Objective Assessment of Walking Impairments in Myotonic Dystrophy by Means of a Wearable Technology and a Novel Severity Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
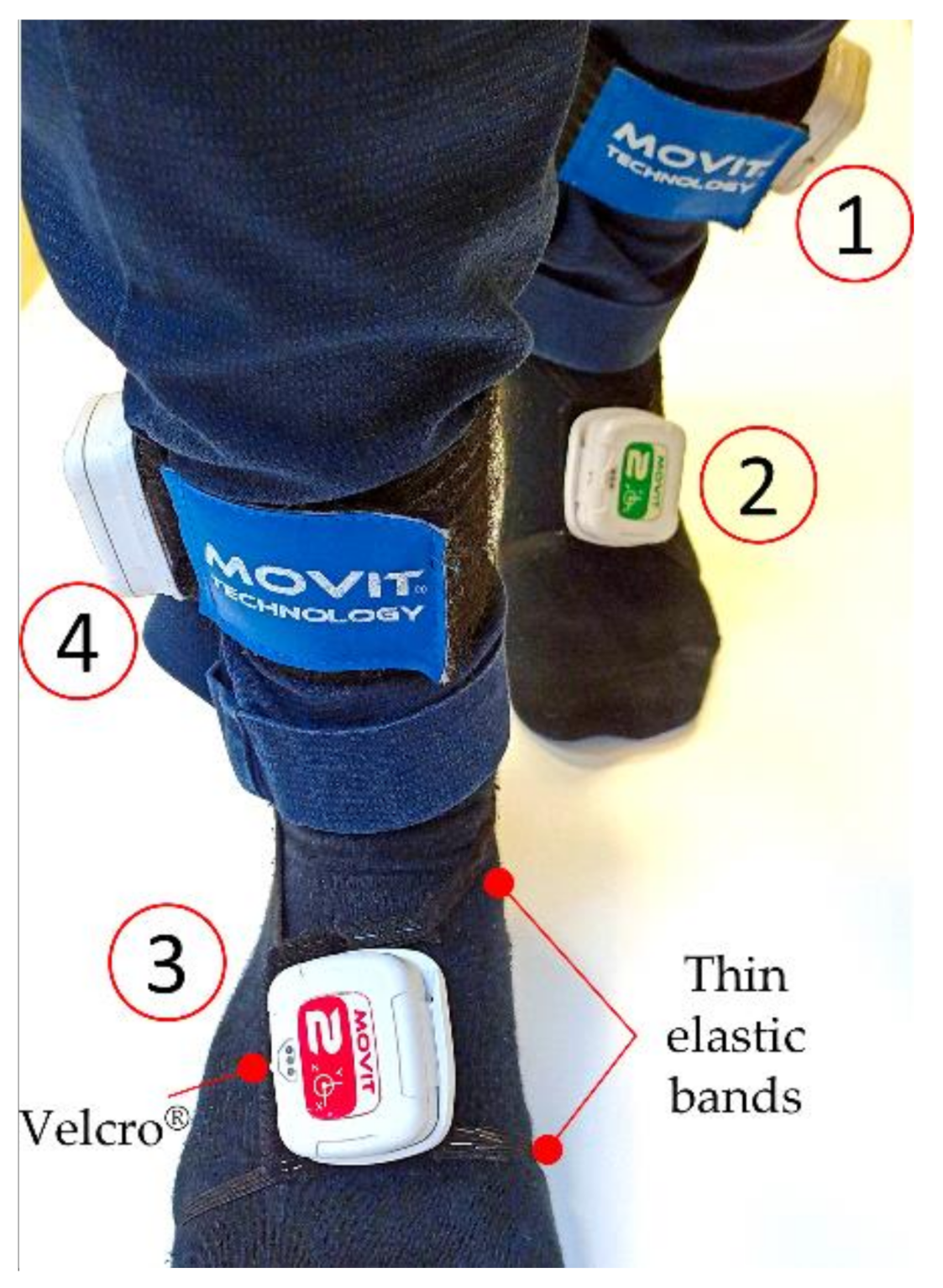
2.2. Wearable Sensors
2.3. Motor Tasks
2.4. Data Analysis
2.4.1. Area Ratio (AR)
2.4.2. Power Ratio (PR)
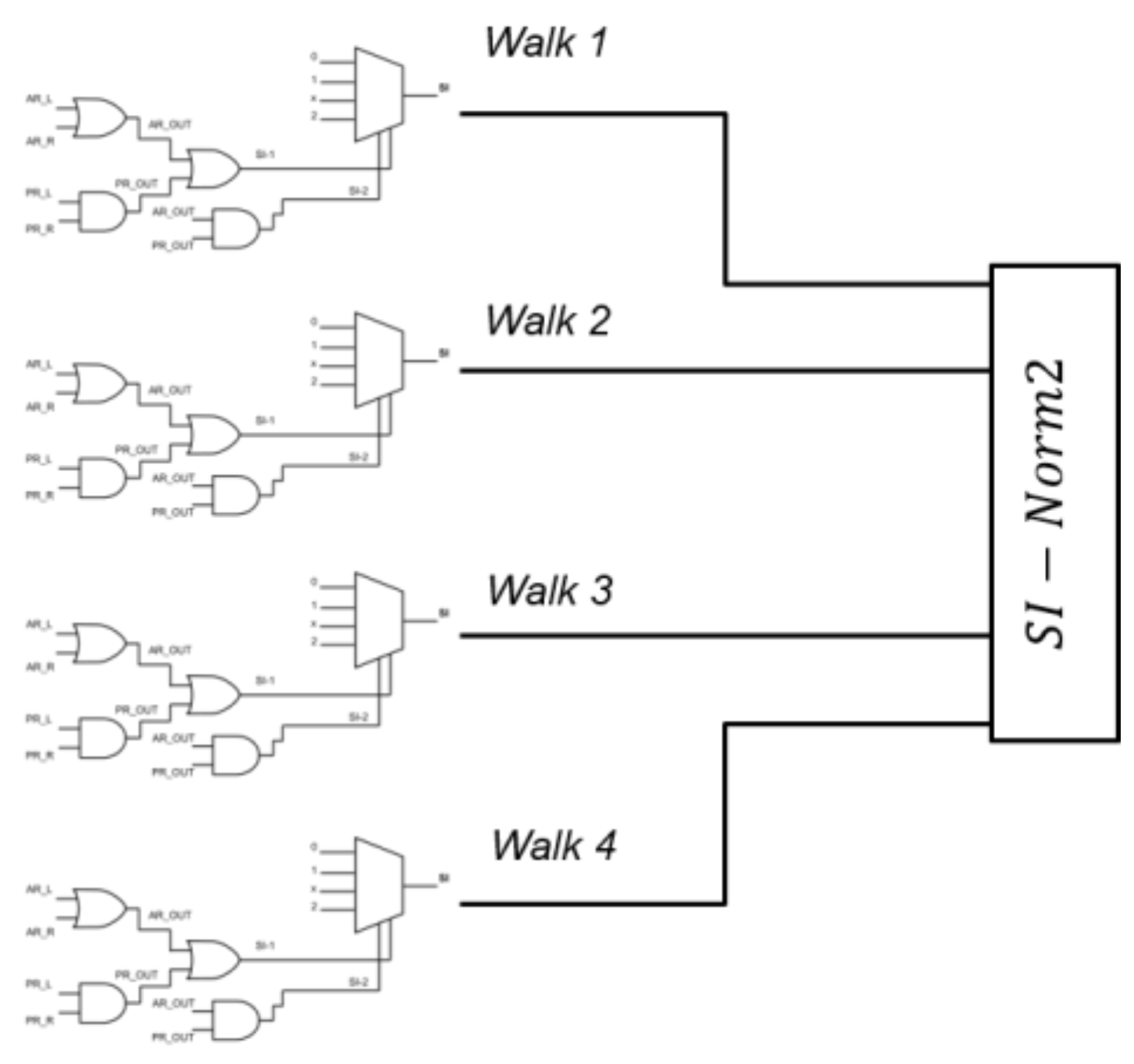
2.4.3. Severity Index (SI) and Si-Norm2
3. Results
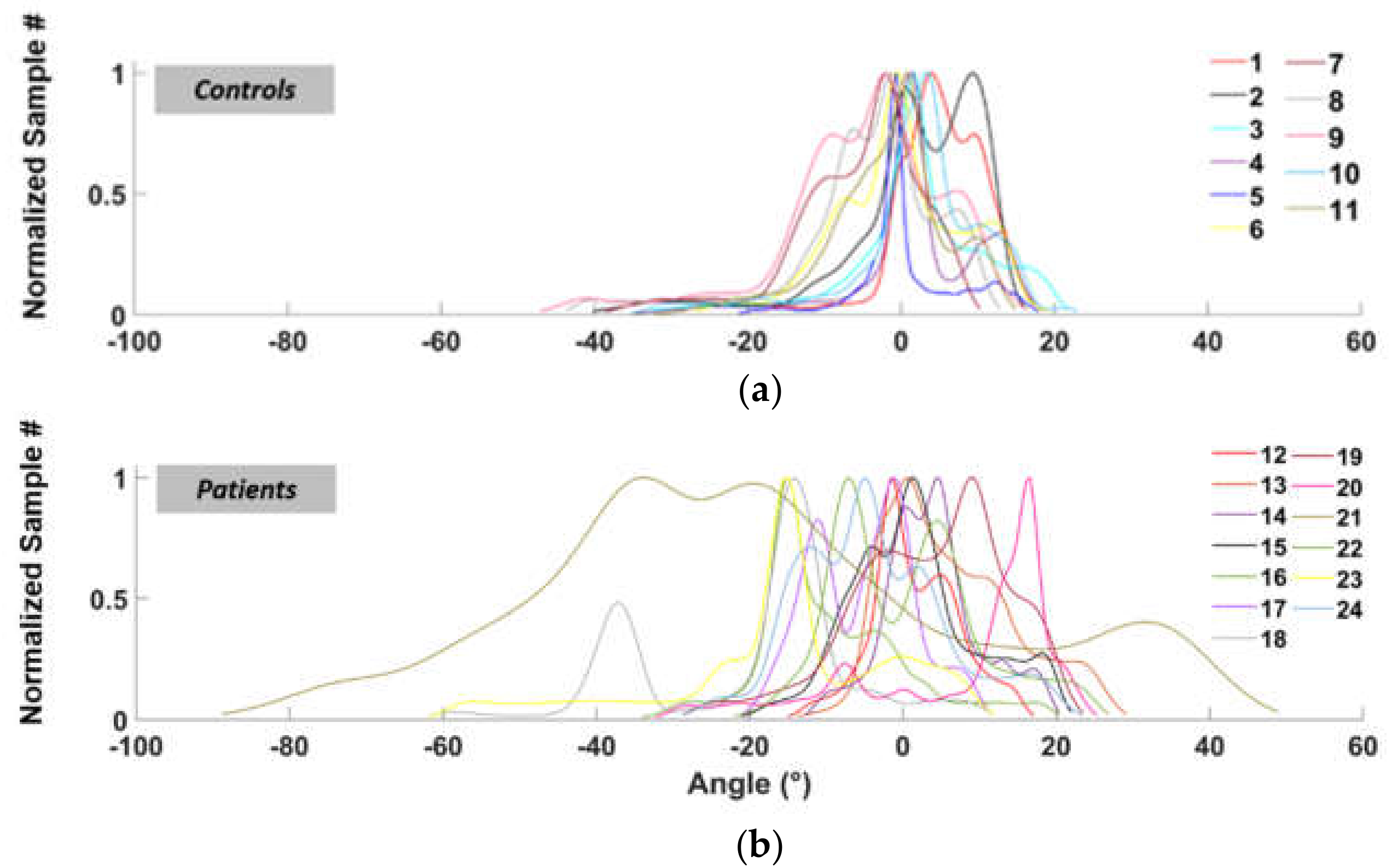
3.1. Area Ratio (AR)
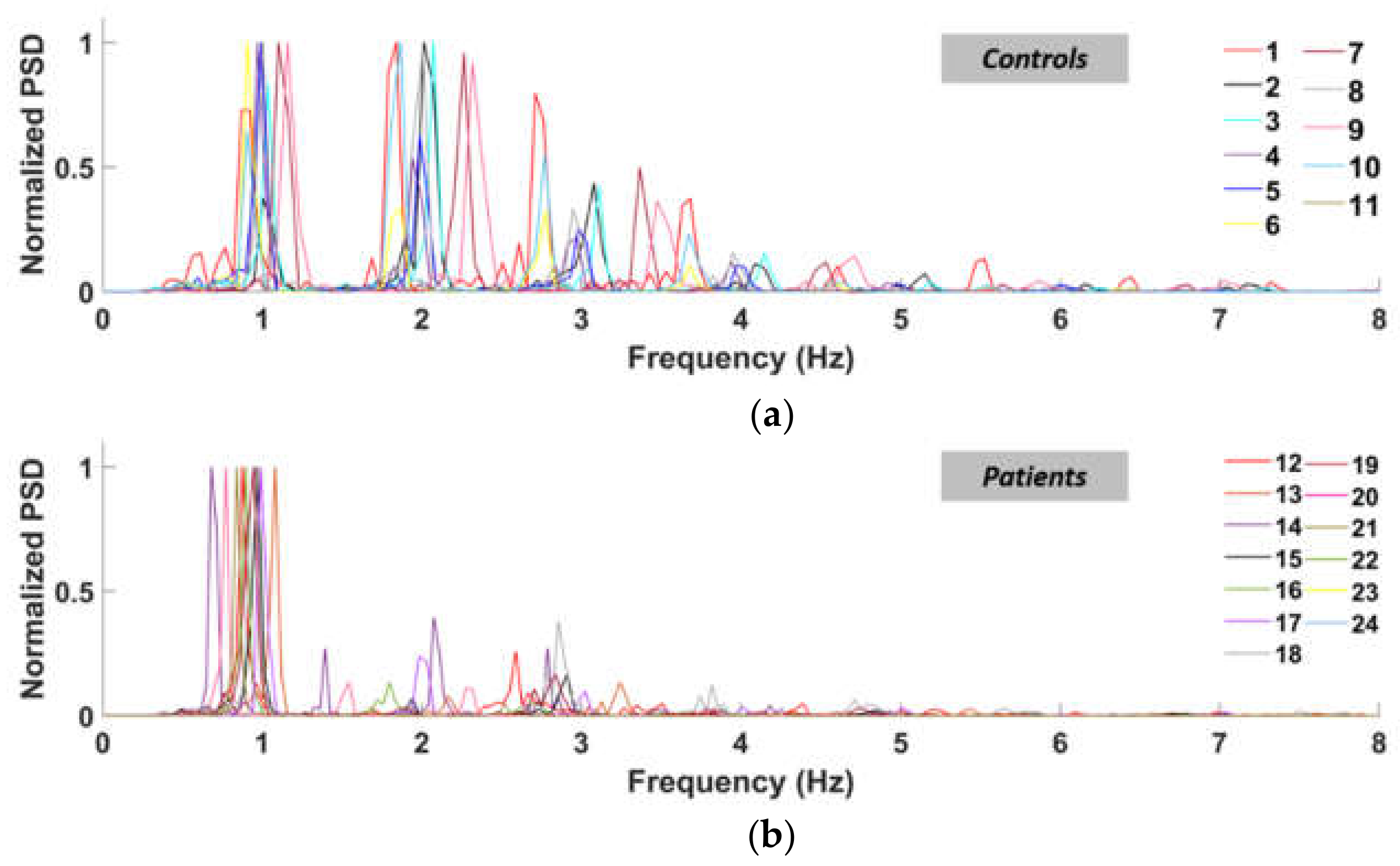
3.2. Power Spectral Density and PR (Power Ratio)
3.3. Severity Index (SI-Norm2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanacore, N.; Rastelli, E.; Antonini, G.; Bianchi, M.L.E.; Botta, A.; Bucci, E.; Casali, C.; Costanzi-Porrini, S.; Giacanelli, M.; Gibellini, M.; et al. An Age-Standardized Prevalence Estimate and a Sex and Age Distribution of Myotonic Dystrophy Types 1 and 2 in the Rome Province, Italy. Neuroepidemiology 2016, 46, 191–197. [Google Scholar] [CrossRef]
- Tomé, S.; Gourdon, G. DM1 Phenotype Variability and Triplet Repeat Instability: Challenges in the Development of New Therapies. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- De Antonio, M.; Dogan, C.; Hamroun, D.; Mati, M.; Zerrouki, S.; Eymard, B.; Katsahian, S.; Bassez, G. Unravelling the Myotonic Dystrophy Type 1 Clinical Spectrum: A Systematic Registry-Based Study with Implications for Disease Classification. Rev. Neurol. 2016, 172, 572–580. [Google Scholar] [CrossRef]
- Okkersen, K.; Jimenez-Moreno, C.; Wenninger, S.; Daidj, F.; Glennon, J.; Cumming, S.; Littleford, R.; Monckton, D.G.; Lochmüller, H.; Catt, M.; et al. Cognitive Behavioural Therapy with Optional Graded Exercise Therapy in Patients with Severe Fatigue with Myotonic Dystrophy Type 1: A Multicentre, Single-Blind, Randomised Trial. Lancet Neurol. 2018, 17, 671–680. [Google Scholar] [CrossRef]
- Mathieu, J.; Boivin, H.; Meunier, D.; Gaudreault, M.; Bégin, P. Assessment of a Disease-Specific Muscular Impairment Rating Scale in Myotonic Dystrophy. Neurology 2001, 56, 336–340. [Google Scholar] [CrossRef]
- Gagnon, C.; Heatwole, C.; Hébert, L.J.; Hogrel, J.-Y.; Laberge, L.; Leone, M.; Meola, G.; Richer, L.; Sansone, V.; Kierkegaard, M. Report of the Third Outcome Measures in Myotonic Dystrophy Type 1 (OMMYD-3) International Workshop Paris, France, June 8, 2015. J. Neuromuscul. Dis. 2018, 5, 523–537. [Google Scholar] [CrossRef]
- Cutellè, C.; Rastelli, E.; Gibellini, M.; Greco, G.; Frezza, E.; Botta, A.; Terracciano, C.; Massa, R. Validation of the Nine Hole Peg Test as a Measure of Dexterity in Myotonic Dystrophy Type 1. Neuromuscul. Disord. 2018, 28, 947–951. [Google Scholar] [CrossRef]
- Jimenez-Moreno, A.C.; Nikolenko, N.; Kierkegaard, M.; Blain, A.P.; Newman, J.; Massey, C.; Moat, D.; Sodhi, J.; Atalaia, A.; Gorman, G.S.; et al. Analysis of the Functional Capacity Outcome Measures for Myotonic Dystrophy. Ann. Clin. Transl. Neurol. 2019, 6, 1487–1497. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; Crugnola, V.; Priano, L.; Menegoni, F.; Trotti, C.; Milano, E.; Mauro, A. Gait Pattern in Myotonic Dystrophy (Steinert Disease): A Kinematic, Kinetic and EMG Evaluation Using 3D Gait Analysis. J. Neurol. Sci. 2011, 314, 83–87. [Google Scholar] [CrossRef]
- Ricci, M.; Di Lazzaro, G.; Pisani, A.; Mercuri, N.B.; Giannini, F.; Saggio, G. Assessment of Motor Impairments in Early Untreated Parkinson’s Disease Patients: The Wearable Electronics Impact. IEEE J. Biomed Health Inform. 2020, 24, 120–130. [Google Scholar] [CrossRef]
- Mazzetta, I.; Zampogna, A.; Suppa, A.; Gumiero, A.; Pessione, M.; Irrera, F. Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson’s Disease Using Electromyography and Inertial Signals. Sensors 2019, 19, 948. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, A.; Manoni, A.; Asci, F.; Liguori, C.; Irrera, F.; Suppa, A. Shedding Light on Nocturnal Movements in Parkinson’s Disease: Evidence from Wearable Technologies. Sensors 2020, 20, 5171. [Google Scholar] [CrossRef]
- Ricci, M.; Terribili, M.; Giannini, F.; Errico, V.; Pallotti, A.; Galasso, C.; Tomasello, L.; Sias, S.; Saggio, G. Wearable-Based Electronics to Objectively Support Diagnosis of Motor Impairments in School-Aged Children. J. Biomech. 2019, 83, 243–252. [Google Scholar] [CrossRef]
- Zampogna, A.; Mileti, I.; Palermo, E.; Celletti, C.; Paoloni, M.; Manoni, A.; Mazzetta, I.; Costa, G.D.; Pérez-López, C.; Camerota, F.; et al. Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders. Sensors 2020, 20, 3247. [Google Scholar] [CrossRef] [PubMed]
- Popp, W.L.; Schneider, S.; Bär, J.; Bösch, P.; Spengler, C.M.; Gassert, R.; Curt, A. Wearable Sensors in Ambulatory Individuals With a Spinal Cord Injury: From Energy Expenditure Estimation to Activity Recommendations. Front. Neurol. 2019, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Prospective Fall-Risk Prediction Models for Older Adults Based on Wearable Sensors. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Errico, V.; Ricci, M.; Pallotti, A.; Giannini, F.; Saggio, G. Ambient assisted living for tetraplegic people by means of an electronic system based on a novel sensory headwear: Increased possibilities for reduced abilities. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Reeder, B.; Chung, J.; Stevens-Lapsley, J. Current Telerehabilitation Research with Older Adults at Home: An Integrative Review. J. Gerontol. Nurs. 2016, 42, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Leoni, A.; Ulisse, I.; Pantoli, L.; Errico, V.; Ricci, M.; Orengo, G.; Giannini, F.; Saggio, G. Energy Harvesting Optimization for Built-in Power Replacement of Electronic Multisensory Architecture. AEU Int. J. Electron. Commun. 2019, 107, 170–176. [Google Scholar] [CrossRef]
- Saggio, G.; Cavallo, P.; Ricci, M.; Errico, V.; Zea, J.; Benalcázar, M.E. Sign Language Recognition Using Wearable Electronics: Implementing k-Nearest Neighbors with Dynamic Time Warping and Convolutional Neural Network Algorithms. Sensors 2020, 20, 3879. [Google Scholar] [CrossRef]
- Miozzi, C.; Errico, V.; Saggio, G.; Gruppioni, E.; Marrocco, G. UHF RFID-Based EMG for Prosthetic Control: Preliminary Results. In Proceedings of the 2019 IEEE International Conference on RFID Technology and Applications (RFID-TA), Pisa, Italy, 25–27 September 2019; pp. 310–313. [Google Scholar]
- Muñoz, B.; Valderrama, J.; Orozco, J.; Castaño, Y.; Montilla, L.; Rincon, D.; Navarro, A. Smart Tracking and Wearables: Techniques in Gait Analysis and Movement in Pathological Aging. Smart Healthc. 2019. [Google Scholar] [CrossRef]
- Chapron, K.; Plantevin, V.; Thullier, F.; Bouchard, K.; Duchesne, E.; Gaboury, S. A More Efficient Transportable and Scalable System for Real-Time Activities and Exercises Recognition. Sensors 2018, 18, 268. [Google Scholar] [CrossRef]
- Storm, F.A.; Cesareo, A.; Reni, G.; Biffi, E. Wearable Inertial Sensors to Assess Gait during the 6-Minute Walk Test: A Systematic Review. Sensors 2020, 20, 2660. [Google Scholar] [CrossRef]
- Naro, A.; Portaro, S.; Milardi, D.; Billeri, L.; Leo, A.; Militi, D.; Bramanti, P.; Calabrò, R.S. Paving the Way for a Better Understanding of the Pathophysiology of Gait Impairment in Myotonic Dystrophy: A Pilot Study Focusing on Muscle Networks. J. Neuroeng. Rehabil. 2019, 16, 116. [Google Scholar] [CrossRef]
- Jimenez-Moreno, A.C.; Charman, S.J.; Nikolenko, N.; Larweh, M.; Turner, C.; Gorman, G.; Lochmüller, H.; Catt, M. Analyzing Walking Speeds with Ankle and Wrist Worn Accelerometers in a Cohort with Myotonic Dystrophy. Disabil. Rehabil. 2019, 41, 2972–2978. [Google Scholar] [CrossRef]
- Saggio, G.; Tombolini, F.; Ruggiero, A. Technology-Based Complex Motor Tasks Assessment: A 6-DOF Inertial-Based System Versus a Gold-Standard Optoelectronic-Based One. IEEE Sens. J. 2021, 21, 1616–1624. [Google Scholar] [CrossRef]
- Das, K.D.; Saji, A.J.; Kumar, C.S. Frequency Analysis of Gait Signals for Detection of Neurodegenerative Diseases. In Proceedings of the 2017 International Conference on Circuit, Power and Computing Technologies (ICCPCT), Kollam, India, 20–21 April 2017; pp. 1–6. [Google Scholar]



| SI-1 | SI-2 | SI | Severity Grade |
|---|---|---|---|
| 0 | 0 | 0 | Regular |
| 1 | 0 | 1 | Mild |
| 1 | 1 | 2 | Severe |
| 0 | 1 | X | Impossible |
| # | CS_1L | CS_1R | FP_2L | FP_2R | CS_3L | CS_3R | FP_4L | FP_4R | |
|---|---|---|---|---|---|---|---|---|---|
| CONTROLS | 1 | 0.673 | 0.777 | 0.594 | 0.672 | 0.375 | 0.749 | 0.957 | 0.715 |
| 2 | 0.582 | 0.771 | 0.251 | 0.268 | 0.720 | 0.578 | 0.506 | 0.420 | |
| 3 | 0.867 | 0.820 | 0.866 | 0.873 | 0.761 | 0.797 | 0.760 | 0.750 | |
| 4 | 0.880 | 0.838 | 0.859 | 0.492 | 0.506 | 0.650 | 0.976 | 0.632 | |
| 5 | 0.832 | 1.021 | 0.689 | 0.627 | 0.558 | 0.568 | 0.863 | 0.408 | |
| 6 | 0.998 | 0.911 | 0.648 | 0.599 | 0.881 | 0.673 | 0.564 | 0.515 | |
| 7 | 1.542 | 0.240 | 1.486 | 0.734 | 0.596 | 1.182 | 0.471 | 1.055 | |
| 8 | 1.415 | 1.781 | 0.727 | 0.887 | 0.764 | 1.683 | 1.162 | 0.791 | |
| 9 | 1.189 | 1.174 | 1.015 | 1.385 | 1.086 | 0.732 | 0.774 | 0.946 | |
| 10 | 1.495 | 0.908 | 1.366 | 0.683 | 1.226 | 1.090 | 1.712 | 1.076 | |
| 11 | 1.335 | 1.549 | 1.181 | 1.986 | 1.570 | 0.688 | 1.092 | 2.118 | |
| PATIENTS | 12 | 0.468 | 0.860 | 0.634 | 0.504 | 0.430 | 1.739 | 0.616 | 0.794 |
| 13 | 0.419 | 0.392 | 9.681 | 0.771 | 0.961 | 0.680 | 0.802 | 0.570 | |
| 14 | 1.423 | 1.941 | 12.028 | 1.919 | 1.358 | 2.025 | 1.698 | 2.105 | |
| 15 | 1.048 | 1.905 | 0.220 | 1.511 | 1.886 | 0.591 | 1.487 | 0.473 | |
| 16 | 0.456 | 1.834 | 0.698 | 1.374 | 0.465 | 0.605 | 0.404 | 1.911 | |
| 17 | 2.246 | 4.154 | 0.915 | 1.333 | 2.432 | 0.526 | 0.231 | 1.189 | |
| 18 | 1.488 | 0.948 | 1.690 | 1.523 | 1.970 | 1.173 | 1.631 | 1.843 | |
| 19 | 1.887 | 4.532 | 0.813 | 0.821 | 0.589 | 0.713 | 0.673 | 0.660 | |
| 20 | 1.736 | 2.138 | 2.100 | 2.864 | 2.752 | 1.986 | 2.141 | 2.459 | |
| 21 | 1.144 | 1.621 | 0.636 | 1.555 | 0.804 | 1.900 | 0.636 | 1.545 | |
| 22 | 1.992 | 2.170 | 1.538 | 1.924 | 1.738 | 2.188 | 1.071 | 1.697 | |
| 23 | 1.025 | 1.000 | 0.906 | 0.855 | 3.117 | 3.192 | 1.461 | 0.796 | |
| 24 | 0.939 | 0.837 | 0.617 | 0.620 | 0.458 | 2.261 | 0.464 | 0.494 |
| # | CS_1L | CS_1R | FP_2L | FP_2R | CS_3L | CS_3R | FP_4L | FP_4R | |
|---|---|---|---|---|---|---|---|---|---|
| CONTROLS | 1 | 0.381 | 0.500 | 0.902 | 1.242 | 0.188 | 0.449 | 0.140 | 0.818 |
| 2 | 0.203 | 0.239 | 0.207 | 0.416 | 0.348 | 0.357 | 0.407 | 0.345 | |
| 3 | 0.521 | 0.433 | 0.586 | 0.615 | 0.559 | 0.462 | 0.761 | 0.572 | |
| 4 | 0.775 | 0.640 | 0.899 | 0.688 | 0.813 | 0.658 | 0.883 | 0.596 | |
| 5 | 0.812 | 0.871 | 0.682 | 0.741 | 0.605 | 0.412 | 0.540 | 0.447 | |
| 6 | 0.921 | 0.860 | 0.764 | 0.817 | 0.741 | 1.089 | 0.672 | 1.090 | |
| 7 | 0.622 | 0.788 | 0.864 | 0.887 | 0.908 | 0.962 | 0.837 | 1.063 | |
| 8 | 0.478 | 0.613 | 0.603 | 0.560 | 0.490 | 0.589 | 0.836 | 0.940 | |
| 9 | 0.472 | 0.642 | 0.679 | 0.731 | 0.664 | 0.547 | 0.315 | 0.192 | |
| 10 | 0.343 | 0.171 | 0.365 | 0.165 | 0.310 | 0.388 | 0.373 | 0.423 | |
| 11 | 0.602 | 0.825 | 2.071 | 0.558 | 0.685 | 0.691 | 0.847 | 0.638 | |
| PATIENTS | 12 | 1.022 | 1.388 | 1.180 | 1.077 | 1.051 | 1.322 | 1.216 | 1.149 |
| 13 | 1.971 | 1.446 | 1.928 | 1.249 | 1.827 | 1.471 | 2.512 | 1.476 | |
| 14 | 1.227 | 2.945 | 0.976 | 1.874 | 1.379 | 2.695 | 0.917 | 1.637 | |
| 15 | 1.880 | 1.097 | 1.449 | 1.059 | 1.906 | 0.683 | 1.764 | 0.673 | |
| 16 | 1.115 | 1.971 | 1.145 | 1.679 | 0.984 | 2.017 | 1.030 | 1.772 | |
| 17 | 1.987 | 1.612 | 0.635 | 1.291 | 2.008 | 2.339 | 2.132 | 2.079 | |
| 18 | 1.853 | 1.240 | 1.536 | 1.296 | 1.461 | 2.641 | 1.397 | 2.706 | |
| 19 | 0.775 | 0.261 | 0.365 | 0.154 | 0.667 | 0.474 | 0.602 | 0.318 | |
| 20 | 3.142 | 2.506 | 3.191 | 2.550 | 2.535 | 1.945 | 2.959 | 1.882 | |
| 21 | 1.624 | 1.738 | 1.886 | 1.904 | 1.582 | 1.719 | 1.887 | 1.905 | |
| 22 | 1.851 | 2.071 | 1.342 | 2.223 | 2.864 | 4.560 | 1.288 | 1.757 | |
| 23 | 7.622 | 8.977 | 6.692 | 9.246 | 2.907 | 2.640 | 2.648 | 2.939 | |
| 24 | 2.111 | 1.167 | 1.828 | 1.125 | 1.584 | 1.220 | 1.457 | 1.288 |
| SUBJECT # | AGE | GENDER | MIRS | SI-Norm2 | |
|---|---|---|---|---|---|
| Controls | 1 | 62 | M | - | 1 |
| 2 | 59 | F | - | 0 | |
| 3 | 48 | F | - | 0 | |
| 4 | 41 | M | - | 0 | |
| 5 | 28 | M | - | 1 | |
| 6 | 30 | M | - | 0 | |
| 7 | 46 | F | - | 4 | |
| 8 | 46 | F | - | 3 | |
| 9 | 29 | F | - | 4 | |
| 10 | 46 | F | - | 4 | |
| 11 | 28 | F | - | 4 | |
| Patients | 12 | 48 | M | 3 | 7 |
| 13 | 47 | F | 3 | 7 | |
| 14 | 53 | F | 3 | 10 | |
| 15 | 55 | M | 3 | 10 | |
| 16 | 38 | F | 3 | 12 | |
| 17 | 38 | F | 3 | 16 | |
| 18 | 55 | M | 3 | 16 | |
| 19 | 52 | F | 3 | 2 | |
| 20 | 52 | M | 4 | 16 | |
| 21 | 57 | M | 4 | 16 | |
| 22 | 33 | M | 4 | 16 | |
| 23 | 53 | M | 4 | 13 | |
| 24 | 22 | F | 4 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saggio, G.; Manoni, A.; Errico, V.; Frezza, E.; Mazzetta, I.; Rota, R.; Massa, R.; Irrera, F. Objective Assessment of Walking Impairments in Myotonic Dystrophy by Means of a Wearable Technology and a Novel Severity Index. Electronics 2021, 10, 708. https://doi.org/10.3390/electronics10060708
Saggio G, Manoni A, Errico V, Frezza E, Mazzetta I, Rota R, Massa R, Irrera F. Objective Assessment of Walking Impairments in Myotonic Dystrophy by Means of a Wearable Technology and a Novel Severity Index. Electronics. 2021; 10(6):708. https://doi.org/10.3390/electronics10060708
Chicago/Turabian StyleSaggio, Giovanni, Alessandro Manoni, Vito Errico, Erica Frezza, Ivan Mazzetta, Rosario Rota, Roberto Massa, and Fernanda Irrera. 2021. "Objective Assessment of Walking Impairments in Myotonic Dystrophy by Means of a Wearable Technology and a Novel Severity Index" Electronics 10, no. 6: 708. https://doi.org/10.3390/electronics10060708
APA StyleSaggio, G., Manoni, A., Errico, V., Frezza, E., Mazzetta, I., Rota, R., Massa, R., & Irrera, F. (2021). Objective Assessment of Walking Impairments in Myotonic Dystrophy by Means of a Wearable Technology and a Novel Severity Index. Electronics, 10(6), 708. https://doi.org/10.3390/electronics10060708








