L-(+)-Lactic Acid from Reed: Comparing Various Resources for the Nutrient Provision of B. coagulans
Abstract
:1. Introduction
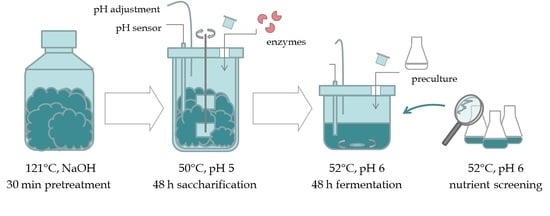
2. Materials and Methods
2.1. Raw Material & Processing
2.2. Chemical and Enzymatic Pretreatment
2.3. Bacterial Strain and Preculture Conditions
2.4. Fermentation Studies with Varying Nutrient Sources
2.5. Analytics
2.6. Calculations
3. Results
3.1. Reed Analysis & Pretreatment
3.2. Shaking Flask Fermentation Experiments
3.3. Lab-Scale Fermentation Experiments
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- de Jong, E.; Stichnothe, H.; Bell, G.; Jørgensen, H. Bio-Based Chemicals: A 2020 Update; IEA Bioenergy Task 42; IEA Bioenergy, 2020; ISBN 978-1-910154-69-4. Available online: https://www.ieabioenergy.com/wp-content/uploads/2020/02/Bio-based-chemicals-a-2020-update-final-200213.pdf (accessed on 20 July 2020).
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–555. [Google Scholar] [CrossRef]
- Komesu, A.; Allan Rocha de Oliveira, J.; Helena da Silva Martins, L.; Lee, H.D.; Lee, M.Y.; Hwang, Y.S.; Cho, Y.H.; Kim, H.W. Lactic Acid Production to Purification: A Review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef] [Green Version]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent advances in bioplastics: Application and biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [Green Version]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Microbial production of specialty organic acids from renewable and waste materials. Crit. Rev. Biotechnol. 2015, 35, 497–513. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The utilisation of reed (Phragmites australis): A review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Brix, H. Functions of macrophytes in constructed wetlands. Water Sci. Technol. 1994, 29, 71–78. [Google Scholar] [CrossRef]
- van der Sluis, T.; Poppens, R.; Kraisvitnii, P.; Rii, O.; Lesschen, J.P.; Galytska, M.; Elbersen, W. Reed Harvesting from Wetlands for Bioenergy; Alterra Report 2460; Alterra Wageningen UR: Wageningen, Netherlands, 2013. [Google Scholar]
- Baibagyssov, A.; Thevs, N.; Nurtazin, S.; Waldhardt, R.; Beckmann, V.; Salmurzauly, R. Biomass Resources of Phragmites australis in Kazakhstan: Historical Developments, Utilization, and Prospects. Resources 2020, 9, 74. [Google Scholar] [CrossRef]
- Perttunen, J.; Myllykoski, L.; Keiski, R.L. Lactic Acid Fermentation of Hemicellulose Liquors and Their Activated Carbon Pretreatments. In Engineering and Manufacturing for Biotechnology; Hofman, M., Thonart, P., Eds.; Kluwer Academic Publishers: Berlin, Germany, 2001; ISBN 0-7923-6927-0. [Google Scholar]
- Zhang, Y.; Li, M.; Nie, T.; Ni, Z. A Process Study of Lactic Acid Production from Phragmites australis Straw by a Thermophilic Bacillus coagulans Strain under Non-Sterilized Conditions. Processes 2018, 6, 175. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, K.M.; Liu, S.; Hughes, S.R.; Rich, J.O. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 2010, 32, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Hu, G.; Pan, L.; Wang, Z.; Zhou, Y.; Wang, Y.; Ruan, Z.; He, M. Highly efficient production of optically pure L-lactic acid from corn stover hydrolysate by thermophilic Bacillus coagulans. Bioresour. Technol. 2016, 219, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, L.; Ju, J.; Yu, B.; Xu, P.; Ma, Y. Efficient production of polymer-grade L-lactic acid from corn stover hydrolyzate by thermophilic Bacillus sp. strain XZL4. Springerplus 2012, 1, 43. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, Z.; Lin, Y.; Zhao, S.; Mei, Y.; Liang, Y.; Peng, N. High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresour. Technol. 2015, 182, 251–257. [Google Scholar] [CrossRef]
- Peng, L.; Wang, L.; Che, C.; Yang, G.; Yu, B.; Ma, Y. Bacillus sp. strain P38: An efficient producer of l-lactate from cellulosic hydrolysate, with high tolerance for 2-furfural. Bioresour. Technol. 2013, 149, 169–176. [Google Scholar] [CrossRef]
- Pleissner, D.; Venus, J. Utilization of protein-rich residues in biotechnological processes. Appl. Microbiol. Biotechnol. 2016, 100, 2133–2140. [Google Scholar] [CrossRef]
- Michelson, T.; Kask, K.; Jõgi, E.; Talpsep, E.; Suitso, I.; Nurk, A. L(+)-Lactic acid producer Bacillus coagulans SIM-7 DSM 14043 and its comparison with Lactobacillus delbrueckii ssp. lactis DSM 20073. Enzym. Microb. Technol. 2006, 39, 861–867. [Google Scholar] [CrossRef]
- de la Torre, I.; Ladero, M.; Santos, V.E. Production of D-lactic acid by L. delbrueckii growing on orange peel waste hydrolysates and model monosaccharide solutions: Effects of pH and temperature on process kinetics. Biomass Convers. Biorefinery 2019, 9, 565–575. [Google Scholar] [CrossRef]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef]
- Meng, Y.; Xue, Y.; Yu, B.; Gao, C.; Ma, Y. Efficient production of l-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20. Bioresour. Technol. 2012, 116, 334–339. [Google Scholar] [CrossRef]
- Assavasirijinda, N.; Ge, D.; Yu, B.; Xue, Y.; Ma, Y. Efficient fermentative production of polymer-grade d-lactate by an engineered alkaliphilic Bacillus sp. strain under non-sterile conditions. Microb. Cell Fact. 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhao, B.; Li, F.; Xu, K.; Ma, C.; Tao, F.; Li, Q.; Xu, P. Highly efficient production of d-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl. Microbiol. Biotechnol. 2011, 89, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.Y.; Wu, J.C. Use of dry yeast cells as a cheap nitrogen source for lactic acid production by thermophilic Bacillus coagulans WCP10-4. Front. Chem. Sci. Eng. 2015, 9, 381–385. [Google Scholar] [CrossRef]
- Ma, K.; Maeda, T.; You, H.; Shirai, Y. Open fermentative production of l-lactic acid with high optical purity by thermophilic Bacillus coagulans using excess sludge as nutrient. Bioresour. Technol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Venus, J.; Schneider, R.; Socaciu, C. Lactic acid production by Lactobacillus paracasei 168 in discontinuous fermentation using lucerne green juice as nutrient substitute. Chem. Eng. Technol. 2010, 33, 468–474. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Schneider, R.; Vaz Rossell, C.E.; Maciel Filho, R.; Venus, J. Polymer grade l-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour. Technol. Rep. 2019, 6, 26–31. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis; Technical Report NREL/TP-510-42620; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; Technical Report NREL/TP-510-42628; National Renewable Energy Laboratory: Golden, CO, USA, 1996. [Google Scholar]
- de Toledo, V.D.A.A.; Ruvolo-Takasusuki, M.C.C.; de Oliveira, A.J.B.; Dechechi Chambó, E.; Sanches Lopes, S.M. Spectrophotometry as a Tool for Dosage Sugars in Nectar of Crops Pollinated by Honeybees. In Macro to Nano Spectroscopy; Uddin, J., Ed.; Intech Open: London, UK, 2012. [Google Scholar]
- VDLUFA (Association of German Agricultural Analytic and Research Institutes). VDLUFA Methodenbuch Band III Die Chemische Untersuchung Von Futtermitteln, 3rd ed.; VDLUFA-Verlag: Darmstadt, Germany, 1976; ISBN 978-3-941273-14-6. [Google Scholar]
- Wyman, C.; Decker, S.; Himmel, M.; Brady, J.; Skopec, C.; Viikari, L. Hydrolysis of Cellulose and Hemicellulose. In Polysaccharides; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0429131660. [Google Scholar]
- Templeton, D.W.; Scarlata, C.J.; Sluiter, J.B.; Wolfrum, E.J. Compositional analysis of lignocellulosic feedstocks. 2. Method uncertainties. J. Agric. Food Chem. 2010, 58, 9054–9062. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Pisello, A.L.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable ethanol production from common reed (Phragmites australis) through simultaneuos saccharification and fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef] [Green Version]
- Seidl, P.R.; Goulart, A.K. Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr. Opin. Green Sustain. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.; Beers, R.J. Growth of Bacillus coagulans in chemically defined media. J. Bacteriol. 1967, 94, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Gómez, J.P.; Unger, P.; Schneider, R.; Venus, J. From Upstream to Purification: Production of Lactic Acid from the Organic Fraction of Municipal Solid Waste. Waste Biomass Valoriz. 2020. [Google Scholar] [CrossRef] [Green Version]
- Altaf, M.; Naveena, B.J.; Ready, G. Screening of inexpensive nitrogen sources for production of L(+) lactic acid from starch by amylolytic lactobacillus amylophilus GV6 in single step fermentation. Food Technol. Biotechnol. 2005, 43, 235–239. [Google Scholar]




| Cellulose (%) | Hemicellulose (%) | Lignin (%) | Reference (-) |
|---|---|---|---|
| 38.13 | 20.51 | 23.02 | [39] |
| 42.45 | 29.34 | 17.28 | [14] |
| 40.90 | 27.65 | 19.46 | This study |
| Nutrient Source (-) | Abbr. (-) | DM105 °C (%) | NKjel (mg gFM−1) | NKjel (mg mL−1) | NKjel in SF (mg 0.1 L−1) |
|---|---|---|---|---|---|
| Yeast Extract | YE | 87.3 | 115.5 | - | 57.8 |
| Malt Extract | ME | 89.4 | 13.4 | - | 6.7 |
| Peptone fr. Soymeal | PS | 92.5 | 94.5 | - | 47.3 |
| Tryptone | TR | 95.0 | 133.2 | - | 66.6 |
| Baker’s Yeast | BaY | 97.7 | 68.7 | - | 34.4 |
| Brewer’s Yeast | BrY | 90.8 | 82.2 | - | 41.1 |
| Insect Meal | IM | 95.7 | 58.1 | - | 29.1 |
| Lucerne Green Juice | GJ | 5.2 | - | 2.24 | 22.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroedter, L.; Schneider, R.; Remus, L.; Venus, J. L-(+)-Lactic Acid from Reed: Comparing Various Resources for the Nutrient Provision of B. coagulans. Resources 2020, 9, 89. https://doi.org/10.3390/resources9070089
Schroedter L, Schneider R, Remus L, Venus J. L-(+)-Lactic Acid from Reed: Comparing Various Resources for the Nutrient Provision of B. coagulans. Resources. 2020; 9(7):89. https://doi.org/10.3390/resources9070089
Chicago/Turabian StyleSchroedter, Linda, Roland Schneider, Lisa Remus, and Joachim Venus. 2020. "L-(+)-Lactic Acid from Reed: Comparing Various Resources for the Nutrient Provision of B. coagulans" Resources 9, no. 7: 89. https://doi.org/10.3390/resources9070089