Elucidation of Response and Electrochemical Mechanisms of Bio-Inspired Rubber Sensors with Supercapacitor Paradigm
Abstract
:1. Introduction
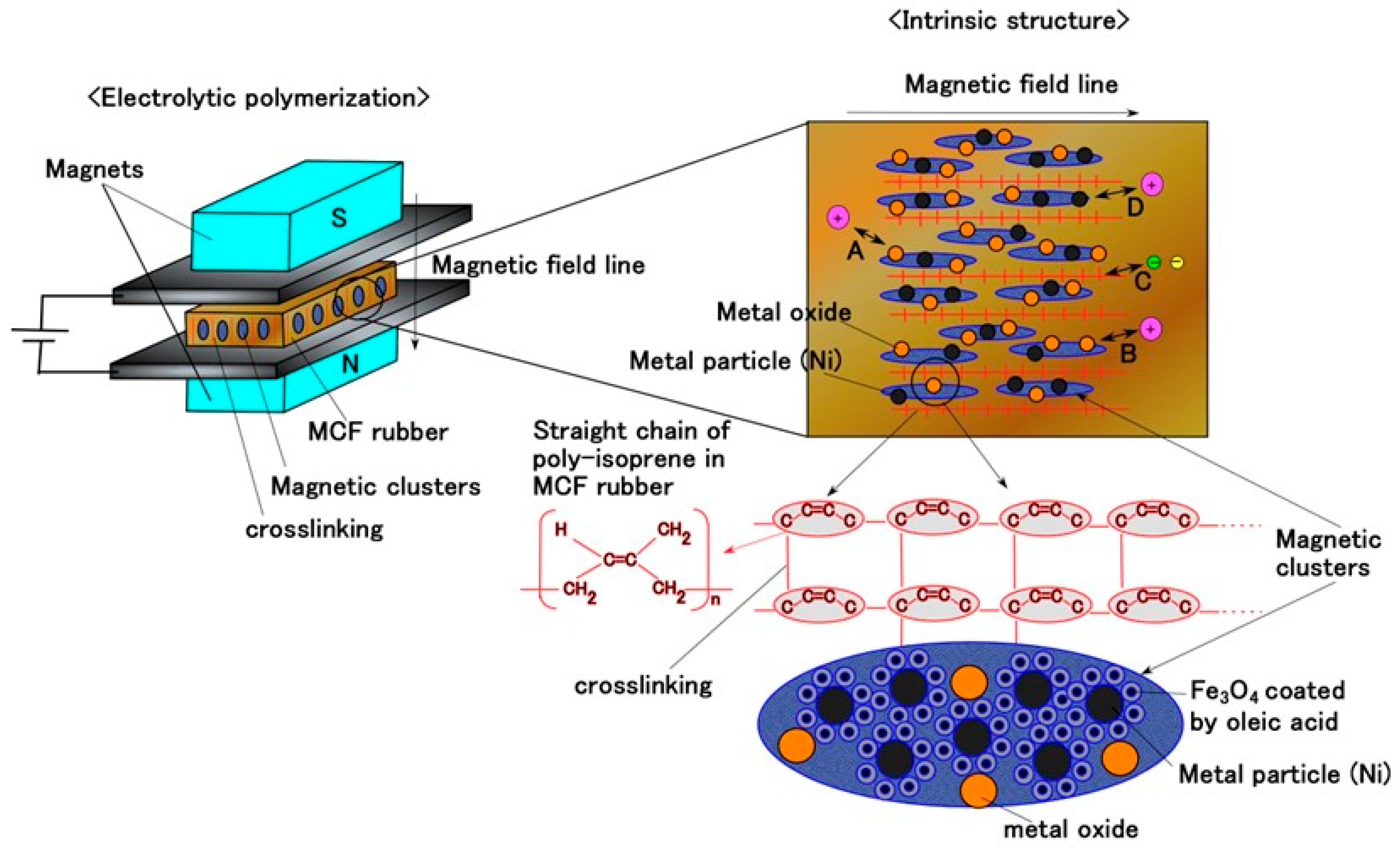
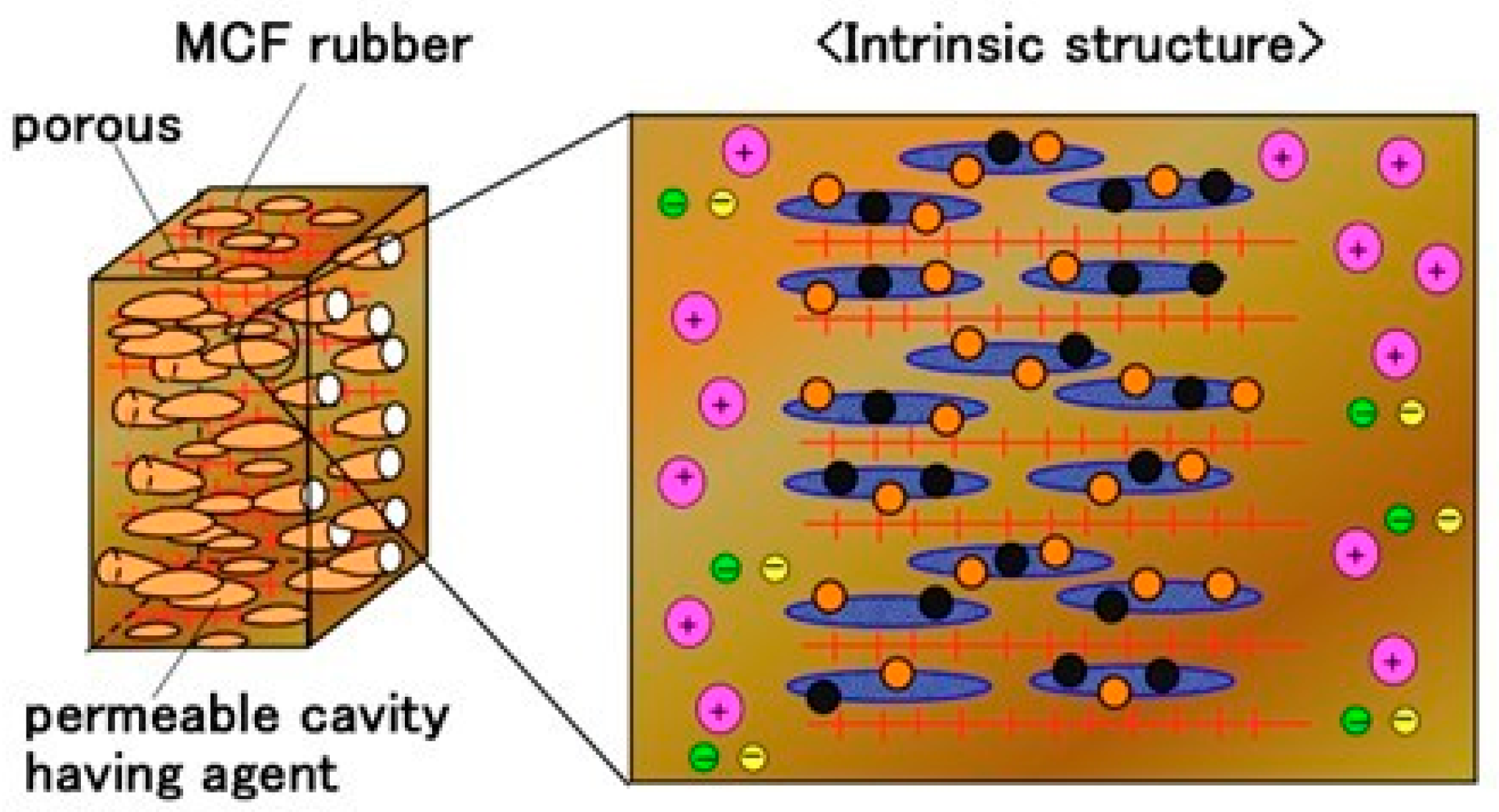
2. Intrinsic Mechanism
2.1. Materials
2.2. Experimental Procedure
2.3. Results and Discussion
3. Bio-Inspired Responsive Rubber
3.1. Materials
3.2. Experimental Procedure
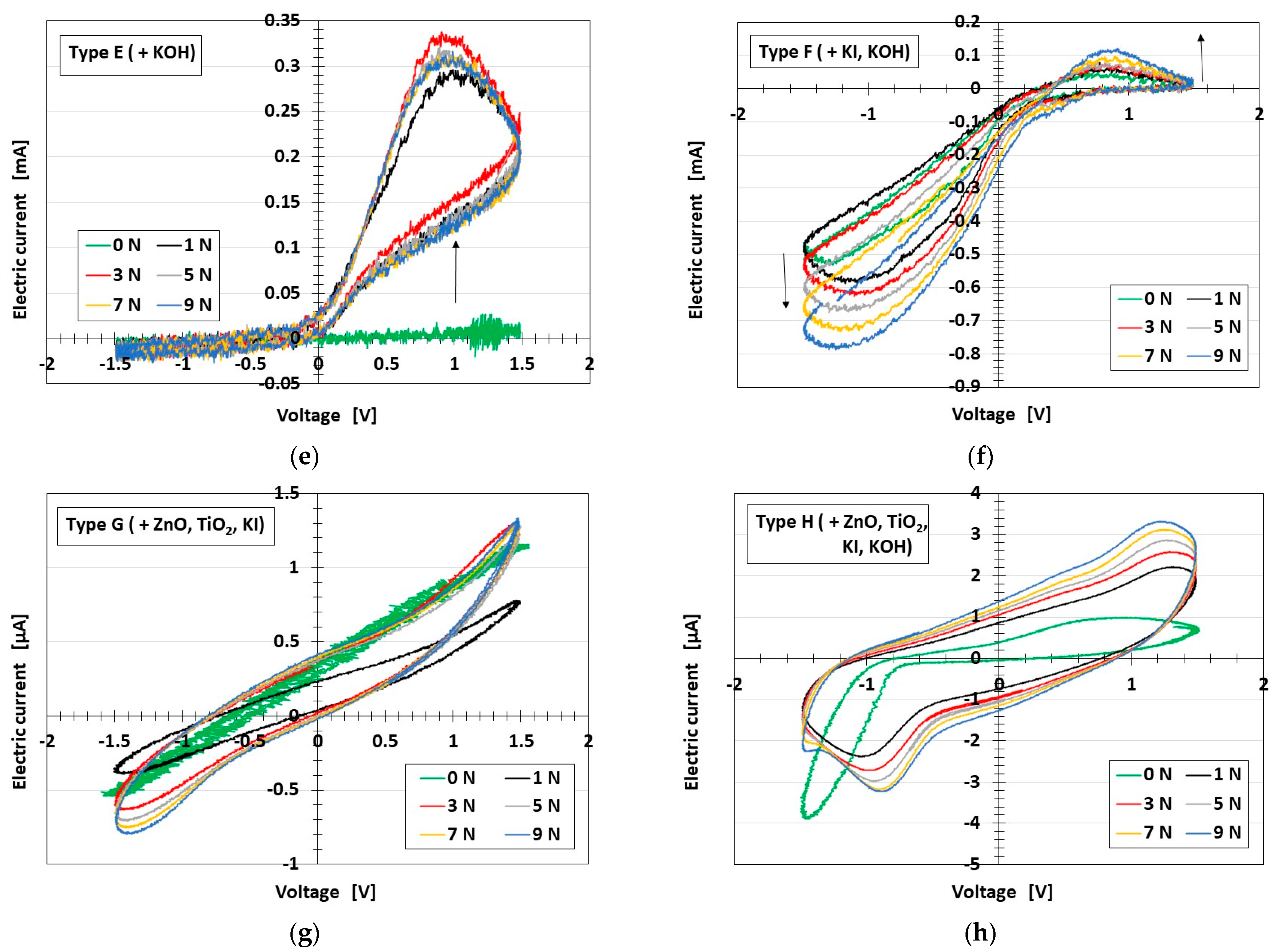
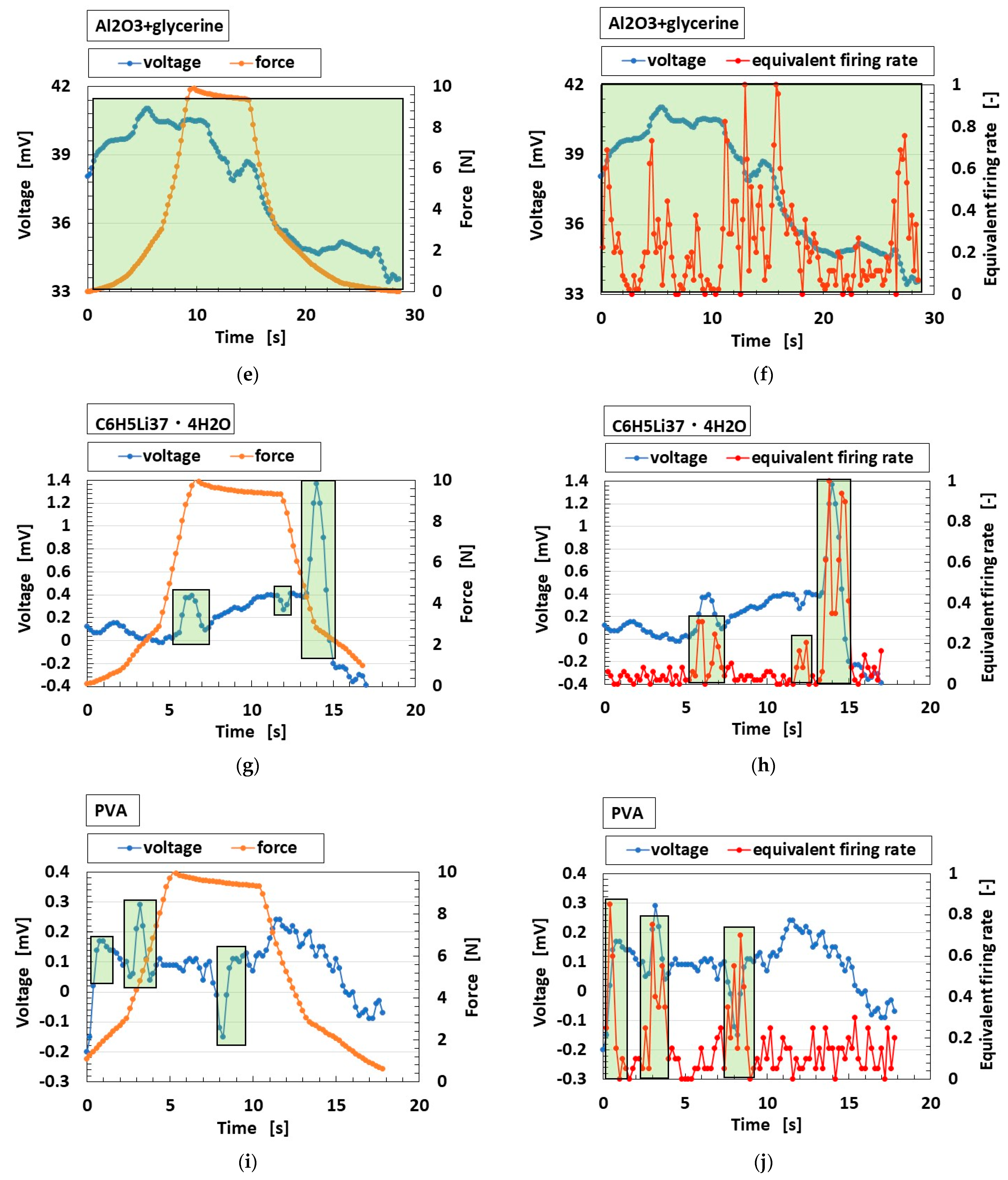
3.3. Results and Discussion
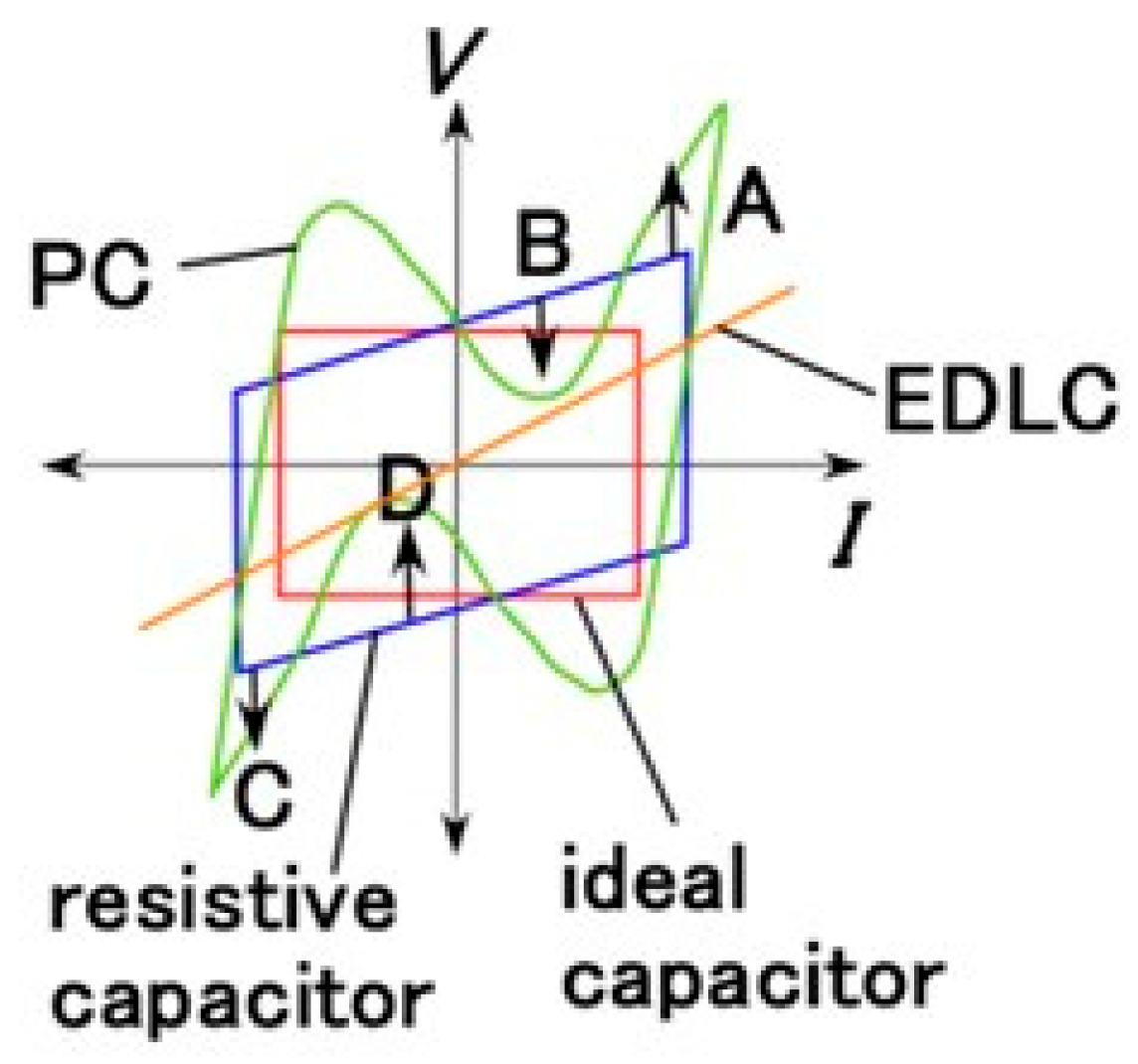
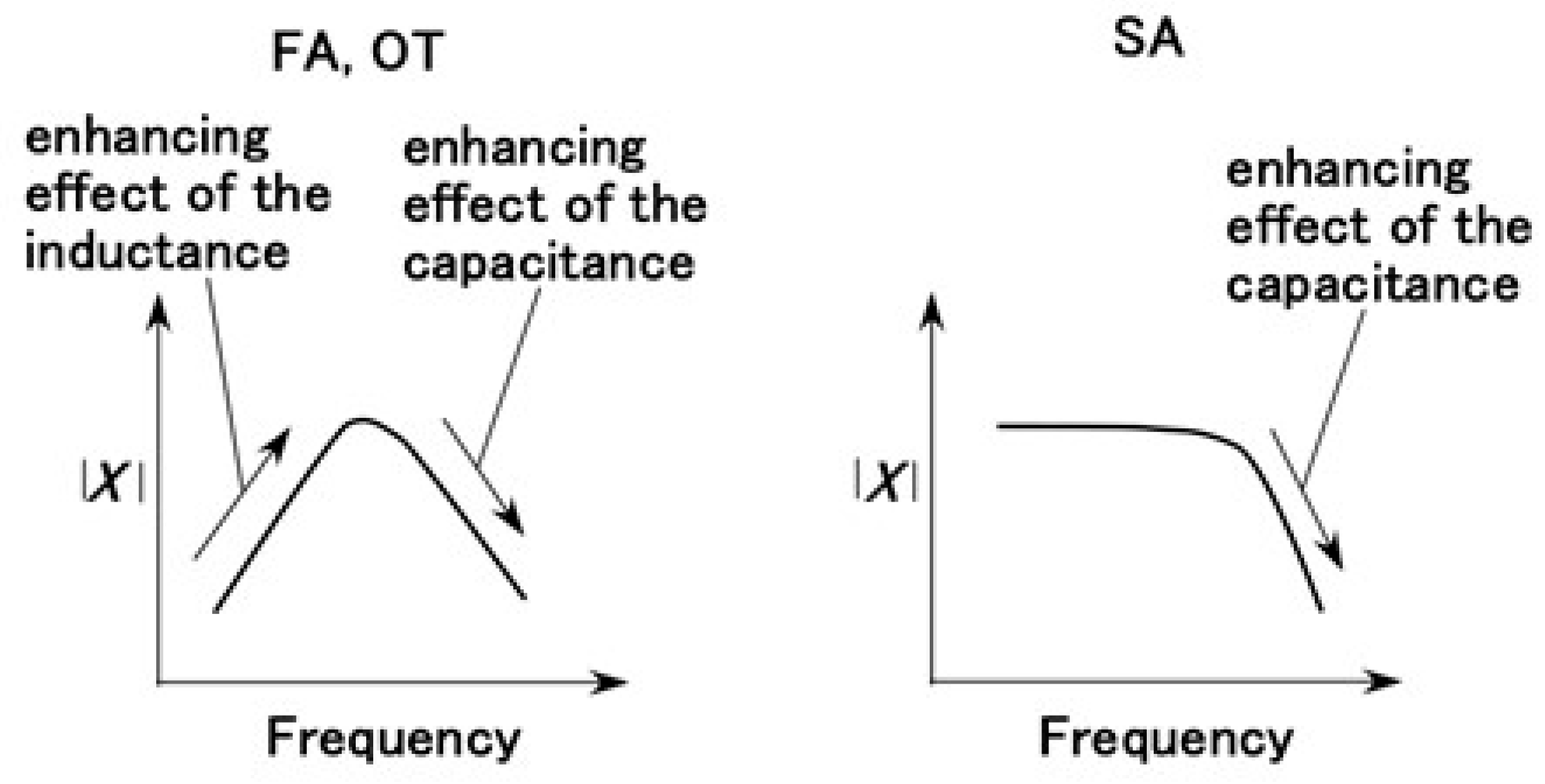
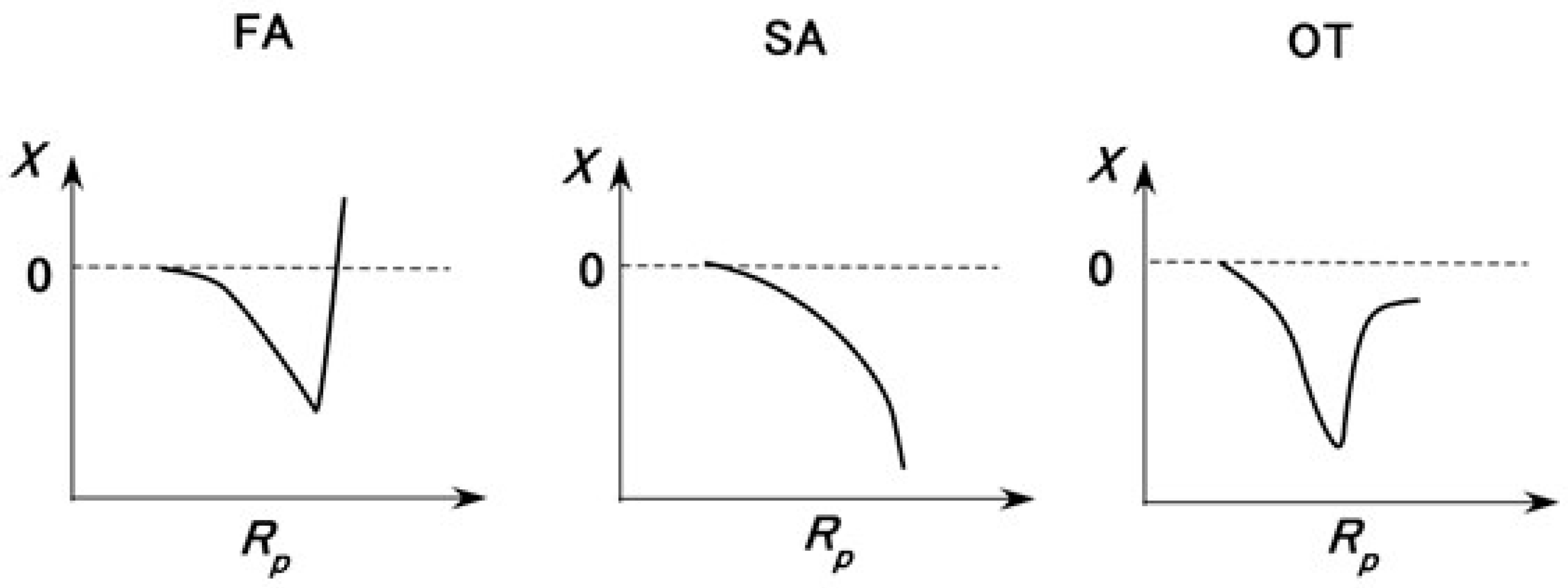
3.3.1. Electrochemical Mechanism
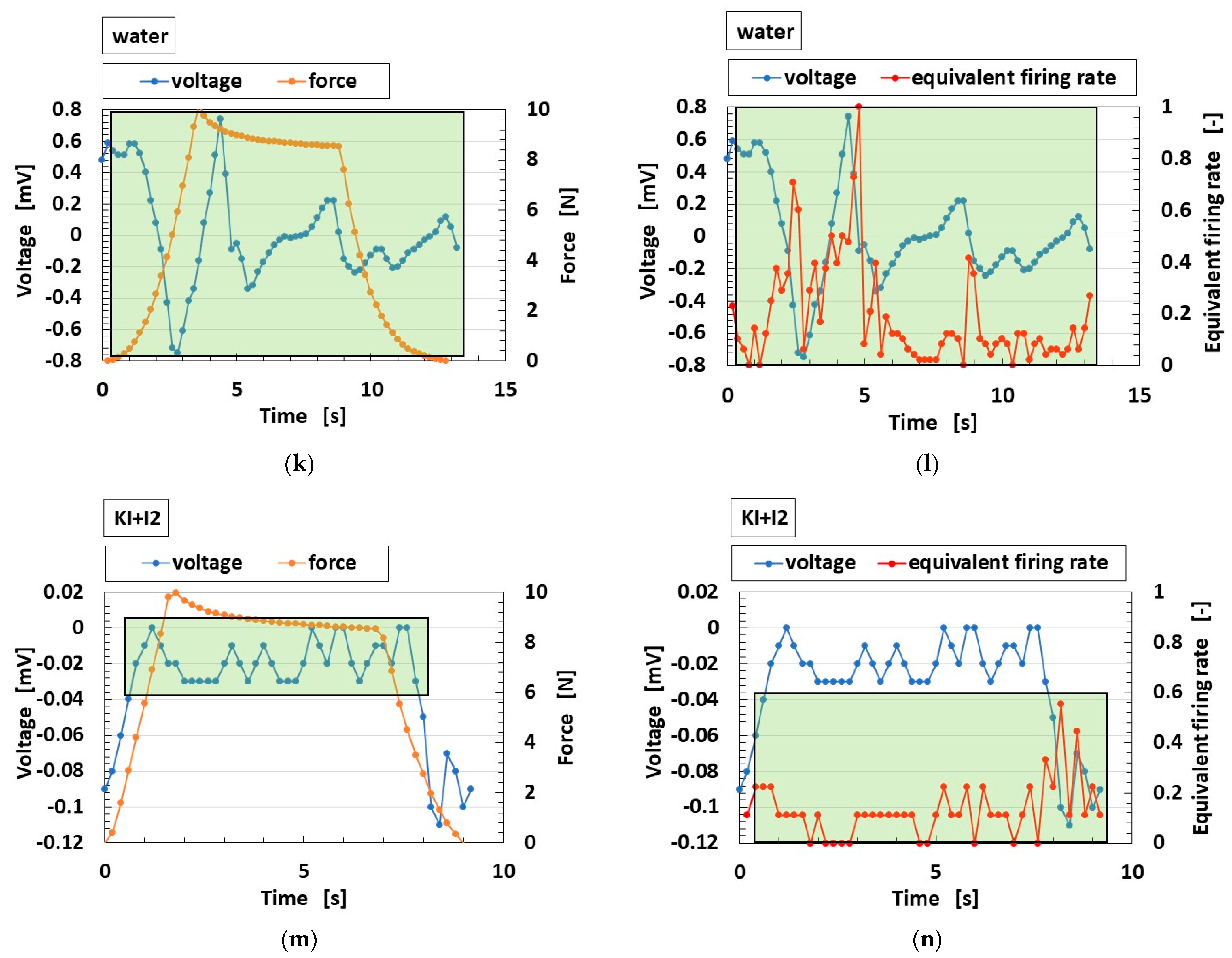
3.3.2. Firing Rate
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Khandelwal, G.; Dahiya, R. Self-powered active sensing based on triboelectric generators. Adv. Mater. 2022, 34, 2200724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S.; Zhu, G.; Niu, S.; Liu, Y.; Bai, P.; Jing, Q.; Wang, Z.L. Nanometer resolution self-powered static and dynamic motion sensor based on micro-grated triboelectrification. Adv. Mater. 2014, 26, 1719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, L.; Deng, W.; Jin, L.; Chun, F.; Pan, H.; Gu, B.; Zhang, H.; Lv, Z.; Yang, W.; et al. Self-powered acceleration sensor based on liquid metal triboelectric nanogenerator for vibration monitoring. ACS Nano 2017, 11, 7440. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, J.; Xing, F.; Zhang, M.; Cao, X.; Wang, N.; Wang, L. From dual-mode triboelectric nanogenerator to smart tactile sensor: A multiplexing design. ACS Nano 2017, 11, 3950. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Wang, B.; Yang, T.; Yang, B.; Xie, G.; Zhou, Y.; Zhang, S.; Tai, H.; Cai, Z.; et al. Alveolus-inspired active membrane sensors for self-powered wearable chemical sensing and breath analysis. ACS Nano 2020, 14, 6067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Su, Y.; Chen, J.; Hu, C.; Wu, Z.; Liu, Y.; Wong, C.P.; Bando, Y.; Wang, Z.L. Triboelectric nanogenerator as self-powered active sensors for detecting liquid/gaseous water/ethanol. Nano Energy 2013, 2, 693. [Google Scholar] [CrossRef]
- Khandelwal, G.; Ediriweera, M.K.; Kumari, N.; Raj, N.P.M.J.; Cho, S.K.; Kim, S.-J. Metal-amino acid nanofibers based triboelectric nanogenerator for self-powered thioacetamide sensor. ACS Appl. Mater. Interfaces 2021, 13, 18887. [Google Scholar] [CrossRef]
- Qi, S.; Guo, H.; Chen, J.; Fu, J.; Hu, C.; Yu, M.; Wang, Z.L. Magnetorheological elastomers enabled high-sensitive self-powered tribo-sensor for magnetic field detection. Nanoscale 2018, 10, 4745. [Google Scholar] [CrossRef]
- Su, L.; Zhao, Z.X.; Li, H.Y.; Yuan, J.; Wang, Z.L.; Cao, G.Z.; Zhu, G. High-performance organolead halide perovskite-based self-powered triboelectric photodetector. ACS Nano 2015, 9, 11310. [Google Scholar] [CrossRef]
- Jie, Y.; Wang, N.; Cao, X.; Xu, Y.; Li, T.; Zhang, X.; Wang, Z.L. Self-powered triboelectric nanosensor with poly(tetrafluoroethylene) nanoparticle arrays for dopamine detection. ACS Nano 2015, 9, 8376. [Google Scholar] [CrossRef]
- Jung, Y.K.; Kim, K.N.; Baik, J.M.; Kim, B.-S. Self-powered triboelectric aptasensor for label-free highly specific thrombin detection. Nano Energy 2016, 30, 77. [Google Scholar] [CrossRef]
- Pao, Y.-P.; Yu, C.-C.; Lin, Y.-Z.; Chatterjee, S.; Saha, S.; Tiwari, N.; Huang, Y.-T.; Wu, C.-C.; Choi, D.; Lin, Z.-H. Carbohydrate-protein interactions studied by solid-liquid contact electrification and its use for label-free bacterial detection. Nano Energy 2021, 85, 106008. [Google Scholar] [CrossRef]
- Li, W.D.; Ke, K.; Jia, J.; Pu, J.H.; Zhao, X.; Bao, R.Y.; Liu, Z.Y.; Bai, L.; Zhang, K.; Yang, M.B.; et al. Recent advances in multiresponsive flexible sensors towards e-skin: A delicate design for versatile sensing. Small 2021, 18, 2103734. [Google Scholar] [CrossRef]
- Mehrali, M.; Bagherifard, S.; Akbari, M.; Thakur, A.; Mirani, B.; Mehrali, M.; Hasany, M.; Orive, G.; Das, P.; Emneus, J.; et al. Blending electronics with the human body: A pathway toward a cybernetic future. Adv. Sci. 2018, 5, 1700931. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.S.; Thireau, J.; Boudaden, J.; Lal, S.; Gulzar, U.; Zhang, Y.; Gil, T.; Azemard, N.; Ramm, P.; Kiessling, T.; et al. Review-energy autonomous wearable sensors for smart healthcare: A review. J. Electrochem. Soc. 2020, 167, 037516. [Google Scholar] [CrossRef]
- Sang, M.; Kim, K.; Shin, J.; Yu, K.J. Ultra-thin flexible encapsulating materials for soft bio-integrated electronics. Adv. Sci. 2022, 9, 2202980. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.; Solomon, S.; Gao, W. Flexible electronics and devices as human-machine interfaces for medical robotics. Adv. Mater. 2022, 34, 2107902. [Google Scholar] [CrossRef]
- Bubniene, U.S.; Ratautatite, V.; Ramanavicius, A.; Bucinskas, V. Conducting polymers for the design of tactile sensors. Polymers 2022, 14, 2984. [Google Scholar] [CrossRef]
- Kim, T.Y.; Suh, W.; Jeong, U. Approaches to deformable physical sensors: Electronic versus iontronic. Mater. Sci. Eng. R. Rep. 2021, 146, 100640. [Google Scholar] [CrossRef]
- Ding, Q.; Wu, Z.; Tao, K.; Wei, Y.; Wang, W.; Yang, B.R.; Xie, X.; Wu, J. Environment tolerant, adaptable and stretchable organohydrogels: Preparation, optimization, and applications. Mat. Horiz. 2022, 9, 1356–1386. [Google Scholar] [CrossRef]
- Bao, S.; Gao, J.; Xu, T.; Li, N.; Chen, W.; Lu, W. Anti-freezing and antibacterial conductive organohydrogel co-reinforced by 1D silk nanofibers and 2D graphitic carbon nitride nanosheets as flexible sensor. Chem. Eng. J. 2021, 411, 128470. [Google Scholar] [CrossRef]
- Chen, J.; Lee, P.S. Electrochemical supercapacitors: From mechanism understanding to multifunctional applications. Adv. Energy Mater. 2021, 11, 2003311. [Google Scholar] [CrossRef]
- Swain, N.; Tripathy, A.; Thirumurugan, A.; Saravanakumar, B.; Mende, L.S.; Ramadoss, A. A brief review on stretchable, compressible, and deformable supercapacitor for smart devices. Chem. Eng. J. 2022, 446, 136876. [Google Scholar] [CrossRef]
- Shimada, K. Enhancement of MCF rubber utilizing electric and magnetic fields, and clarification of electrolytic polymerization. Sensors 2017, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Ikeda, R.; Kikura, H.; Takahashi, H. Development of novel magnetic responsive intelligent fluid, hybrid fluid (HF), for production of soft and tactile rubber. World J. Mech. 2021, 11, 187–203. [Google Scholar] [CrossRef]
- Hamann, W. Mammalian cutaneous mechanoreceptors. Prog. Biophys. Mol. Biol. 1995, 64, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R. Review: Methods of firing rate estimation. BioSystems 2019, 183, 103980. [Google Scholar] [CrossRef]
- Kobayakawa, T.; Gotow, N. Interaction between olfaction and gustation by using synchrony perception task. i-Perception 2011, 2, 964. [Google Scholar] [CrossRef]
- Gotow, N.; Kobayakawa, T. Simultaneity judgment using olfactory-visual, visual-gustatory, and olfactory-gustatory combinations. PLoS ONE 2017, 12, e0174958. [Google Scholar] [CrossRef]
- Shimada, K. Morphological configuration of sensory biomedical receptors based on structures integrated by electric circuits and utilizing magnetic-responsive hybrid fluid (HF). Sensors 2022, 22, 9952. [Google Scholar] [CrossRef]
- Jang, S.H.; Park, Y.L.; Yin, H. Influence of coalescence on the anisotropic mechanical and electrical properties of nickel powder/polydimethylsiloxane composites. Materials 2016, 9, 239. [Google Scholar] [CrossRef]
- Patil, A.R.; Dongale, T.D.; Kamat, R.K.; Rajpure, K.Y. Binary metal oxide-based resistive switching memory devices: A status review. Mater. Today Commu. 2023, 34, 105356. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Han, Y.; Zhou, Z.; Yuan, G.; Zhang, X. Cellulose nanowhisker modulated 3D hierarchical conductive structure of carbon black/natural rubber nanocomposites for liquid and strain sensing application. Compos. Sci. Technol. 2016, 124, 44–51. [Google Scholar] [CrossRef]
- Sahatiya, P.; Badhulika, S. Eraser-based eco-friendly fabrication of a skin-like large-area matrix of flexible carbon nanotube strain and pressure sensors. Nanotechnology 2017, 28, 095501. [Google Scholar] [CrossRef]
- Bao, R.; Wang, C.; Dong, L.; Yu, R.; Zhao, K.; Wang, Z.L.; Pan, C. Flexible and controllable piezo-phototronic pressure mapping sensor matrix by ZnO NW/p-polymer LED array. Adv. Func. Mater. 2015, 25, 2884–2891. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Dai, Y. preparation and electrochemical performance of polymer-derived SiBCN-graphene composite as anode material for lithium ion batteries. Ceram. Int. 2017, 43, 1210–1216. [Google Scholar] [CrossRef]
- Ding, X.; Shao, H.; Wang, H.; Yang, W.; Fang, J.; Zhang, D.; Lin, T. Schottky DC generators with considerable enhanced power output and energy conversion efficiency based on polypyrrole-TiO2 nanocomposite. Nano Energy 2021, 89, 106367. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Liu, X.; Li, P.; Wu, W. Nanocellulose as a promising substrate for advanced sensors and their applications. Int. J. Biolog. Macromol. 2022, 218, 473–487. [Google Scholar] [CrossRef]
- Moftah, A.; Shetiti, A.A. Review of super capacitor technology. Indones. J. Comput. Sci. Electr. Eng. 2015, 3, 226–231. [Google Scholar]
- Jiang, Y.; Liu, J. Definitions of pseudocapacitive materials: A brief review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Li, J. Stretchable ionic-liquid-based gel polymer electroryles for lithium-ion batteries. Ind. Eng. Chem. Res. 2017, 56, 12456–12463. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode materials for supercapacitors: A review of recent advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive materials for electrochemical capacitors: From rational synthesis to capacitance optimization. Natl. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.P.; Shao, Z. Intercalation pseudocapacitance in electrochemical energy storage: Recent advances in fundamental understanding and materials development. Mater. Today Adv. 2020, 7, 100072. [Google Scholar] [CrossRef]
- Xie, L.; Su, F.; Xie, L.; Guo, X.; Wang, Z.; Kong, Q.; Sun, G.; Ahmad, A.; Li, X.; Yi, Z.; et al. Effect of pore structure and doping species on charge storage mechanisms in porous carbon-based supercapacitors. Mater. Chem. Front. 2020, 4, 2610–2634. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Wain, A.J. The butler-Volmer equation in electrochemical theory: Origins, value, and practical application. J. Electroanalyt. Chem. 2020, 872, 114145. [Google Scholar] [CrossRef]
- Yu, Y.; Meng, Q.; Liu, T. Multifunctional and durable graphene-based composite sponge doped with antimonene nanosheets. J. Mater. Res. Technol. 2022, 17, 2466–2479. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, L.; Wan, P. Polymer nanocomposite meshes for flexible electronic devices. Prog. Polym. Sci. 2020, 107, 101279. [Google Scholar] [CrossRef]
- Shimada, K. Correlations among firing rates of tactile, thermal, gustatory, olfactory and auditory sensations mimicked by artificial hybrid fluid (HF) rubber mechanoreceptors. Sensors 2023, 23, 4593. [Google Scholar] [CrossRef]
- Lu, Y.; Ru, S.; Li, H.; Wang, G.; Xu, S. Laser-structured microarray electrodes for durable stretchable lithium-ion battery. J. Colloids Inter. Sci. 2023, 631, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Ikeda, R.; Kikura, H.; Takahashi, H. Enhancement of diversity on production and application utilizing electrolytically polymerized rubber sensors with MCF: 1st report on consummate fabrication combining varied kinds of constituents with porous permeant stocking-like rubber. Sensors 2020, 20, 4658. [Google Scholar] [CrossRef] [PubMed]









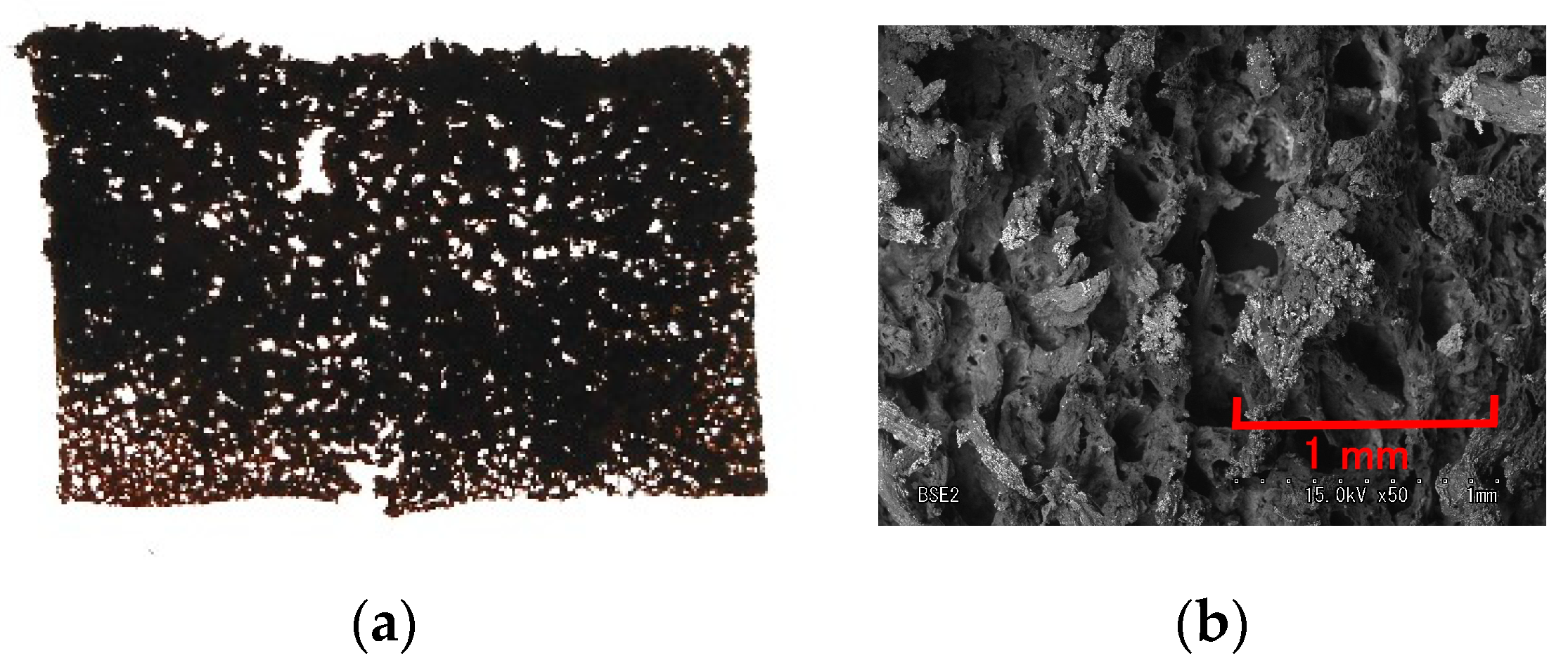
| Type A (Normal) | Type B (+ZnO) | Type C (+TiO2) | Type D (+KI) |
|---|---|---|---|
| Ni: 1.5 g | Ni: 1.5 g | Ni: 1.5 g | Ni: 1.5 g |
| MF: 0.75 g | MF: 0.75 g | MF: 0.75 g | MF: 0.75 g |
| NR latex: 4.5 g | NR latex: 4.5 g | NR latex: 4.5 g | NR latex: 4.5 g |
| - | ZnO: 0.25 g | TiO2: 0.25 g | KI solution: 0.5 g |
| Type E (+KOH) | Type F (+KI, KOH) | Type G (+ZnO, TiO2, KI) | Type H (+ZnO, TiO2, KI, KOH) |
| Ni: 1.5 g | Ni: 1.5 g | Ni: 1.5 g | Ni: 1.5 g |
| MF: 0.75 g | MF: 0.75 g | MF: 1.125 g | MF: 0.75 g |
| NR latex: 4.5 g | NR latex: 4.5 g | NR latex: 4.5 g | NR latex: 4.5 g |
| KOH solution: 1.7 g | KI solution: 0.5 g | ZnO: 0.5 g | ZnO: 0.25 g |
| - | KOH solution: 0.75 g | TiO2: 0.5 g | TiO2: 0.25 g |
| - | - | KI solution: 0.5 g | KI solution: 1.1 g |
| - | - | - | KOH solution: 0.75 g |
| Ni: 1.5 g |
| MF: 0.75 g Na2WO4·2H2O: 0.5 g |
| S-500: 3 g |
| CR latex: 3 g KF96: 3 g PVA: 3 g Compounding fillers or permeating agents (glycerine *1, glycerine + BaTiO3 *2, glycerine *1 + Al2O3 *2, C6H5Li3O7·4H2O *1, water *1, KI + I2 *1, PVA *1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, K. Elucidation of Response and Electrochemical Mechanisms of Bio-Inspired Rubber Sensors with Supercapacitor Paradigm. Electronics 2023, 12, 2304. https://doi.org/10.3390/electronics12102304
Shimada K. Elucidation of Response and Electrochemical Mechanisms of Bio-Inspired Rubber Sensors with Supercapacitor Paradigm. Electronics. 2023; 12(10):2304. https://doi.org/10.3390/electronics12102304
Chicago/Turabian StyleShimada, Kunio. 2023. "Elucidation of Response and Electrochemical Mechanisms of Bio-Inspired Rubber Sensors with Supercapacitor Paradigm" Electronics 12, no. 10: 2304. https://doi.org/10.3390/electronics12102304








