Pituitary Actions of EGF on Gonadotropins, Growth Hormone, Prolactin and Somatolactins in Grass Carp
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Cell Culture, RNA Isolation, Reverse Transcription and Real-Time PCR
2.3. Measurement of PRL, GH and LH Secretion by Fluorescence-Based ELISA
2.4. Data Transformation and Statistical Analysis
3. Results
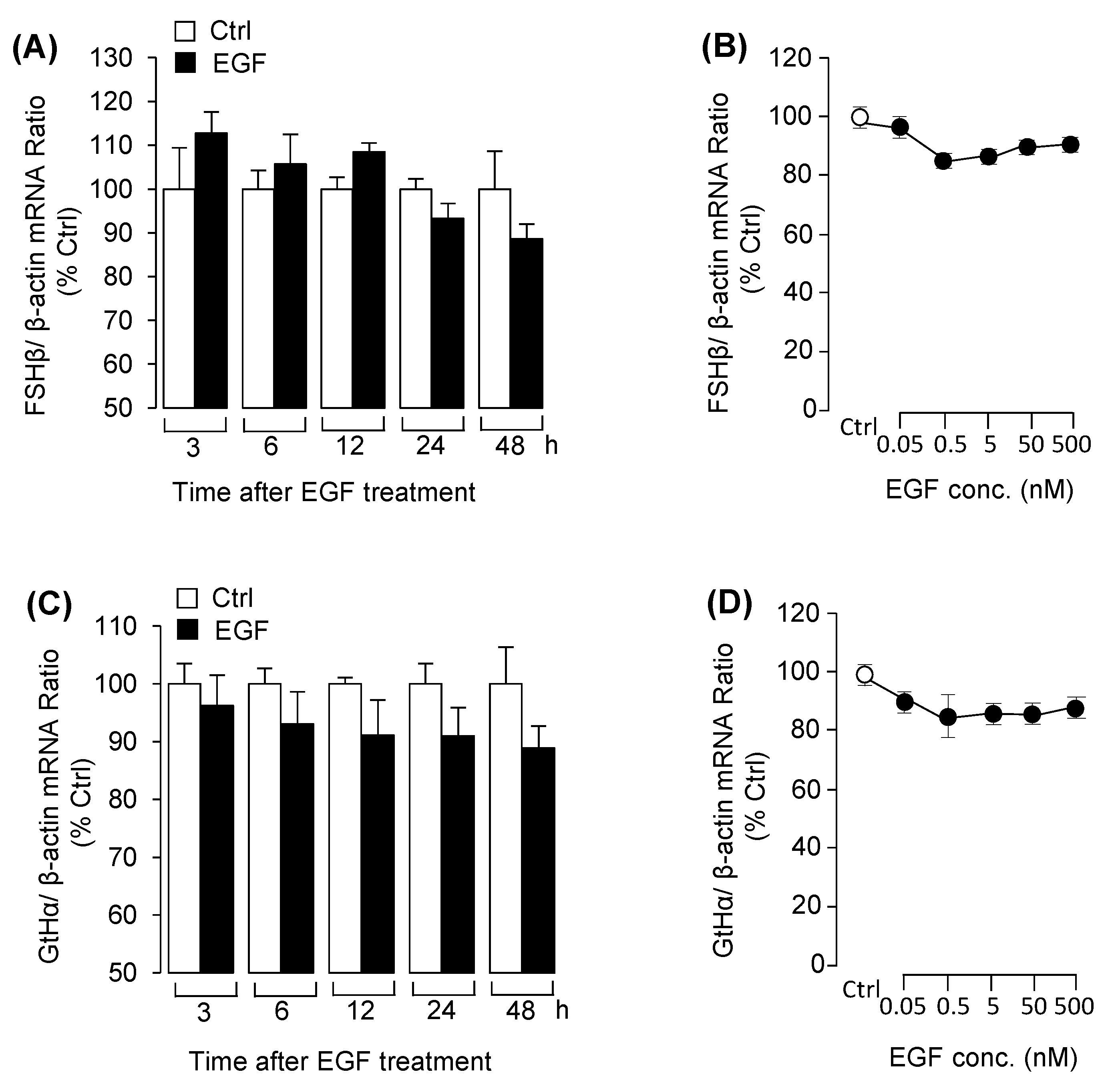
3.1. EGF Reduced LHβ mRNA Expression in Grass Carp Pituitary Cells
3.2. EGF Induced Pituitary GH mRNA Expression, But No Effect on GH Secretion
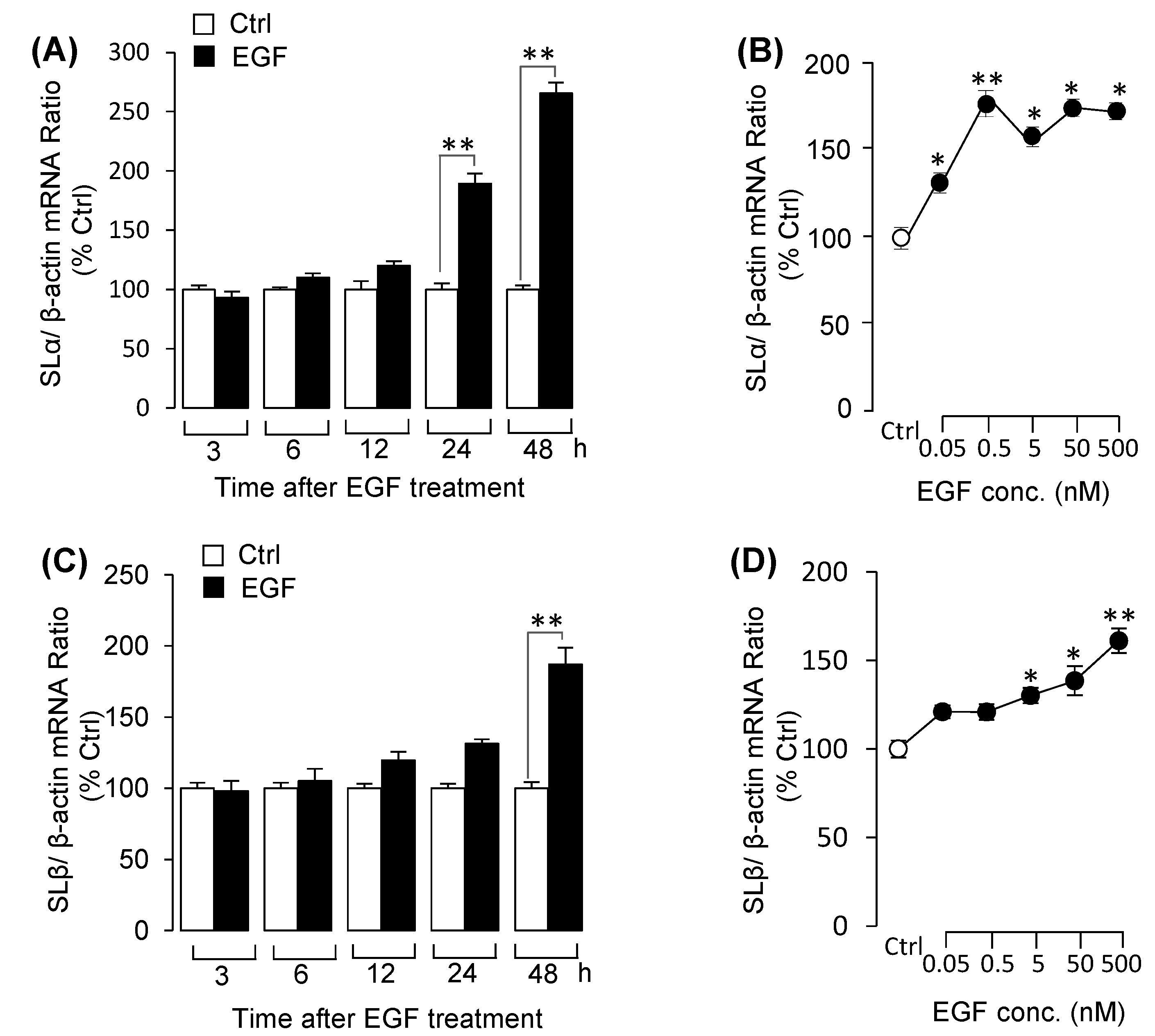
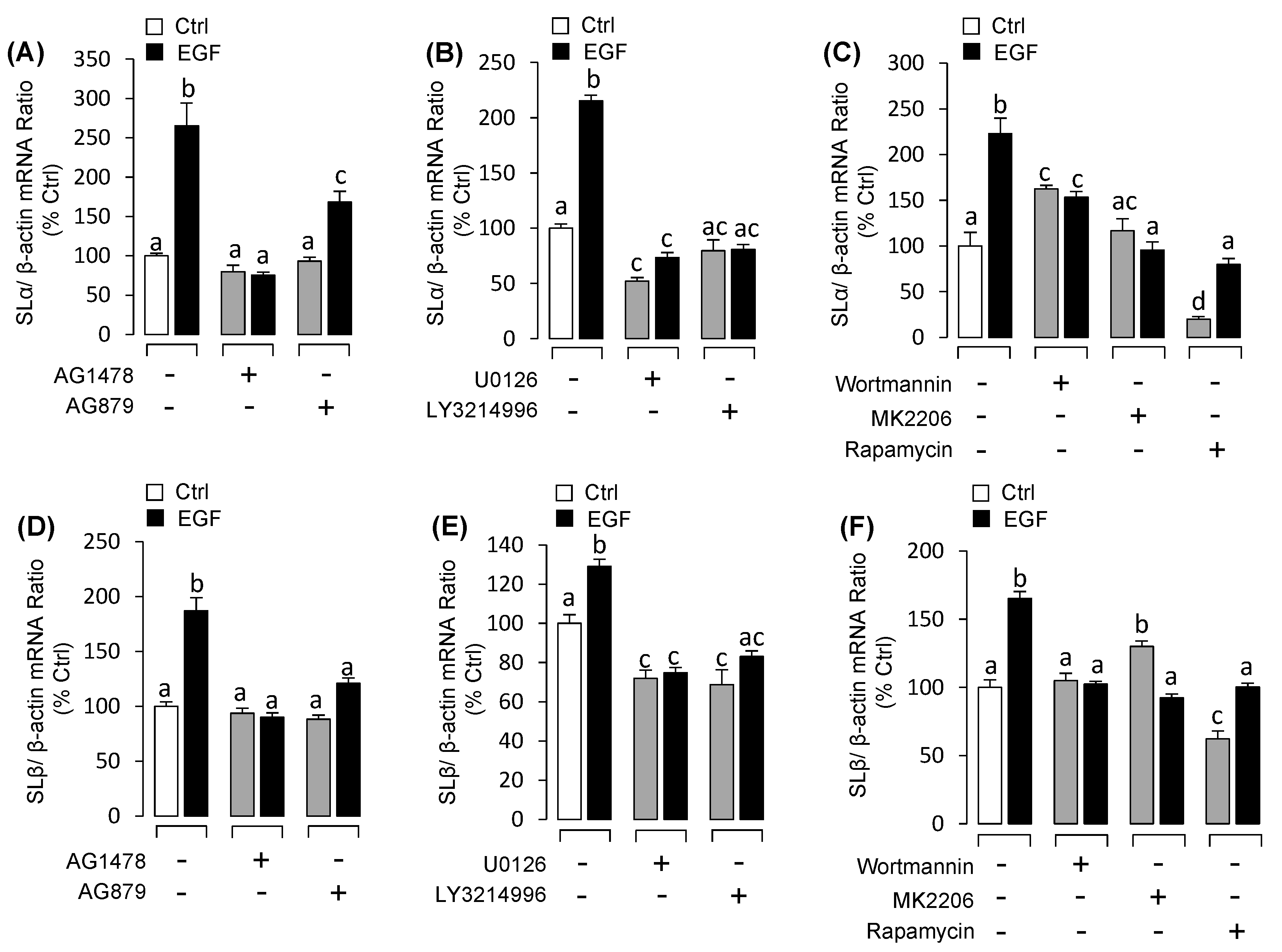
3.3. EGF Stimulated Both SLα and SLβ mRNA Expression in Grass Carp Pituitary Cells
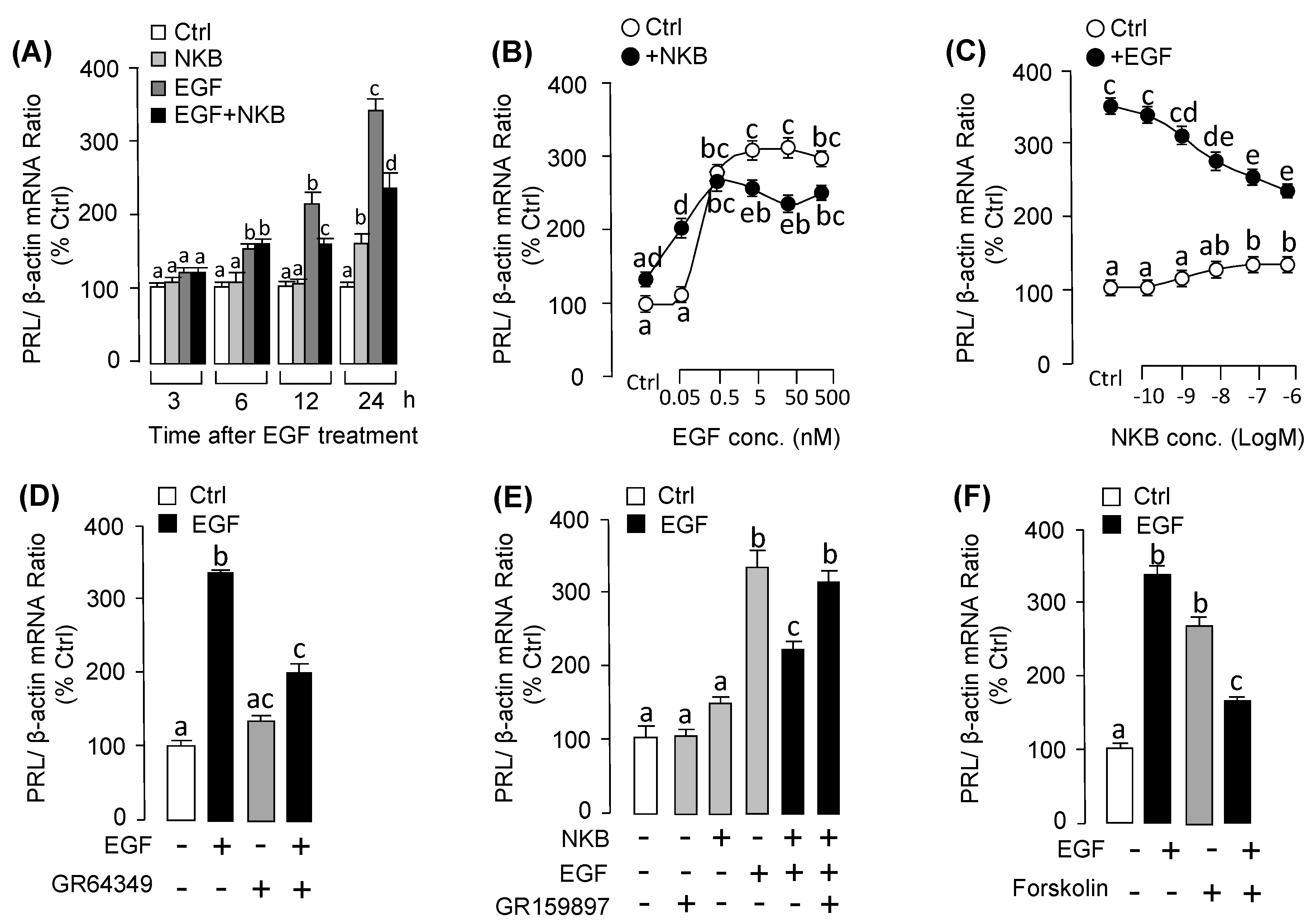
3.4. EGF Strongly Induced PRL Secretion and mRNA Expression in Grass Carp Pituitary Cells
3.5. NKB Suppressed EGF-Induced PRL mRNA Expression in Grass Carp Pituitary Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| Akt | protein kinase B |
| cAMP | cyclic adenosine monophosphate |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinase |
| FSH | follicle-stimulating hormone |
| GH | growth hormone |
| GPCR | G protein coupled receptor |
| GtHα | gonadotropin subunit alpha |
| HB-EGF | Heparin-binding EGF-like growth factor |
| LH | luteinizing hormone |
| MAPK | mitogen-activated protein kinase |
| mTOR | mammalian target of rapamycin |
| NKB | neurokinin B |
| NKR | neurokinin receptor |
| PI3K | phosphatidylinositol-3-kinase |
| PKA | protein kinase A |
| PRL | prolactin |
| PRL | prolactin |
| SLα | somatolactin α |
| SLβ | somatolactin β |
| TAC3 | tachykinin 3 |
References
- Hull, K. Growth hormone: Roles in female reproduction. J. Endocrinol. 2001, 168, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.; Dickey, J.T.; Campbell, B. Biochemistry and Physiology of fish gonadotropins. Fish Physiol. Biochem. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Higham, C.E.; Johannsson, G.; Shalet, S.M. Hypopituitarism. Lancet 2016, 388, 2403–2415. [Google Scholar] [CrossRef]
- Frank, S.J. Mechanistic aspects of crosstalk between GH and PRL and ErbB receptor family signaling. J. Mammary Gland Biol. Neoplasia 2008, 13, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, O. GH, IGF-I, and growth. J. Pediatr. Endocrinol. Metab. 2004, 4, 1321–1326. [Google Scholar]
- Roth, J.; Glick, S.M.; Yalow, R.S.; Bersonsa, S.A. Hypoglycemia: A potent stimulus to secretion of growth hormone. Science 1963, 140, 987–988. [Google Scholar] [CrossRef]
- Chandrashekar, V.; Zaczek, D.; Bartke, A. The consequences of altered somatotropic system on reproduction. Biol. Reprod. 2004, 71, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Ai, N.; Chen, W.; Wong, Q.W.; Ge, W. Loss of Growth Hormone Gene (gh1) in Zebrafish Arrests Folliculogenesis in Females and Delays Spermatogenesis in Males. Endocrinology 2019, 160, 568–586. [Google Scholar] [CrossRef] [Green Version]
- Benedet, S.; Björnsson, B.T.; Taranger, G.L.; Taranger, G.L.; Andersson, E. Cloning of somatolactin alpha, beta forms and the somatolactin receptor in Atlantic salmon: Seasonal expression profile in pituitary and ovary of maturing female broodstock. Reprod. Biol. Endocrinol. 2008, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Ge, W. Differential regulation of gonadotropin receptors (fshr and lhcgr) by epidermal growth factor (EGF) in the zebrafish ovary. Gen. Comp. Endocrinol. 2013, 181, 288–294. [Google Scholar] [CrossRef]
- Fukamachi, S.; Yada, T.; Meyer, A.; Kinoshita, M. Effects of constitutive expression of somatolactin alpha on skin pigmentation in medaka. Gene 2009, 442, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.; Sugimoto, M.; Zhu, Y. Production and purification of recombinant somatolactin beta and its effects on melanosome aggregation in zebrafish. Gen. Comp. Endocrinol. 2006, 145, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, D.; Tran, N.T.; Nguyen, N. The effects of the members of growth hormone family knockdown in zebrafish development. Gen. Comp. Endocrinol. 2007, 150, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.A.; Hashem, A.M.; Ibrahim, A.A.; Mousa, M.A. Effect of stress during handling, seawater acclimation, confinement, and induced spawning on plasma ion levels and somatolactin-expressing cells in mature female Liza ramada. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2012, 317, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Sudo, R.; Suetake, H.; Suzuki, Y.; Aoyama, J.; Tsukamoto, K. Profiles of mRNA expression for prolactin, growth hormone, and somatolactin in Japanese eels, Anguilla japonica: The effect of salinity, silvering and seasonal change. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 10–16. [Google Scholar] [CrossRef]
- Shu, Y.Q.; Lou, Q.Y.; Dai, Z.R.; Dai, X.G.; He, J.Y.; Hu, W.; Yin, Z. The basal function of teleost prolactin as a key regulator on Ion uptake identified with zebrafish knockout models. Sci. Rep. 2016, 6, 18597. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, B.; Ge, W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol. Endocrinol. 2015, 29, 76–98. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.C.; Martínez, C.; Cáceres, M.; Martínez, J. Research on growth factors in periodontology. Periodontology 2015, 67, 234–250. [Google Scholar] [CrossRef]
- Schneider, M.R.; Wolf, E. The epidermal growth factor receptor ligands at a glance. J. Cell. Physiol. 2009, 218, 460–466. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, R.M.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, S.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Yatabe, Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010, 277, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Mitsuhashi, T.; Kubota, K.; Kuzuya, N.; Uchimura, H. Epidermal Growth Factor Stimulates Growth Hormone Secretion from Superfused Rat Adenohypophyseal Fragments. Endocrinology 1984, 2, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Saito, T. The effects of epidermal growth factor on cell proliferation and prolactin production by GH3 rat pituitary cells. J. Cell. Physiol. 1984, 120, 249–256. [Google Scholar] [CrossRef]
- Przylipiak, A.; Kiesel, L.; Rabe, T.; Helm, K.; Przylipiak, M.; Runnebaum, B. Epidermal growth factor stimulates luteinizing hormone and arachidonic acid release in rat pituitary cells. Mol. Cell. Endocrinol. 1988, 57, 157–162. [Google Scholar] [CrossRef]
- Altschuler, L.R.; Parisi, M.N.; Cageao, L.F.; Chiocchio, S.R.; Fernandez-Pol, J.A.; Zaninovich, A.A. Epidermal Growth Factor Stimulates Thyrotropin Secretion in the Rat. Neuroendocrinology 1993, 57, 23–27. [Google Scholar] [CrossRef]
- Cooper, O.; Vlotides, G.; Fukuoka, H.; Greene, M.; Melmed, S. Expression and function of ErbB receptors and ligands in the pituitary. Endocr. Relat. Cancer 2011, 18, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.W.; Ge, W. Differential regulation of gonadotropins (FSH and LH) and growth hormone (GH) by neuroendocrine, endocrine, and paracrine factors in the zebrafish—An in vitro approach. Gen. Comp. Endocrinol. 2009, 160, 183–193. [Google Scholar] [CrossRef]
- Qin, X.F.; Ye, C.; Zhou, X.Y.; Jia, J.Y.; Xu, S.H.; Hu, Q.Y.; Hu, G.F. NK3R Mediates the EGF-Induced SLα Secretion and mRNA Expression in Grass Carp Pituitary. Int. J. Mol. Sci. 2018, 20, 91. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; He, M.; Wang, X.Y.; Wong, A.O.L. Grass carp somatolactin: II. Pharmacological study on postreceptor signaling mechanisms for PACAP-induced somatolactin-α and -β gene expression. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 477–490. [Google Scholar] [CrossRef]
- Wong, A.O.; Ng, S.; Lee, E.K.; Leung, R.C.; Ho, W.K. Somatostatin inhibits (D-Arg6, Pro9-NEt) salmon gonadotropin-releasing hormone- and dopamine D1-stimulated growth hormone release from perifused pituitary cells of Chinese grass carp, Ctenopharyngodon idellus. Gen. Comp. Endocrinol. 1998, 110, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Y.; Xu, S.H.; Ye, C.; Jia, J.Y.; Zhou, L.L.; Hu, G.F. Novel Pituitary Actions of Epidermal Growth Factor: Receptor Specificity and Signal Transduction for UTS1, EGR1, and MMP13 Regulation by EGF. Int. J. Mol. Sci. 2019, 20, 5172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib, S.D.; Wierman, M.E.; Shupnik, M.A.; Chin, W.W. Molecular Biology of the Pituitary Gonadotropins. Endocr. Rev. 1990, 11, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, H.; Cao, X.; Motola, S.; Popliker, M.; Conti, M.; Tsafriri, A. Epidermal Growth Factor Family Members: Endogenous Mediators of the Ovulatory Response. Endocrinology 2006, 146, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Su, Y.Q.; Ariga, M.; Law, E.; Jin, S.L. EGF-Like Growth Factors As Mediators of LH Action in the Ovulatory Follicle. Science 2004, 303, 682–684. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Mizutani, T.; Yamada, K.; Kajitani, T.; Yazawa, T.; Yoshino, M.; Miyamoto, K. Expression of epiregulin and amphiregulin in the rat ovary. J. Mol. Endocrinol. 2004, 33, 281–291. [Google Scholar] [CrossRef]
- Ono, M.; Takayama, Y.; Rand-Weaver, M.; Sakata, S.; Yasunaga, T.; Noso, T.; Kawauchi, H. cDNA cloning of somatolactin, a pituitary protein related to growth hormone and prolactin. Proc. Natl. Acad. Sci. USA 1990, 87, 4330–4334. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y. Cloning of somatolactin alpha and beta cDNAs in zebrafish and phylogenetic analysis of two distinct somatolactin subtypes in fish. J. Endocrinol. 2004, 182, 509–518. [Google Scholar] [CrossRef]
- Murdoch, G.H.; Potter, E.; Nicolaisen, A.K.; Evans, R.M.; Rosenfeld, M.G. Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature 1982, 300, 192–194. [Google Scholar] [CrossRef]
- Trott, J.F.; Vonderhaar, B.K.; Hovey, R.C. Historical Perspectives of Prolactin and Growth Hormone as Mammogens, Lactogens and Galactagogues—Agog for the Future! J. Mammary Gland Biol. Neoplasia 2008, 13, 3–11. [Google Scholar] [CrossRef]
- Shu, T.T.; Shu, Y.Q.; Gao, Y.P.; Jin, X.; He, J.Y.; Zhai, G.; Yin, Z. Depletion of Tissue-Specific Ion 544 Transporters Causes Differential Expression of PRL Targets in Response to Increased Levels of 545 Endogenous PRL. Front. Endocrinol. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Jonathan, N.; Chen, S.L.; Dunckley, J.A.; LaPensee, C.; Kansra, S. Estrogen receptor-alpha mediates the epidermal growth factor-stimulated prolactin expression and release in lactotrophs. Endocrinology 2009, 150, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biran, J.; Palevitch, O.; Ben-Dor, S.; Levavi-Sivan, B. Neurokinin Bs and neurokinin B receptors in zebrafish- potential role in controlling fish reproduction. Proc. Natl. Acad. Sci. USA 2012, 109, 10269–10274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.F.; He, M.L.; Ko, W.K.; Lin, C.Y.; Wong, A.O.L. Novel pituitary actions of TAC3 gene products in fish model: -Receptor specificity and signal transduction for prolactin and somatolactin alpha regulation by neurokinin B (NKB) and NKB-related peptide in carp pituitary cells. Endocrinology 2014, 155, 3582–3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.; Conti, M. G-protein-coupled receptor signaling and the EGF network in endocrine systems. Trends Endocrinol. Metab. 2005, 16, 320–326. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Qin, Q.; Xu, S.; Zhou, L.; Xia, C.; Shi, X.; Zhang, H.; Jia, J.; Ye, C.; Yin, Z.; et al. Pituitary Actions of EGF on Gonadotropins, Growth Hormone, Prolactin and Somatolactins in Grass Carp. Biology 2020, 9, 279. https://doi.org/10.3390/biology9090279
Hu Q, Qin Q, Xu S, Zhou L, Xia C, Shi X, Zhang H, Jia J, Ye C, Yin Z, et al. Pituitary Actions of EGF on Gonadotropins, Growth Hormone, Prolactin and Somatolactins in Grass Carp. Biology. 2020; 9(9):279. https://doi.org/10.3390/biology9090279
Chicago/Turabian StyleHu, Qiongyao, Qinbo Qin, Shaohua Xu, Lingling Zhou, Chuanhui Xia, Xuetao Shi, Huiying Zhang, Jingyi Jia, Cheng Ye, Zhan Yin, and et al. 2020. "Pituitary Actions of EGF on Gonadotropins, Growth Hormone, Prolactin and Somatolactins in Grass Carp" Biology 9, no. 9: 279. https://doi.org/10.3390/biology9090279




