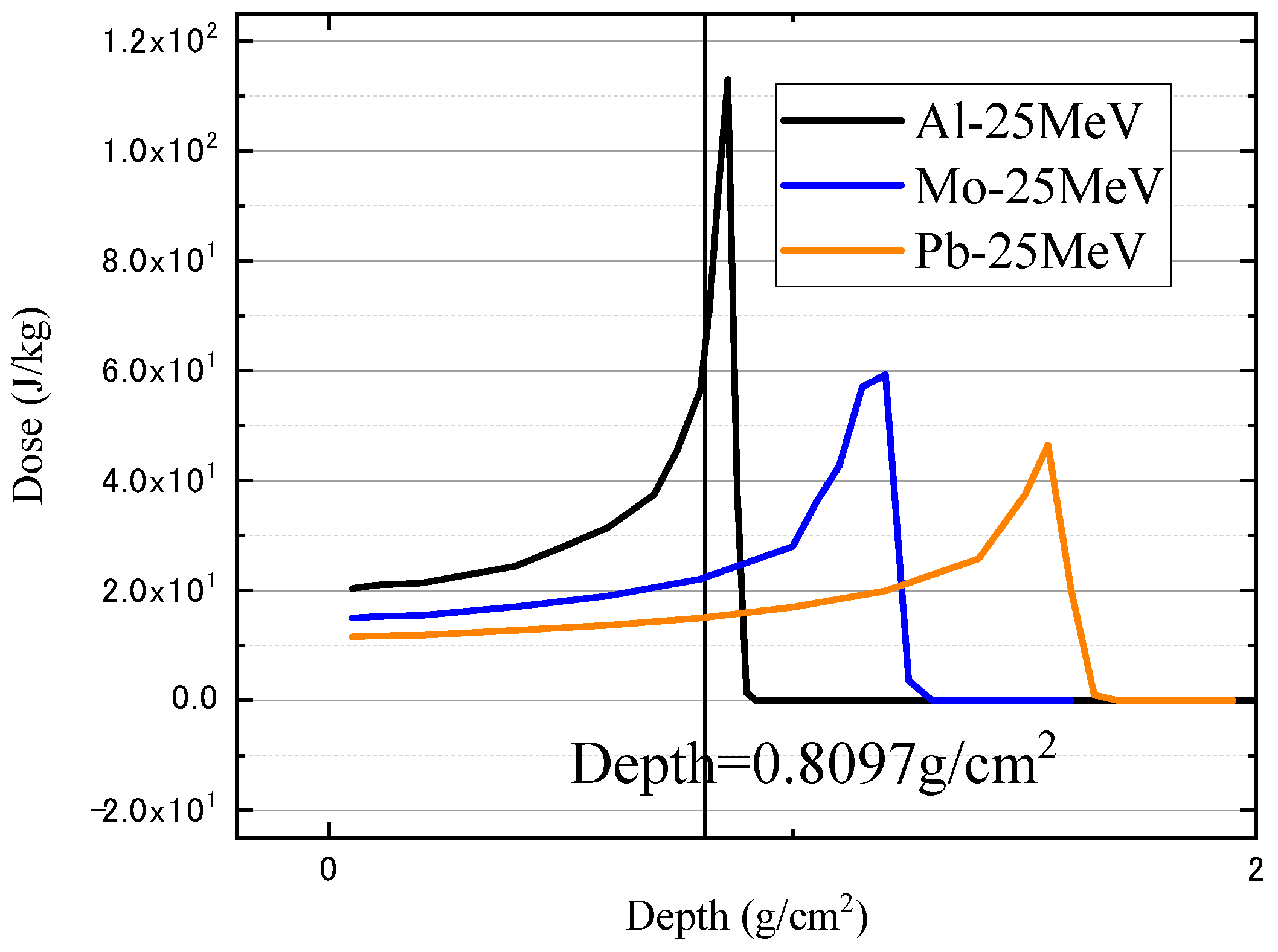
| Material | Atomic Number (Z) | Density (g/cm3) | Equivalent Areal Density (g/cm2) | Geometric Thickness (mm) | Electron Density (1023 e/g) |
|---|---|---|---|---|---|
| Aluminum | 13 | 2.699 | 0.8097 | 3.000 | 2.901 |
| Titanium | 22 | 4.540 | 0.8097 | 1.783 | 2.719 |
| Molybdenum | 42 | 10.220 | 0.8097 | 0.792 | 2.636 |
| Tantalum | 73 | 16.654 | 0.8097 | 0.486 | 2.429 |
| Tungsten | 74 | 19.300 | 0.8097 | 0.420 | 2.424 |
| Lead | 82 | 11.350 | 0.8097 | 0.713 | 2.383 |
| Particle | Number of Primary Particles | Fluence/(Flux) Intensity (cm−2s−1) | Energy (MeV) |
|---|---|---|---|
| Electron | cm−2s−1 | 2–7 | |
| Proton | cm−2s−1 | 25–300 |
| Material | Al | Ti | Mo | Ta | W | Pb |
|---|---|---|---|---|---|---|
| Dose | ||||||
| 1 | 0.999 | 0.924 | 0.792 | 0.777 | 0.721 |
| Material | Al | Ti | Mo | Ta | W | Pb |
|---|---|---|---|---|---|---|
| Dose | ||||||
| 1 | 0.936 | 0.902 | 0.879 | 0.878 | 0.875 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; He, C.; Feng, J.; Xun, M.; Sun, J.; Li, Y.; Guo, Q. Analysis of Difference in Areal Density Aluminum Equivalent Method in Ionizing Total Dose Shielding Analysis of Semiconductor Devices. Electronics 2023, 12, 4181. https://doi.org/10.3390/electronics12194181
Liu M, He C, Feng J, Xun M, Sun J, Li Y, Guo Q. Analysis of Difference in Areal Density Aluminum Equivalent Method in Ionizing Total Dose Shielding Analysis of Semiconductor Devices. Electronics. 2023; 12(19):4181. https://doi.org/10.3390/electronics12194181
Chicago/Turabian StyleLiu, Mingyu, Chengfa He, Jie Feng, Mingzhu Xun, Jing Sun, Yudong Li, and Qi Guo. 2023. "Analysis of Difference in Areal Density Aluminum Equivalent Method in Ionizing Total Dose Shielding Analysis of Semiconductor Devices" Electronics 12, no. 19: 4181. https://doi.org/10.3390/electronics12194181




